
Intrahepatic cholangiocarcinoma: a dose threshold evaluation in those undergoing transarterial radioembolization
Highlight box
Key findings
• Realized tumor dose may correlate with radiologic response and patients who achieve a complete radiologic response may obtain a survival benefit. When treating with glass microspheres a target dose of ≥541.7 Gy to the tumor may be warranted.
What is known and what is new?
• Phase 2 data has suggested that the combination of systemic therapy and transarterial radioembolization may be a viable treatment option for well selected patients with intrahepatic cholangiocarcinoma.
• This study demonstrates that there may be a correlation between tumoral dose and radiologic response, and identifies a potential target dose for patients undergoing glass microsphere yttrium-90 treatment.
What is the implication, and what should change now?
• Those treating intrahepatic cholangiocarcinoma patients with glass microspheres should consider targeting a tumoral dose of ≥541.7 Gy. However, further data on dose thresholds in this patient population is needed.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a rare primary hepatic malignancy, however its incidence has been increasing in Western countries (1-3). While chemotherapy is the gold standard treatment for non-resectable ICC patients, the results of chemotherapy alone are less than desirable (4). This has led to the investigation of other treatment algorithms for this patient cohort. Most of these treatment algorithms have investigated the addition of locoregional therapies to systemic chemotherapy given the systemic nature of the disease (5-8).
One of the treatment strategies which has shown some promise is transarterial radioembolization (TARE) (5-17). However, data on dose thresholds, arguably the most important aspect of the procedure itself, is still limited (18-21). Furthermore, the majority of dose threshold studies are macroaggregated albumin (MAA) based (18-20). Utilizing MAA distribution to determine realized dose to tumor is fraught with error and thus these studies are limited (21). Understanding dose response thresholds is of particular importance as a recent production of prospective data suggesting the combination of TARE with chemotherapy is safe and may be efficacious (8). Given the promise of the technique and the known importance of dose to response as a general concept, further evaluation of dose response curves is needed.
Finally, other important factors such as particle load and specific activity per particle have to the authors knowledge not been investigated in relationship to treatment outcomes and ICC. The two commercially available yttrium-90 products differ significantly in specific activity and particle load, with resin (SIR-Spheres, Sirtex medical, Woburn) having relatively low specific activity and associated high particle load and glass (Therasphere, Boston Scientific, Marlborough, MA) having relatively high specific activity and low particle load. Furthermore, even within the two products there can be significant variation in these parameters with resin offering flex dosing, allowing for variation in specific activity to some degree and glass particles being created on Sunday’s allowing users to reduce the specific activity by delivering later in the two-week time span.
Therefore, the aim of this retrospective single center study was to evaluate the dose response relationship of TARE in patients with ICC to evaluate the hypothesis that a relationship between dose and response does exist. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-210/rc).
Methods
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board (IRB) of the University of Minnesota (No. STUDY00003416). Informed consent was waived by the IRB for this retrospective study but all patients were consented for the procedure. After IRB approval all ICC patients treated with TARE between 1/1/2013–6/1/2021 (n=24) at a single-institution were reviewed. The primary endpoint was radiologic-response (RR) at 3-month. Patients without 3-month follow-up imaging (n=1) and those without single-photon-emission-computerized tomography (SPECT) computed-tomography (CT) following yttrium-90 delivery (n=3) were excluded. If patients had multiple TARE treatments of a single-lesion, which were separated by at least 3-month, each was included and analyzed separately for the primary endpoint. In total 20-patients who underwent treatment of 26-tumor were included with demographic data available in Table 1. No patients had extra hepatic disease at the time of treatment, 18/20 (90%) had 1 tumor, 1/20 (5%) had 3 and 1/20 (5%) had greater than 5.
Table 1
| Variable | Entire cohort | Resin only | Glass only |
|---|---|---|---|
| Age (years) | 62.6±10.5 | 65.4±6.5 | 61.8±8.8 |
| Sex | |||
| Men | 13/20, 65% | 4/7, 57.1% | 9/13, 69.2% |
| Women | 7/20, 35% | 3/7, 42.9% | 4/13, 30.8% |
| Total Bilirubin (mg/dL) | 0.8±0.4 | 0.8±0.3 | 0.8±0.3 |
| Albumin (g/dL) | 3.4±0.4 | 3.3±0.5 | 3.4±0.4 |
| INR | 1.1±0.2 | 1.2±0.3 | 1.1±0.1 |
| Creatinine (mg/dL) | 0.8±0.3 | 0.7±0.2 | 0.8±0.3 |
| Platelets (109/L) | 150.6±75.2 | 134.4±87 | 154±71.2 |
| Lymphocytes (109/L) | 1±0.5 | 0.8±0.3 | 1.1±0.4 |
| Child-Pugh | 6.2±1.3 | 6.7±1.5 | 6.1±1.1 |
| Cancer Antigen 19-9 (U/mL) | 127.5 (102.25) | 165 (165.3) | 129 (105) |
| Prior chemotherapy | 11/20, 55% | 3/7, 42.9% | 8/13, 61.5% |
| Concomitant chemotherapy | 17/26, 65.4% | 6/9, 66.7% | 11/17, 64.7% |
| Prior radiation | 1/20, 5% | 1/7, 14.3% | 0/13, 0% |
| Prior TACE | 1/20, 5% | 1/7, 14.3% | 0/13, 0% |
| Microsphere utilized | N/A | N/A | |
| Resin | 9/26, 34.6% | ||
| Glass | 17/26, 65.4% | ||
| Delivery target: | |||
| Lobar | 8/26, 30.8% | 5/9, 55.6% | 3/17, 17.7% |
| 2–3 segments | 6/26, 23.1% | 2/9, 22.2% | 4/17, 23.5% |
| ≤1 segment | 12/26, 46.1% | 2/9, 22.2% | 10/17, 58.8% |
| Activity delivery (mCi) | 80.5±74 | 22±9 | 111.4±74.7 |
| Particle load | 4,000,000 (6,400,350.5) | 11,116,232 (8,105,586) | 2,400,000 (2,000,000) |
| Specific activity (Bq) | 658.5 (1,407.1) | 76 (0) | 1,389.7 (708.1) |
| Lung shunt percent | 3.3±2.9 | 4.4±3.2 | 2.8±2.8 |
| Perfused volume (mL) | 536.8±341.8 | 389.1±140.9 | 588.9±378.5 |
| Tumor size (cm) | 5.5±2.6 | 5.3±1 | 5.5±3 |
| Predicted T:N ratio | 2.2±2.2 | 2.8±2.8 | 2±0.8 |
| Realized T:N ratio | 2.1±0.7 | 1.9±0.6 | 2.2±0.7 |
Data are expressed as mean ± standard deviation, median (IQR) or n, %. INR, international ratio; T:N ratio, tumor to normal ratio; TACE, transarterial chemoembolization.
Electronic-medical-records were evaluated for laboratory, imaging, and clinical factors. Furthermore, type of yttrium-90 microsphere utilized (resin or glass), activity, particle-load, and delivery points were recorded. All resin treatments utilized day of calculation doses and glass microspheres were delivered on 2 (9/17, 52.9%), 3 (1/17, 5.9%), 4 (3/17, 17.6%), and 5 (4/17, 23.5%) days after calibration respectively. Adverse events were evaluated using common terminology criteria for adverse events version 5.
RR was evaluated using the European Association for the Study of Liver (EASL) criteria and response evaluation criteria in solid tumors (RECIST) version1.1. However, because EASL has been shown to more accurately predict oncologic outcomes as compared to RECIST when patients are treated with TARE, RECIST data is presented in supplemental data (22). An objective RR (ORR) was defined as a complete or partial response. RR was evaluated with independent review by a body-radiologist; comparing their interpretation to the initial report with discrepancies resolved by mutual consensus. OS, time-to-progression (TTP) and local-TTP were considered to be time from first TARE procedure until death, progression at any site, or target lesion progression respectively.
Particle-load: specific activity calculations
Where activity delivered was calculated by measuring the predelivery activity with a dose calibrator (Mirion Technologies Inc.) on the day of and immediately prior to the delivery.
TARE technique
TARE was performed in previously described manner (5-17). However, in brief after presentation at a multi-disciplinary conference patients which were not surgical candidates were considered for TARE. After consultation a mapping angiogram utilizing technetium-99m MAA (Tc99mMAA) followed by SPECT-CT to evaluate liver distribution, lung shunt fraction (LSF), and extra-hepatic distribution. Performing physician preference determined dose calculation method [medical internal radiation dose (MIRD), partition, body-surface-area (BSA) and a voxel based multi-compartment method were all utilized during the study period] and dose target. The microsphere (resin or glass) utilized was also at the performing physicians discretion, however, there was a tendency to utilize resin more early in the study period and glass more late in the study period. Patients returned for delivery and underwent post-delivery bremsstrahlung SPECT-CT.
Calculation of absorbed dose
Pretreatment contrast enhanced magnetic resonance imaging (MRI)s or CTs were utilized along with a dosimetry software [Simplicit90y™ (Mirada Medical, Denver, CO)] to calculate absorbed dose. A single physician with ≥6 years of experience drew 3D-regions of interest (ROI) (23). The lowest relevant isodose curve was used to contour the normal and tumoral tissues within the perfused-volume. Anatomic structures within the yttrium-90 bremsstrahlung SPECT-CT were utilized to evaluate and adjust the fit of the Co-registration. The activity delivered and LSF (calculated using standard planar method) was entered into Simplicit90y™, and the below calculation was utilized.
Dose to tumor
Where Dt is the tumor absorbed dose in Gy, Ai is the perfused volume injected activity in GBq, Ci represents the perfused volume counts, Ct represents the counts in the segmented tumor volume, p represents tumor tissue density (kg/cm3), Vt is the tumor volume (cm3), LSF is lung shunt fraction, and R is residual activity.
Statistical analysis
Quantitative variables are expressed as mean ± standard deviations or median [interquartile-range (IQR)]. Qualitative variables are expressed as frequencies followed by percentages in parentheses. Student T-test or ANOVA were used to analyze continuous variables and Chi-Squared or Fisher exact tests were utilized to evaluate categorical data. To determine the relationship between RR and various variables a receiver operating characteristic (ROC) curve and area under the curve (AUC) analysis was performed. Youden-index analysis was utilized to identify thresholds on the ROC. Further analysis with univariate and multivariate regression was performed. Kaplan-Meier survival curves and Cox proportional hazard ratios were also calculated to analyze overall survival and time to progression variables. R (Version 3.4.1, The R Foundation for Statistical Computing) was used for the analysis and P values of <0.05 were considered statistically significant.
Results
Results for the RECIST classification of response can be found in supplemental materials (Appendix 1).
RR by microparticle type
RR can be found in for the entire, glass only, and resin only cohorts in Table 2. When comparing glass to resin there was no significant difference in the likely hood of obtaining an ORR (16/17, 94.1% glass vs. 6/9, 66.7% resin, P=0.1). When looking at the likelihood of obtaining a complete response (CR) again no significant difference was seen between glass (8/17, 47.1%) and resin (1/9, 11.1%) (P=0.098).
Table 2
| Radiologic response | EASL, n (%) |
|---|---|
| Entire cohort | n=26 |
| Progressive disease | 2 (7.7) |
| Stable disease | 2 (7.7) |
| Partial response | 13 (50.0) |
| Complete response | 9 (34.6) |
| Glass only | n=17 |
| Progressive disease | 0 |
| Stable disease | 1 (5.8) |
| Partial response | 8 (47.1) |
| Complete response | 8 (47.1) |
| Resin only | n=9 |
| Progressive disease | 2 (22.2) |
| Stable disease | 1 (11.1) |
| Partial response | 5 (55.6) |
| Complete response | 1 (11.1) |
EASL, European Association for the Study of Liver.
RR and relationship to dose
Glass only
The mean tumor dose (MTD) for stable disease (SD) (1/17, 5.8%), partial response (PR) (8/17, 47.1%), and CR (8/17, 47.1%) by EASL was 294±0, 465.4±292.4 and 951.8±666.5 Gy respectively (P=0.039).
Resin only
The MTD for PD (2/9, 22.2%), SD (1/9, 11.1%), PR (5/9, 55.6%), and CR (1/9, 11.1%) by EASL was 126.8±9.4, 128.4±0, 144.5±105 and 132.8±0 Gy, respectively (P=0.42).
ROC analysis for ORR
A ROC curve analysis could not be performed for those who did and did not achieve an ORR by EASL in the glass only cohort as only a single patient failed to achieve an ORR. For the resin only cohort the AUC was 0.583 (P=0.75). Youden index analysis demonstrated a cutoff point of >103.2 Gy resulted in a sensitivity of 50% and a specificity of 100% for achieving an ORR.
ROC analysis for CR
A ROC curve analysis evaluating tumor dose in those who achieved a CR by EASL was performed for the glass only patients. Similar analysis could not be performed for resin only patients as only a single patient achieved a CR. The AUC of this analysis was 0.738 (P=0.038), for the glass only patients, with Youden-index analysis demonstrated a cutoff point of >541.7Gy (sensitivity: 55.56%; specificity: 92.86%).
RR and relationship to particle-load and specific activity
The median particle-load and specific activity by RR category can be found in Table 3. No significant differences were found between the particle load or specific activity in the glass cohort when compared between those with a PD or SD, PR, and CR. Similarly, no significant differences were seen between these groups in the resin only cohort when looking at particle load. Specific activity for the resin cohort could not be analyzed as all treatments had the same specific activity.
Table 3
| Variable | PD or SD | PR | CR | P value |
|---|---|---|---|---|
| Glass only | ||||
| Particle load (particles) | 2,000,000 (IQR: 0) | 4,000,000 (IQR: 1,100,000) | 2,200,000 (IQR: 600,000) | 0.1 |
| Specific activity (Bq) | 605 (IQR: 0) | 814.9 (IQR: 800.5) | 1,477.8 (IQR: 270) | 0.12 |
| Resin only | ||||
| Particle load (particles) | 11,116,232 (IQR: 6,813,721) | 9,379,321 (IQR: 6,716,057) | 45,041,559 (IQR: 0) | 0.3 |
| Specific activity (Bq) | – | – | – | – |
PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response; EASL, European Association for the Study of Liver.
Univariate and multivariate regression analysis of achieving a CR by EASL
Results of this analysis are presented in Table 4, however, no tested variables remained significant on multivariate analysis. The entire cohort was included in this analysis given the small numbers and the breath of particle load and specific activity which is introduced by including both microsphere types.
Table 4
| Variable | Univariate regression | Multivariate regression | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Tumor dose | 1.26 (1.08–1.58) | 0.017 | 1.22 (0.99–1.61) | 0.28 | |
| Specific activity | 1.16 (1.01–1.31) | 0.045 | 1.01 (0.98–1.37) | 0.48 | |
| Perfused volume | 0.99 (0.94–1.16) | 0.28 | – | ||
| Particle load | 1 (0.98–1.03) | 0.74 | – | ||
| Tumor size | 0.75 (0.53–1.05) | 0.086 | 0.94 (0.61–1.45) | 0.79 | |
| Pretreatment CA 19-9 | 0.97 (0.94–1.45) | 0.18 | – | ||
| Ytrrium-90 material (resin or glass) | 0.14 (0.01–1.38) | 0.07 | 2.99 (0.05–3.89) | 0.61 | |
| Prior chemotherapy | 7.3 (1.46–14.71) | 0.008 | 6.88 (0.49–15.40) | 0.15 | |
Please note that all listed variables were included in the multiple regression model presented. OR, odds ratio.
TTP and OS analysis
Figure 1 demonstrates KM OS curves for those who did and did not achieve a CR by EASL criteria (P=0.01, Cox HR: 4.79 (95% CI: 1.41–16.25).

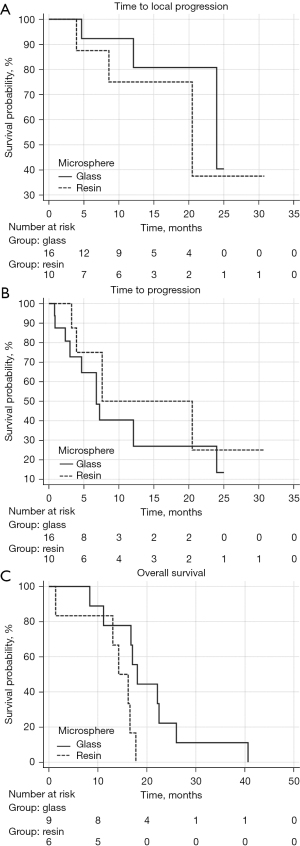
Figure 2 demonstrates the KM curves for local TTP, TTP, and OS comparing the outcomes between patients receiving the two different device types. There was no significant difference in the local TTP [HR: 1.69 (95% CI: 0.32–8.88), P=0.53] and TTP [HR: 1.64 (95% CI: 0.58–4.64), P=0.35]. However, OS [HR: 5.02 (95% CI: 1.23–20.55), P=0.025] was significantly longer in the glass as compared to resin cohorts.
Adverse events
The only Grade 3 or greater adverse events occurred in a single patient who had grade 3 elevation in total bilirubin, aspartate aminotransferase (AST), and alanine transaminase (ALT) at 1 month. Of the 17 treatments in 13 patients which were done concomitantly with chemotherapy 7/17 (41.2%) required a delay in chemotherapy, however all patients reinitiated chemotherapy after a delay.
Discussion
ICC is a rare primary hepatic malignancy which can be challenging to treat. While initial studies have demonstrated promise when adding TARE to the treatment algorithm, further data is needed (8-17). In particular more data on dose thresholds is needed, as emphasized by recent studies in hepatocellular carcinoma (HCC) which have demonstrated the importance of dose thresholds (24,25). This study demonstrated a significant difference in the tumoral dose seen across various radiologic response categories in the glass but not the resin cohorts. Furthermore, the study found an AUC of 0.738 (P=0.038) when evaluating glass patients who did and did not have a complete radiologic response, confirming the apparent association between dose and radiologic response. Using Youden’s index to evaluate a ROC curve, this study would suggest that providers should target at least 541.7 Gy to the tumor when using glass. The tumoral values are higher than a prior study which demonstrated that a minimum of 158 Gy was needed to induce a response (18). This difference may be secondary to the small cohorts in both studies, however further data is needed. The study also confirms the OS benefit of achieving a CR in this patient cohort.
The current study also evaluated the importance of particle load on radiologic response. Particle load has been shown to be an important factor in areas such as tumor to normal ratio (TNR) in prior publications evaluating treatment of other primary liver cancers (23). While significant differences were not seen among RR and particle load in either the glass or resin cohort, the subject warrants further evaluation. A related factor, specific activity could not be evaluated in the resin cohort as all resin treatments utilized day of calibrations. While there was a trend toward higher specific activity being associated with better RR in glass treatments this was not significant. Again, this variable warrants further evaluation, especially given the emerging evidence supporting their importance in TARE for HCC (23,26,27). In line with this there was a significant difference noted in OS between those receiving glass and resin. These differences may have occurred for a number of reasons, however, the devices primarily differ in their relative particle loads and specific activities, again emphasizing the importance of these variables in future investigations.
One important consideration with any treatment are adverse events. There were very few adverse events in this cohort, consistent with prior studies (5-17). A specific concern expressed by the medical oncology community is the inability to continue systemic therapy during/after TARE. This study did show that of the 13 patients who underwent 17 TARE treatments while undergoing systemic therapy delay in the next cycle of therapy occurred after 7 (41.2%) treatments. However, perhaps more importantly all patients did continue with chemotherapy after a delay. These results are fairly consistent with Edeline et al. who showed fairly low levels of toxicity in those patients undergoing simultaneous TARE and systemic therapy, particularly when whole liver TARE was not performed (8). These findings should help give confidence to multidisciplinary teams when offering a combined treatment strategy.
This study has a number of limitations, most importantly it is a single center retrospective study with a limited patient cohort. No pathologic data was available for the cohort, and thus radiologic response was used as a surrogate. Radiologic response is known not to mirror pathologic response perfectly (25,28). Finally, patients were treated at a quaternary referral center and the experience there may not reflect other centers experience.
Conclusions
In conclusion, a relationship between tumor dose and radiologic response in patients treated for ICC with TARE appears to exist. Furthermore, activity per particle and particle load may be associated with response as well and further evaluation of these variables may be of benefit.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-210/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-210/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-210/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-210/coif). SY have received consulting fees, been a part of the speaker bureau, and received funds for travel from Boston Scientific, and also received consulting fees from Mirada as well as served in leadership roles for both the Society of Interventional Radiology and the GEST meeting. DD have received consulting fees, been a part of the speaker bureau, and received funds for travel from Sirtex as well as served in leadership roles for both the Society of Interventional Radiology and the GEST meeting. JG have received consulting fees, been a part of the speaker bureau, and received funds for travel from Sirtex as well as served in leadership roles for both the Society of Interventional Radiology and the GEST meeting. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the IRB of the University of Minnesota (No. STUDY00003416). Informed consent was waived by the IRB for this retrospective study but all patients were consented for the procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Poultsides GA, Zhu AX, Choti MA, et al. Intrahepatic cholangiocarcinoma. Surg Clin North Am 2010;90:817-37. [Crossref] [PubMed]
- Oh DY, He AR, Qin S, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid 2022. doi:
10.1056/EVIDoa2200015 10.1056/EVIDoa2200015 - Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134-44. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758-65. [Crossref] [PubMed]
- Avila S, Smani DA, Koay EJ. Radiation dose escalation for locally advanced unresectable intrahepatic and extrahepatic cholangiocarcinoma. Chin Clin Oncol 2020;9:10. [Crossref] [PubMed]
- Schartz DA, Porter M, Schartz E, et al. Transarterial Yttrium-90 Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol 2022;33:679-86. [Crossref] [PubMed]
- Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2020;6:51-9. [Crossref] [PubMed]
- Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. [Crossref] [PubMed]
- Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. [Crossref] [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [Crossref] [PubMed]
- Jia Z, Paz-Fumagalli R, Frey G, et al. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: preliminary results. J Cancer Res Clin Oncol 2017;143:481-9. [Crossref] [PubMed]
- Buettner S, Braat AJAT, Margonis GA, et al. Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Analysis. J Vasc Interv Radiol 2020;31:1035-1043.e2. [Crossref] [PubMed]
- White J, Carolan-Rees G, Dale M, et al. Yttrium-90 Transarterial Radioembolization for Chemotherapy-Refractory Intrahepatic Cholangiocarcinoma: A Prospective, Observational Study. J Vasc Interv Radiol 2019;30:1185-92. [Crossref] [PubMed]
- Köhler M, Harders F, Lohöfer F, et al. Prognostic Factors for Overall Survival in Advanced Intrahepatic Cholangiocarcinoma Treated with Yttrium-90 Radioembolization. J Clin Med 2019;9:56. [Crossref] [PubMed]
- Paz-Fumagalli R, Core J, Padula C, et al. Safety and initial efficacy of ablative radioembolization for the treatment of unresectable intrahepatic cholangiocarcinoma. Oncotarget 2021;12:2075-88. [Crossref] [PubMed]
- Bargellini I, Mosconi C, Pizzi G, et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc Intervent Radiol 2020;43:1305-14. [Crossref] [PubMed]
- Manceau V, Palard X, Rolland Y, et al. A MAA-based dosimetric study in patients with intrahepatic cholangiocarcinoma treated with a combination of chemotherapy and (90)Y-loaded glass microsphere selective internal radiation therapy. Eur J Nucl Med Mol Imaging 2018;45:1731-41. [Crossref] [PubMed]
- Lam MG, Banerjee A, Goris ML, et al. Fusion dual-tracer SPECT-based hepatic dosimetry predicts outcome after radioembolization for a wide range of tumour cell types. Eur J Nucl Med Mol Imaging 2015;42:1192-201. [Crossref] [PubMed]
- Levillain H, Duran Derijckere I, Ameye L, et al. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: a multicenter study. Eur J Nucl Med Mol Imaging 2019;46:2270-9. [Crossref] [PubMed]
- Willowson KP, Eslick EM, Bailey DL. Individualised dosimetry and safety of SIRT for intrahepatic cholangiocarcinoma. EJNMMI Phys 2021;8:65. [Crossref] [PubMed]
- Camacho JC, Kokabi N, Xing M, et al. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol 2014;25:256-65. [Crossref] [PubMed]
- Young S, Chen T, Flanagan S, et al. Realized tumor to normal ratios in hepatocellular carcinoma patients undergoing transarterial radioembolization: a retrospective evaluation. Eur Radiol 2022;32:4160-7. [Crossref] [PubMed]
- Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17-29. [Crossref] [PubMed]
- Gabr A, Riaz A, Johnson GE, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging 2021;48:580-3. [Crossref] [PubMed]
- Toskich B, Vidal LL, Olson MT, et al. Pathologic Response of Hepatocellular Carcinoma Treated with Yttrium-90 Glass Microsphere Radiation Segmentectomy Prior to Liver Transplantation: A Validation Study. J Vasc Interv Radiol 2021;32:518-526.e1. [Crossref] [PubMed]
- Montazeri SA, De la Garza-Ramos C, Lewis AR, et al. Hepatocellular carcinoma radiation segmentectomy treatment intensification prior to liver transplantation increases rates of complete pathologic necrosis: an explant analysis of 75 tumors. Eur J Nucl Med Mol Imaging 2022;49:3892-7. [Crossref] [PubMed]
- Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014;60:192-201. [Crossref] [PubMed]


