
Effect of human epidermal growth factor receptor 2 overexpression in metastatic colorectal cancer on standard chemotherapy outcomes
Highlight box
Key findings
• The tumor response and survival of patients with metastatic colorectal cancer (mCRC) after standard chemotherapy did not differ by the HER2 expression.
What is known and what is new?
• In mCRC, the prognostic relevance of the HER2 remains controversial.
• The tumor response, progression-free survival, and overall survival among patients with mCRC who underwent first- and second-line chemotherapy did not significantly differ according to HER2 expression.
What is the implication, and what should change now?
• The status of HER2 expression need not be considered when choosing regimens as the current first- and second-line treatments.
Introduction
Background
Colorectal cancer (CRC) is the third most frequently diagnosed cancer worldwide. It is the second leading cause of cancer death, accounting for 10% of the total (1). Approximately 20% of patients are initially diagnosed with metastatic CRC (mCRC), while around 70% of patients with early-stage disease progress to metastatic disease (2,3). The use of chemotherapy with additional targeted therapies, including anti-epidermal growth factor receptor (EGFR) antibody (cetuximab or panitumumab) or anti-vascular endothelial growth factor (VEGF) antibody (bevacizumab), has improved the overall survival (OS) of patients with mCRC by approximately 25–35 months (4-11).
The human epidermal growth factor receptor-2 (HER2; also known as the c-erbB-2 protein) is encoded by the ERBB2 gene located on chromosome 17q21. The HER2 protein acts as a receptor tyrosine kinase and activates the phosphatidylinositol 3-kinase (PI3K/AKT) and RAS/RAF/MEK/ERK pathways. Therefore, ERBB2 genes and the HER2 protein regulate cell proliferation and survival (12). Dysregulated overexpression or amplification of HER2 drives oncogenesis (13), occurring in about 20% of invasive breast cancer (14), about 20% of gastric cancer (15), and about 1–30% of lung (16). The presence of HER2 overexpression/amplification is associated with a worse prognosis than HER2-negative cancer. It has become clinically valuable as a predictive marker of response to specific treatments; the HER2 protein is also a treatment target (14-17).
Rationale and knowledge gap
In mCRC, the prevalence of HER2 overexpression or amplification reported in various studies ranges from about 1.0% to 14.0% (18-21). Several studies have investigated the role of HER2 overexpression or amplification in mCRC: some have reported that HER2 gene amplification was associated with liver metastases (22), lung metastases (23), or central nervous system metastases (24). However, the prognostic relevance of HER2 in mCRC remains controversial, in contrast to that in breast cancer. In CRC, overexpression or amplification of HER2 is a predictive biomarker of resistance to anti-EGFR therapy (23,25) and an independent prognostic factor (22,26). However, other studies have reported no association between HER2 expression and prognosis (19,27). Moreover, all of the HER2 dual blockade treatment, trastuzumab-lapatinib (HERACLES trial), trastuzumab-pertuzumab (MYPATHWAY trial), and trastuzumab deruxtecan (DESTINY-CRC01 trial), have proven effective as third-line and beyond. The first- and second-line treatment standard for mCRC remains the combination of anti-EGFR/anti-VEGF and 5-fluorouracil backbone chemotherapy, regardless of HER2 overexpression (3,28-30).
Objective
In this study, we aimed to evaluate the impact of HER2 overexpression on treatment outcomes when employing standard first- and second-line chemotherapy and to analyze the prognostic utility of HER2 overexpression in patients with mCRC. We present this article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-375/rc).
Methods
Patients
This retrospective study included patients with mCRC who received standard first- and second-line chemotherapy at Samsung Medical Center, Seoul, Korea, between January 15, 2017, and February 5, 2022. Simultaneously, the patients were available for the c-erbB-2 immunohistochemistry (IHC) test. Clinical data, including physical examinations, pathology reports, imaging, laboratory data, and demographic information, were collected from the patients’ electronic medical records. History of prior surgical, adjuvant, or palliative treatment, toxicity profile, treatment response, and survival data were documented. The last survival and treatment response data collection date was September 13, 2022. This study was approved by the Institutional Review Board (IRB) of the Samsung Medical Center in Seoul (IRB No. 2022-12-067), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As this study was performed retrospectively based on existing medical records, the requirement for written consent from the patients was waived by the IRB.
HER-2 (c-erbB-2) immunohistochemistry test
The IHC HER2 test was performed using a VENTANA anti-HER2/neu rabbit monoclonal primary antibody (clone 4B5, Ventana Medical System; Roche, Tucson, AZ, USA) at the Department of Pathology, Samsung Medical Center, Seoul. The results of the HER2 IHC assay were interpreted according to the HERACLES Diagnostic Criteria (31). An HER2 positive tumor was defined as an IHC intensity score of 3+ in >50% of the tumor cells, an IHC intensity score of 3+ in 10–50% of the tumor cell and fluorescence in situ hybridization (FISH) positivity, or an IHC intensity score of 2+ in >50% of the tumor cells and FISH positivity. FISH positivity was defined as an HER2:CEP17 ratio higher than two in >50% of the tumor cells.
Outcomes and statistical analyses
Response to treatment was assessed by physicians according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The ORR was defined as the percentage of patients with a complete response (CR) or partial response (PR). Meanwhile, the disease control rate (DCR) was defined as the percentage of patients with CR, PR, or stable disease (SD). The PFS was defined as the duration from initiating the first treatment cycle to disease progression or any cause of death. The OS was defined as the duration from initiating the first treatment cycle to any cause of death. Categorical variables were compared using Fisher’s exact test. Using Cox regression, we conducted univariate and multivariate analyses to assess prognostic factors. We estimated all univariate models and included independent variables with P<0.1 in the multivariate model. Also, clinically significant variables were included using background knowledge. We estimated PFS and OS using Kaplan-Meier curve analyses. We performed the log-rank test to assess survival differences according to HER2 status. A P value <0.05 was considered to reflect statistical significance. All statistical analyses were performed using SPSS® version 25 (IBM, Armonk, NY, USA).
Results
Patient characteristics
A total of 111 patients diagnosed with mCRC and available for the HER2 IHC test between January 15, 2017, and February 05, 2022, were included in the analysis (Figure 1). Among them, 6 patients were excluded for lack of data on chemotherapies and tumor response; 1 was HER2-positive, and 5 were HER2-negative. Patient characteristics are presented in Table 1. The median age was 58 years (21–84 years), and 65 (61.9%) patients were male. Microsatellite instability (MSI-high) was detected in 4 (3.8%) patients. The median total tumor mutational burden (TMB) was 6.3 mutations per Megabase (mt/Mb) in all patients, and 22 (21.0%) patients were classified into the TMB-high group (≥10 mt/Mb). Almost all patients received FOLFIRI (5-fluorouracil, leucovorin, and irinotecan) and FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) as first- and second-line chemotherapy, respectively.
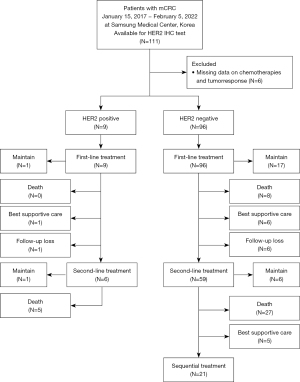
Table 1
| Characteristics | No. of patients (N=105, 100%) | HER2-negative (N=96, 91%) | HER2-positive (N=9, 9%) | P |
|---|---|---|---|---|
| Age (years) | 58 [21–84] | 58 [21–84] | 59 [34–67] | 0.483 |
| Sex | ||||
| Male | 65 (61.9) | 59 (61.5) | 6 (66.7) | 1.000 |
| Female | 40 (38.1) | 37 (38.5) | 3 (33.3) | |
| ECOG | ||||
| 0–1 | 96 (91.4) | 87 (90.6) | 9 (100.0) | 1.000 |
| ≥2 | 9 (8.6) | 9 (9.4) | 0 | |
| Primary site of disease | ||||
| Colon | 71 (67.6) | 63 (65.6) | 8 (88.9) | 0.266 |
| Rectum | 34 (32.4) | 33 (34.4) | 1 (11.1) | |
| Tumor sidedness | ||||
| Right | 23 (21.9) | 20 (20.8) | 3 (33.3) | 0.407 |
| Left† | 82 (78.1) | 76 (79.2) | 6 (66.7) | |
| KRAS mutation‡ | ||||
| No | 59 (56.2) | 53 (55.2) | 6 (66.7) | 0.728 |
| Yes | 46 (43.8) | 43 (44.8) | 3 (33.3) | |
| NRAS mutation‡ | ||||
| No | 103 (98.1) | 94 (97.9) | 9 (100.0) | 1.000 |
| Yes | 2 (1.9) | 2 (2.1) | 0 | |
| BRAF mutation‡ | ||||
| No | 97 (92.4) | 89 (92.7) | 8 (88.9) | 0.524 |
| Yes | 8 (7.6) | 7 (7.3) | 1 (11.1) | |
| MMR status‡ | ||||
| MSS | 101 (96.2) | 92 (95.8) | 9 (100.0) | 1.000 |
| MSI-high | 4 (3.8) | 4 (4.2) | 0 | |
| TMB‡ (mt/mb) | 6.30 [0–125] | 6.3 [0–125] | 7.00 [2.3–10.2] | |
| TMB-low | 83 (79.0) | 75 (78.1) | 8 (88.9) | 0.681 |
| TMB-high | 22 (21.0) | 21 (21.9) | 1 (11.1) | |
| Biological targeted agents | ||||
| Antiangiogenic inhibitors containing | 99 (94.3) | 90 (93.8) | 9 (100.0) | 1.000 |
| Anti-EGFR inhibitor containing | 9 (8.6) | 8 (8.3) | 1 (11.1) | 0.569 |
| First-line chemotherapy regimen | ||||
| Bevacizumab + FOLFIRI | 27 (25.7) | 25 (26.0) | 2 (22.2) | |
| Bevacizumab + FOLFOX | 63 (60.0) | 57 (59.4) | 6 (66.7) | |
| Cetuximab + FOLFIRI | 9 (8.6) | 8 (8.3) | 1 (11.1) | |
| Cetuximab + FOLFOX | 0 | 0 | 0 | |
| Other‡ | 6 (5.4) | 6 (6.3) | 0 | |
| Second-line chemotherapy regimen | (n=64) | (n=58) | (n=6) | |
| Bevacizumab + FOLFIRI | 28 (43.8) | 24 (41.4) | 4 (66.7) | |
| Bevacizumab + FOLFOX | 19 (29.7) | 17 (29.3) | 2 (33.3) | |
| Cetuximab + FOLFIRI | 1 (1.6) | 1 (1.7) | 0 | |
| Cetuximab + FOLFOX | 0 | 0 | 0 | |
| Aflibercept + FOLFIRI | 10 (15.6) | 10 (17.2) | 0 | |
| Aflibercept + FOLFOX | 1 (1.6) | 1 (1.7) | 0 | |
| Other§ | 5 (7.8) | 5 (8.6) | 0 |
Data are presented as n (%) or median [range]. †, tumors located at the descending colon, sigmoid colon, and rectum were defined as left-sided colorectal cancer. ‡, KRAS, NRAS, and BRAF mutation, MMR status, and TMB were tested using next-generation sequencing. The cut-off value for TMB-high was 10 mutations/megabase. §, other chemotherapies included capecitabine, XELOX (capecitabine, leucovorin, and oxaliplatin), or FOLFIRI or FOLFOX without target agents. BRAF, B-Raf Proto-Oncogene; ECOG, European Cooperative Oncology Group score; FOLFIRI, 5-fluoruracil, leucovorin, and irinotecan; FOLFOX, 5-fluoruracil, leucovorin, and oxaliplatin; HER2, human epidermal growth factor receptor-2; KRAS, Kirsten ras oncogene homolog; MMR, mismatch repair; MSI, microsatellite instability; MSS, microsatellite stable; NRAS, neuroblastoma RAS viral oncogene homolog; TMB, tumor mutational burden.
In addition, as first- and second-line chemotherapy, 85.7% and 44.8% of patients received bevacizumab, while 8.6% and 1.0% received cetuximab, respectively. Among the 105 patients, 9 (9%) had HER2-positive tumors. Tumor-sidedness was not significantly different between the HER2-positive and HER2-negative groups. The primary tumor site was more often in the colon in the HER2-positive group (88.9%, 8/9) than in the HER2-negative group (65.6%, 63/96), but this was not statistically significant. Furthermore, the distributions of KRAS, NRAS, and BRAF mutations did not differ regarding HER2 status.
Efficacy of chemotherapy according to HER2 expression
We compared the efficacy of first- or second-line chemotherapy concerning HER2 overexpression. For patients who underwent first-line chemotherapy (Table 2), the ORR and DCR were 43.8% [46/105, 95% confidence interval (CI): 34.1–53.8%] and 92.4% (97/105, 95% CI: 85.5–96.7%), respectively. Among the 9 patients with HER2-positive tumors, 3 (11.1%) achieved CR, 2 achieved PR, and 2 maintained SD, resulting in an ORR of 55.6% (5/9, 95% CI: 21.2–86.3%) and a DCR of 77.8% (7/9, 95% CI: 40.0–97.2%). Among patients with mCRC who underwent first-line chemotherapy, the ORR (P=0.501) and DCR (P=0.140) did not differ between HER2-positive or HER2-negative tumors. Concerning second-line chemotherapy, there were no significant differences in ORR (P=1.000) and DCR (P=1.000) in mCRC patients with HER2-positive and negative tumors (Table 3).
Table 2
| Best response to first line | Total patients (N=105) | HER2 negative (N=96) | HER2 positive (N=9) | P |
|---|---|---|---|---|
| Objective response rate (%, 95% CI†) | 43.8 (34.1, 53.8) | 42.7 (32.7, 53.2) | 55.6 (21.2, 86.3) | 0.501 |
| Disease control rate (%, 95% CI†) | 92.4 (85.5, 96.7) | 93.8 (86.9, 97.7) | 77.8 (40.0, 97.2) | 0.140 |
| Complete response | 4 (3.8) | 1 (1.0) | 3 (33.3) | |
| Partial response | 42 (40.0) | 40 (41.7) | 2 (22.2) | |
| Stable disease | 51 (48.6) | 49 (51.0) | 2 (22.2) | |
| Progressive disease | 8 (7.6) | 6 (6.3) | 2 (22.2) |
Objective response rate and disease control rate were compared using Fisher’s exact test. †, The 95% confident interval was calculated using the Clopper-Pearson method. HER2, human epidermal growth factor receptor-2; CI, confident interval.
Table 3
| Best response to second line | Total patients (N=64) | HER2 negative (N=59) | HER2 positive (N=5) | P |
|---|---|---|---|---|
| Objective response rate (%, 95% CI†) | 18.8 (10.1, 30.5) | 18.6 (9.7, 30.9) | 20.0 (0.5, 71.6) | 1.000 |
| Disease control rate (%, 95% CI†) | 79.7 (67.8, 88.7) | 79.7 (67.2, 89.0) | 80.0 (28.4, 99.5) | 1.000 |
| Complete response | 0 | 0 | 0 | |
| Partial response | 12 (18.8) | 11 (18.6) | 1 (20.0) | |
| Stable disease | 39 (60.9) | 36 (61.0) | 3 (60.0) | |
| Progressive disease | 13 (20.3) | 12 (20.3) | 1 (20.0) |
Objective response rate and disease control rate were compared using Fisher’s exact test. †, The 95% confidential interval was calculated using the Clopper-Pearson method. HER2, human epidermal growth factor receptor-2; CI, confident interval.
Predictive and prognostic analysis according to HER2 expression and other variables
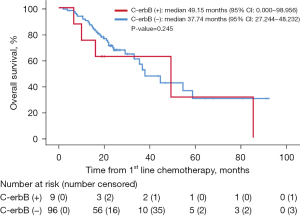
Among patients who underwent first-line chemotherapy, the median PFS was 11.05 months (95% CI: 9.416–12.682), and there was no difference in PFS to first-line chemotherapy between HER2-positive and -negative tumors (P=0.431, Figure 2A). The median PFS to second-line chemotherapy was also not different between HER2-positive and -negative tumors (P=0.861; Figure 2B). The median OS of the 105 patients was 37.74 months (95% CI: 26.562–49.914). Like PFS, the median OS was longer in the HER2-positive group, but this lacked statistical significance (P=0.245, Figure 3).
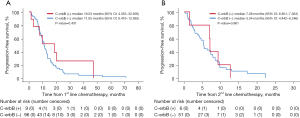
The results of the prognostic analysis of PFS and OS to first-line therapy are provided in Tables 4,5. When HER2 overexpression was assessed as a prognostic marker, there was no evidence as an independent factor for PFS to first-line chemotherapy in multivariate analysis [hazard ratio (HR), 0.82; 95% CI: 0.35–1.94, P=0.652]. BRAF mutation and MSI-high were significant risk factors for the PFS to first-line treatment in univariate and multivariate analyses. The HR of BRAF mutation was 2.49 (95% CI: 1.04–5.96, P=0.041), and the HR of MSI-high was 3.83 (95% CI: 1.24–11.8, P=0.019), in multivariate analysis (Table 4). Similarly, there was no evidence that HER2 overexpression was an independent prognostic factor for OS in the multivariate analysis (HR, 1.50; 95% CI: 0.57–3.90; P=0.411). ECOG and BRAF mutation were statistically significant, with HRs 6.05 (95% CI: 1.98–18.5, P=0.002) and 3.06 (95% CI: 1.03–9.06, P=0.043, Table 5), respectively.
Table 4
| Variables | No. of patients (n=105) | No. of events (n=79) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| Age | 0.99 (0.97, 1.01) | 0.195 | 0.99 (0.97, 1.01) | 0.407 | |||
| Sex | 1.20 (0.76, 1.90) | 0.438 | 1.20 (0.73, 1.96) | 0.480 | |||
| Male | 65 | 49 | |||||
| Female | 40 | 30 | |||||
| ECOG | 1.32 (0.61, 2.89) | 0.481 | 1.57 (0.66, 3.76) | 0.311 | |||
| 0–1 | 96 | 72 | |||||
| ≥2 | 9 | 7 | |||||
| Location | 1.08 (0.67, 1.75) | 0.739 | 0.99 (0.58, 1.69) | 0.965 | |||
| Colon | 71 | 54 | |||||
| Rectum | 34 | 25 | |||||
| Sidedness | |||||||
| Right | 23 | 15 | 1.08 (0.61, 1.90) | 0.793 | 1.06 (0.54, 2.07) | 0.860 | |
| Left† | 82 | 64 | |||||
| Differentiation | (n=83) | (n=66) | |||||
| Well | 8 | 4 | Reference | ||||
| Moderate | 66 | 53 | 0.96 (0.35, 2.69) | 0.944 | |||
| Poor | 9 | 9 | 0.76 (0.22, 2.60) | 0.658 | |||
| KRAS‡ | 1.03 (0.65, 1.64) | 0.892 | |||||
| Wild type | 59 | 46 | |||||
| Mutation | 46 | 33 | |||||
| NRAS‡ | 0.75 (0.18, 3.11) | 0.690 | |||||
| Wild type | 103 | 77 | |||||
| Mutation | 2 | 2 | |||||
| BRAF‡ | 2.53 (1.07, 6.00) | 0.034* | 2.49 (1.04, 5.96) | 0.041* | |||
| Wild type | 97 | 73 | |||||
| Mutation | 8 | 6 | |||||
| MMR status‡ | 4.06 (1.44, 11.5) | 0.008* | 3.83 (1.24, 11.8) | 0.019* | |||
| MSS | 101 | 75 | |||||
| MSI-high | 4 | 4 | |||||
| TMB‡ | 1.13 (0.67, 1.92) | 0.648 | |||||
| Low | 83 | 60 | |||||
| High | 22 | 19 | |||||
| HER2 | 0.73 (0.33, 1.61) | 0.433 | 0.82 (0.35, 1.94) | 0.652 | |||
| Negative | 96 | 72 | |||||
| Positive | 9 | 7 | |||||
†, tumors located at the descending colon, sigmoid colon, and rectum were defined as left-sided colorectal cancer. ‡, KRAS, NRAS, and BRAF mutation, MMR status, and TMB were tested using next-generation sequencing. The cut-off value for TMB-high was 10 mutations/Megabase. *, P value <0.05. HR, hazard ratio; BRAF, B-Raf Proto-Oncogene; ECOG, European Cooperative Oncology Group score; HER2, human epidermal growth factor receptor-2; KRAS, Kirsten ras oncogene homolog; MMR, mismatch repair; MSI, microsatellite instability; MSS, microsatellite stable; NRAS, neuroblastoma RAS viral oncogene homolog; TMB, tumor mutational burden.
Table 5
| Variables | No. of patients (n=105) | No. of events (n=40) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| Age | 1.00 (0.98, 1.03) | 0.758 | 1.00 (0.97, 1.02) | 0.799 | |||
| Sex | 1.18 (0.61, 2.29) | 0.620 | 0.95 (0.46, 1.97) | 0.895 | |||
| Male | 65 | 26 | |||||
| Female | 40 | 14 | |||||
| ECOG | 4.27 (1.74, 10.5) | 0.002* | 6.05 (1.98, 18.5) | 0.002* | |||
| 0–1 | 96 | 34 | |||||
| ≥2 | 9 | 6 | |||||
| Location | 0.66 (0.33, 1.32) | 0.238 | 0.55 (0.24, 1.25) | 0.152 | |||
| Colon | 71 | 29 | |||||
| Rectum | 34 | 11 | |||||
| Sidedness | 0.78 (0.39, 1.56) | 0.474 | 1.06 (0.46, 2.45) | 0.884 | |||
| Right | 23 | 11 | |||||
| Left | 82 | 29 | |||||
| Differentiation | (n=83) | (n=35) | |||||
| Well | 8 | 2 | Reference | ||||
| Moderate | 66 | 26 | 1.41 (0.33, 5.97) | 0.640 | |||
| Poor | 9 | 7 | 2.52 (0.50, 12.6) | 0.260 | |||
| KRAS‡ | 1.09 (0.58, 2.07) | 0.787 | |||||
| Wild type | 59 | 23 | |||||
| Mutation | 46 | 17 | |||||
| NRAS‡ | 1.62 (0.22, 11.9) | 0.634 | |||||
| Wild type | 103 | 39 | |||||
| Mutation | 2 | 1 | |||||
| BRAF‡ | 2.70 (0.93, 7.80) | 0.067 | 3.06 (1.03, 9.06) | 0.043* | |||
| Wild type | 97 | 36 | |||||
| Mutation | 8 | 4 | |||||
| MMR status‡ | 1.68 (0.51, 5.55) | 0.394 | 2.51 (0.59, 10.7) | 0.212 | |||
| MSS | 101 | 37 | |||||
| MSI-high | 4 | 3 | |||||
| TMB‡ | 1.02 (0.50, 2.09) | 0.962 | |||||
| Low | 83 | 30 | |||||
| High | 22 | 10 | |||||
| HER2 | 1.50 (0.57, 3.90) | 0.411 | 1.56 (0.56, 4.34) | 0.394 | |||
| Negative | 96 | 35 | |||||
| Positive | 9 | 5 | |||||
†, tumors located at the descending colon, sigmoid colon, and rectum were defined as left-sided colorectal cancer. ‡, KRAS, NRAS, and BRAF mutation, MMR status, and TMB were tested using next-generation sequencing. The cut-off value for TMB-high was 10 mutations/Megabase. *, P value <0.05. HR, Hazard ratio; BRAF, B-Raf Proto-Oncogene; ECOG, European Cooperative Oncology Group score; HER2, human epidermal growth factor receptor-2; KRAS, Kirsten ras oncogene homolog; MMR, mismatch repair; MSI, Microsatellite instability; MSS, microsatellite stable; NRAS, neuroblastoma RAS viral oncogene homolog; TMB, tumor mutational burden.
Discussion
Key findings
The present study evaluated the impact of HER2 overexpression on the outcomes of patients with mCRC who underwent standard first- and second-line chemotherapy and analyzed the prognostic role of HER2 overexpression. The tumor response, PFS, and OS among patients with mCRC who underwent first- and second-line chemotherapy did not significantly differ according to HER2 expression. Therefore, HER2 overexpression was not predictive of the response rate for first- and second-line chemotherapy in patients with mCRC. Furthermore, HER2 overexpression was not a prognostic factor for OS. These findings suggest that HER2 expression need not be considered when choosing first- or second-line treatment regimens. However, these findings must be interpreted with caution as the study patients did not receive anti-HER2 directed therapy, a useful treatment option for HER2-positive mCRC.
The reported prevalence of HER2 overexpression or amplification in various studies has ranged from 1.0% to 14.0% (18-22,25-27). In the present study, 9% of patients with mCRC had HER2-positive tumors, toward the higher end of incidences reported in previous studies. The definition of HER2 positivity in CRC has not been standardized, partly because the results appear to differ depending on the detection method, which may include IHC and FISH or silver in situ hybridization (SISH). In CRC tissues, HER2 overexpression occurs through mechanisms distinct from those inducing HER2 gene amplification, and HER2 overexpression is detected via IHC more frequently than HER2 gene amplification (22,26,27). In CRC, false positives due to the low prevalence of HER2 overexpression or amplification could be one of the reasons for the differences in reported results.
Comparison with similar research
Notably, there is no consensus on the prognostic value of HER2 overexpression yet. Feng et al. reported that HER2 overexpression was associated with TP53 mutation and that adjuvant 5-fluorouracil prolonged OS in patients with HER2-positive Stage II CRC (32). In the PETACC-8 trial, ERBB2 alterations, including mutation and overexpression/amplification, were associated with a poor prognosis in the adjuvant setting, with shorter times to recurrence and shorter OS (33). However, HER2 overexpression/amplification in early-stage CRC has also been reported not to impact OS (34,35). In patients with mCRC in a palliative care setting, HER2 overexpression/amplification was associated with resistance to chemotherapy, and HER2 has therefore been considered a negative prognostic factor (23,36-39). However, when analyzing the data of 3,256 patients from the QUASAR, FOCUS, and PICCOLO trials, Richman et al. found no difference in OS and PFS among patients with and without HER2 overexpression/amplification (19). These findings are consistent with those of the present study. In the study, the status of HER2 expression was not an independent prognostic factor to PFS to first-line chemotherapy and OS in the univariate and multivariate analyses.
Explanations of findings
HER2 is downstream of the EGFR pathway and has been reported to activate the PI3K/AKT and RAS/RAF/MEK/ERK axis independently of EGFR, resulting in tumor progression (36,40-44). Persistent activation of HER2 and downstream pathways by HER-2 overexpression/amplification is considered one of the resistance mechanisms to anti-EGFR treatment (i.e., cetuximab or panitumumab) (23,25,36-39,45,46). In our analysis, most patients were treated with bevacizumab-containing chemotherapy as the first-line (90/105, 85.7%) and second-line (47/64, 73.4%) treatment regimens. Although both EGFR and HER2 are expressed in cancer cells, VEGF is involved in vascular proliferation in the tumor environment (47) but was not assessed in this study. VEGF expression could be why there was no difference between the HER2-positive and -negative groups in the survival analyses. Thus, while HER2 overexpression or amplification may be a predictive factor for anti-EGFR treatment, it does not appear to be a prognostic factor for mCRC.
In addition to testing for EGFR or RAS mutation, assessment of HER2 overexpression/amplification is needed to identify patients who may be less responsive to anti-EGFR antibody treatment and may benefit from treatment with other drugs (i.e., anti-VEGF antibody). However, in this study, only one of the patients with HER2-positive tumors was administered cetuximab. All the others were administered bevacizumab as a first-line treatment, and comparisons of the efficacy of cetuximab and bevacizumab were not conducted.
Limitations
This study had several limitations. First, this study had a small sample size and was retrospective in nature; our results should therefore be confirmed in a prospective study. Second, only Asian patients with mCRC were enrolled in the study, limiting the generalizability because of differences in the molecular profiles and clinical features between Western and Eastern patients with mCRC. Third, the analyses did not include patients who received anti-HER2-directed therapy.
Conclusions
There were no statistically significant differences in tumor response to first- and second-line chemotherapy, PFS, or OS in patients with mCRC with HER2-negative or -positive tumors. These findings suggest that HER2 overexpression need not be considered when choosing regimens as the current first- and second-line treatments.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HR20C0025).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-375/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-375/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-375/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-375/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Samsung Medical Center in Seoul (No. 2022-12-067), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As this study was performed retrospectively based on existing medical records, the requirement for written consent from the patients was waived by the IRB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. Globocan: cancer fact sheets—colorectal cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-factsheet.pdf. Accessed 13 Nov 2022.
- Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022;72:409-36. [Crossref] [PubMed]
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10-32. [Crossref] [PubMed]
- Botrel TEA, Clark LGO, Paladini L, et al. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer 2016;16:677. [Crossref] [PubMed]
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. [Crossref] [PubMed]
- Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005;23:3706-12. [Crossref] [PubMed]
- Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer 2012;12:89. [Crossref] [PubMed]
- Petrelli F, Borgonovo K, Cabiddu M, et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials. Clin Colorectal Cancer 2013;12:145-51. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology 2009;77:113-9. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [Crossref] [PubMed]
- Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644-6. [Crossref] [PubMed]
- Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med 2011;135:55-62. [Crossref] [PubMed]
- Martínez-Sáez O, Prat A. Current and Future Management of HER2-Positive Metastatic Breast Cancer. JCO Oncol Pract 2021;17:594-604. [Crossref] [PubMed]
- Ma C, Wang X, Guo J, et al. Challenges and future of HER2-positive gastric cancer therapy. Front Oncol 2023;13:1080990. [Crossref] [PubMed]
- Riudavets M, Sullivan I, Abdayem P, et al. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021;6:100260. [Crossref] [PubMed]
- Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020;17:33-48. [Crossref] [PubMed]
- Shabbir A, Mirza T, Khalid AB, et al. Frequency of Her2/neu expression in colorectal adenocarcinoma: a study from developing South Asian Country. BMC Cancer 2016;16:855. [Crossref] [PubMed]
- Richman SD, Southward K, Chambers P, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol 2016;238:562-70. [Crossref] [PubMed]
- Ingold Heppner B, Behrens HM, Balschun K, et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer 2014;111:1977-84. [Crossref] [PubMed]
- Wang G, He Y, Sun Y, et al. Prevalence, prognosis and predictive status of HER2 amplification in anti-EGFR-resistant metastatic colorectal cancer. Clin Transl Oncol 2020;22:813-22. [Crossref] [PubMed]
- Osako T, Miyahara M, Uchino S, et al. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology 1998;55:548-55. [Crossref] [PubMed]
- Sartore-Bianchi A, Amatu A, Porcu L, et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist 2019;24:1395-402. [Crossref] [PubMed]
- Sartore-Bianchi A, Lonardi S, Aglietta M, et al. Central Nervous System as Possible Site of Relapse in ERBB2-Positive Metastatic Colorectal Cancer: Long-term Results of Treatment With Trastuzumab and Lapatinib. JAMA Oncol 2020;6:927-9. [Crossref] [PubMed]
- Martin V, Landi L, Molinari F, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer 2013;108:668-75. [Crossref] [PubMed]
- Park DI, Kang MS, Oh SJ, et al. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int J Colorectal Dis 2007;22:491-7. [Crossref] [PubMed]
- Kavanagh DO, Chambers G, O'Grady L, et al. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer 2009;9:1. [Crossref] [PubMed]
- Sartore-Bianchi A, Lonardi S, Martino C, et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 2020;5:e000911. [Crossref] [PubMed]
- Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2019;20:518-30. [Crossref] [PubMed]
- Siena S, Di Bartolomeo M, Raghav K, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol 2021;22:779-89. [Crossref] [PubMed]
- Valtorta E, Martino C, Sartore-Bianchi A, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol 2015;28:1481-91. [Crossref] [PubMed]
- Feng Y, Li Y, Huang D, et al. HER2 as a potential biomarker guiding adjuvant chemotherapy in stage II colorectal cancer. Eur J Surg Oncol 2019;45:167-73. [Crossref] [PubMed]
- Laurent-Puig P, Balogoun R, Cayre A, et al. ERBB2 alterations a new prognostic biomarker in stage III colon cancer from a FOLFOX based adjuvant trial (PETACC8). Ann Oncol 2016;27:vi151. [Crossref]
- Marx AH, Burandt EC, Choschzick M, et al. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol 2010;41:1577-85. [Crossref] [PubMed]
- Conradi LC, Styczen H, Sprenger T, et al. Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol 2013;37:522-31. [Crossref] [PubMed]
- Wang Q, Shen X, Chen G, et al. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers (Basel) 2022;14:2928. [Crossref] [PubMed]
- Jeong JH, Kim J, Hong YS, et al. HER2 Amplification and Cetuximab Efficacy in Patients With Metastatic Colorectal Cancer Harboring Wild-type RAS and BRAF. Clin Colorectal Cancer 2017;16:e147-52. [Crossref] [PubMed]
- Raghav KPS, Overman MJ, Yu R, et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J Clin Oncol 2016;34:3517. [Crossref]
- Sawada K, Nakamura Y, Yamanaka T, et al. Prognostic and Predictive Value of HER2 Amplification in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer 2018;17:198-205. [Crossref] [PubMed]
- Wells A. EGF receptor. Int J Biochem Cell Biol 1999;31:637-43. [Crossref] [PubMed]
- Li N, Bu X, Wu P, et al. The "HER2-PI3K/Akt-FASN Axis" regulated malignant phenotype of colorectal cancer cells. Lipids 2012;47:403-11. [Crossref] [PubMed]
- Siena S, Sartore-Bianchi A, Marsoni S, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol 2018;29:1108-19. [Crossref] [PubMed]
- Takegawa N, Yonesaka K. HER2 as an Emerging Oncotarget for Colorectal Cancer Treatment After Failure of Anti-Epidermal Growth Factor Receptor Therapy. Clin Colorectal Cancer 2017;16:247-51. [Crossref] [PubMed]
- Loree JM, Bailey AM, Johnson AM, et al. Molecular Landscape of ERBB2/ERBB3 Mutated Colorectal Cancer. J Natl Cancer Inst 2018;110:1409-17. [Crossref] [PubMed]
- Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3:99ra86. [Crossref] [PubMed]
- Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011;1:508-23. [Crossref] [PubMed]
- Press MF, Lenz HJ. EGFR, HER2 and VEGF pathways: validated targets for cancer treatment. Drugs 2007;67:2045-75. [Crossref] [PubMed]


