
The relationship between GLIM-malnutrition, post-operative complications and long-term prognosis in elderly patients undergoing colorectal cancer surgery
Highlight box
Key findings
• Global Leadership Initiative on Malnutrition (GLIM)-malnutrition is significantly associated with post-operative complications and overall survival in elderly patients with colorectal cancer (CRC).
What is known and what is new?
• The GLIM criteria is a new diagnostic criteria for malnutrition that has been used to assess the nutritional status of patients with various tumors.
• In this study, we evaluated the GLIM criteria for the diagnosing malnutrition in elderly patients with CRC. GLIM-malnutrition is significantly associated with post-operative complications and decreased overall survival in elderly patients with CRC.
What is the implication, and what should change now?
• The GLIM criteria is effective to assess malnutrition of elderly patients with CRC early thereby allowing the initiation of appropriate nutritional interventions to reduce post-operative complications, prolong the survival time of patients, and alleviate the overall burden on patients and society.
Introduction
Malnutrition is common amongst elderly people and is becoming a global public health problem as populations age. Colorectal cancer (CRC) has the third highest incidence and second highest mortality rate among all malignancies. In 2020, more than 1.93 million people worldwide were diagnosed with CRC, which caused 935,000 deaths (1). A study has shown that the incidence of malnutrition is higher in elderly patients with CRC than in younger patients, and malnutrition affects the clinical outcome of patients (2). Malnutrition in tumor patients will increase the incidence of post-operative complications, affect the efficacy of drug treatment, and even reduce long-term survival (3,4). Therefore, early diagnosis of malnutrition and nutritional intervention are of great significance in improving the short-term and long-term prognosis of cancer patients (5).
There are many screening and diagnostic tools for malnutrition, including monotypic indicators such as body mass index (BMI) and involuntary weight loss, as well as composite indicators such as Nutrition Risk Screening 2002 (NRS 2002), Malnutrition Universal Screening Tool (MUST), Patient-Generated Subjective Global Assessment (PG-SGA), and Mini Nutritional Assessment-Short Form (MNA-SF). However, the large number of indicators illustrates the lack of a globally consistent consensus on diagnostic criteria for malnutrition (6). Therefore, to establish a globally accepted diagnostic indicator of malnutrition, the Global Leadership Initiative on Malnutrition has introduced a new indicator called the “GLIM criteria” (7). The GLIM criteria utilises a two-step model involving risk screening followed by malnutrition diagnosis assessment. Some studies have shown that, compared with multiple other nutritional diagnostic indicators, the GLIM score is an appropriate index for the diagnosis of malnutrition in cancer patients, such as gastric cancer (8), esophageal cancer (9), and head and neck cancer (10), and is closely related to the patient prognosis (11).
However, there is no study explored the application of GLIM criteria in elderly colorectal patients. Therefore, the purpose of this study was to investigate the effectiveness of GLIM in screening and diagnosing malnutrition in elderly patients who underwent curative CRC surgery. We also analyzed the impact of GLIM-malnutrition on post-operative complications and long-term outcomes of patients. We present this article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-543/rc).
Methods
Patients
Participants in this study were selected from elderly patients who underwent curative CRC surgery at the First Affiliated Hospital of Wenzhou Medical University from January 2015 to December 2018. The inclusion criteria were patients who (I) were ≥65 years old; (II) received elective curative surgery for CRC; and (III) agreed to participate in this study and sign a written informed consent. The exclusion criteria were patients who (I) received chemotherapy and radiotherapy before surgery; (II) had cancer metastasis; (III) underwent palliative surgery; and (IV) were unable to complete grip strength measurement. All surgeries were performed by well-experienced surgeons with a high degree of skill according to the Japanese CRC treatment guidelines. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (ethical No. 2014063) and informed consent was taken from all the patients.
Data acquisition
The following data were prospectively collected and recorded: (I) baseline characteristics: age, sex, body mass index (BMI), hemoglobin concentration (<120 g/L in males or <110 g/L in females was defined as anemia), serum albumin concentration (<35 g/L was defined as hypoalbuminemia), weight loss, handgrip strength (HGS) and history of previous abdominal surgery; (II) various evaluation indicators: NRS 2002 scores; American Society of Anesthesiologists (ASA) grade, Charlson comorbidity index score (CCI); (III) open or laparoscopic surgery; (IV) pathological characteristics of tumor: differentiation and tumor-node-metastasis (TNM) stage of tumor; and (V) post-operative complications within 30 days after surgery, which were classified according to the Clavien-Dindo classification. Only complications classified as grade II or above were accounted for.
GLIM criteria
According to the diagnostic criteria of GLIM-malnutrition (7), we first used the NRS 2002 score to assess the risk of malnutrition in patients. Patients with NRS scores ≥3 were further evaluated for at least one of the following 3 phenotypic criteria: (I) non-volitional weight loss: weight loss >5% within the past 6 months or >10% beyond 6 months; (II) low BMI: <18.5 if <70 years old or <20 if ≥70 years old; and (III) reduced muscle mass: HGS <26 kg for men and <18 kg for women. According to the diagnostic criteria of GLIM, patients still need to meet at least one etiologic criterion (reduced food intake or assimilation and inflammation or disease burden). However, patients with CRC already bear a disease burden. Therefore, in this study, patients with NRS 2002 ≥3 who met at least one of the phenotypic criteria above were diagnosed with GLIM-malnutrition.
Follow-up
All patients had follow-up 1 month after surgery, every 3 months for the first 2 years, and every 6 months thereafter. Follow-up programs include blood tests, imaging studies such as CT or MRI, and endoscopy when appropriate. Overall survival (OS) was calculated from the date of surgery to the date of death from any cause or the last follow-up date. Disease-free survival (DFS) was calculated from the date of surgery to the date of any form of cancer recurrence, death from any cause or the last follow-up date. The last follow-up date was November 2020. The median follow-up time was 37 months.
Statistical analysis
Continuous data with a normal distribution were compared using t-tests and are presented as the mean ± standard deviation (SD). Continuous data with skewed distributions were compared using the Mann-Whitney U test and are presented as interquartile ranges (IQRs). Categorical data were analyzed by the chi-squared test or Fisher’s exact test. Univariate and multivariate logistic regressions were used to analyze potential independent risk factors for post-operative complications. Survival curves were analyzed by the Kaplan-Meier (K-M) method and compared by log-rank tests. Univariate and multivariate COX regressions were used to analyze potential independent risk factors for OS. Only variables with a P value <0.1 in the univariate regression analysis were included in the multivariate regression analysis. Multivariate regression analysis was performed using forward step-wise elimination. All statistical analyses were performed using EmpowerStats software (Version 2.0). Two-tailed P values <0.05 were regarded as statistically significant.
Results
Characteristics of the study participants
The study was first enrolled 512 elderly patients who underwent curative CRC surgery. According to the exclusion criteria, 385 candidates were eventually included in the study (Figure 1). Nutritional risk screening showed that 165 (42.86%) of them were at risk of malnutrition (NRS 2002 ≥3). According to the GLIM diagnostic criteria, 76 patients (19.74%) experienced statistically significant weight loss, 65 patients (16.88%) had a low BMI, and 68 patients (17.66%) had reduced muscle mass. A total of 118 patients (30.65%) were diagnosed with malnutrition according to the GLIM criteria; we called this group the GLIM-malnutrition group.
The Chi-square analysis based on clinical baseline revealed that in elderly patients who underwent curative colorectal resections, GLIM-malnutrition was significantly associated with older age, higher NRS 2002, lower BMI, lower grip strength, tumor location and lower levels of albumin and hemoglobin. However, there were no significant differences between the GLIM-malnutrition group and the non-malnutrition groups in sex, ASA grade, CCI score, previous abdominal surgery, differentiation of tumor, TNM stage, laparoscopic surgery, and hospital stay (Table 1).
Table 1
| Factors | Total (n=385) | Non-malnutrition (n=267) | GLIM-malnutrition (n=118) | P value |
|---|---|---|---|---|
| Age, years | 73 [11] | 71 [10] | 76 [10] | <0.001* |
| Gender | 0.391 | |||
| Female | 154 (40.0) | 103 (38.6) | 51 (43.2) | |
| Male | 231 (60.0) | 164 (61.4) | 67 (56.8) | |
| BMI, kg/m2 | 22.631±3.145 | 23.158±2.968 | 21.439±3.221 | <0.001* |
| HGS, kg | 21.80 [13.55] | 24.30 [12.90] | 17.35 [12.93] | <0.001* |
| Hemoglobin, g/L | 119 [30] | 120 [33] | 115.5 [31.5] | 0.004* |
| Albumin, g/L | 36.90 [5.30] | 37.3 [4.5] | 35.75 [6.05] | <0.001* |
| NRS 2002 | <0.001* | |||
| <3 | 220 (57.1) | 220 (82.4) | 0 (0.0) | |
| ≥3 | 165 (42.9) | 47 (17.6) | 118 (100.0) | |
| CCI | 0.464 | |||
| 0 | 165 (42.9) | 117 (43.8) | 48 (40.7) | |
| 1 | 146 (37.9) | 96 (36.0) | 50 (42.4) | |
| ≥2 | 74 (19.2) | 54 (20.2) | 20 (16.9) | |
| ASA grade | 0.350 | |||
| I | 95 (24.7) | 64 (24.0) | 31 (26.3) | |
| II | 228 (59.2) | 164 (61.4) | 64 (54.2) | |
| III | 62 (16.1) | 39 (14.6) | 23 (19.5) | |
| Previous abdominal surgery | 0.417 | |||
| No | 240 (62.3) | 170 (63.7) | 70 (59.3) | |
| Yes | 145 (37.7) | 97 (36.3) | 48 (40.7) | |
| Tumor location | 0.002* | |||
| Colon | 243 (63.1) | 155 (58.1) | 88 (74.6) | |
| Rectum | 142 (36.9) | 112 (41.9) | 30 (25.4) | |
| Differentiation of tumor | 0.303 | |||
| Median or high | 309 (80.3) | 218 (81.7) | 91 (77.1) | |
| Low | 76 (19.7) | 49 (18.4) | 27 (22.9) | |
| TNM stage | 0.597 | |||
| I | 70 (18.2) | 52 (19.5) | 18 (15.3) | |
| II | 164 (42.6) | 111 (41.6) | 53 (44.9) | |
| III | 151 (39.2) | 104 (39.0) | 47 (39.8) | |
| Laparoscopic surgery | 0.090 | |||
| No | 210 (54.5) | 138 (51.7) | 72 (61.0) | |
| Yes | 175 (45.5) | 129 (48.3) | 46 (39.0) | |
| Length of hospital stay (days) | 21.300±8.283 | 21.030±8.275 | 21.898±8.305 | 0.344 |
Data are presented as mean ± SD, median [IQR], and n (%). *, statistically significant. GLIM, Global Leadership Initiative on Malnutrition; BMI, body mass index; HGS, handgrip strength; NRS 2002, the Nutritional Risk Screening 2002; CCI, Charlson Comorbidity Index; ASA, American Society of Anesthesiologists; TNM, tumor-node-metastasis; SD, standard deviation; IQR, interquartile range.
Relationship between GLIM-malnutrition and post-operative complications
As shown in Table 2, a total of 113 (29.4%) patients with CRC experienced post-operative complications. Compared with the non-malnutrition group (n=68, 25.5%), the incidence of post-operative complications in the GLIM-malnutrition group was significantly higher (n=45, 38.1%), but the incidence of severe complications in the two groups was not significantly different. GLIM-malnutrition is closely related to medical complications (P=0.010), but not to surgical complications. Among specific complications, only the incidence of post-operative venous thrombosis was significantly higher in the GLIM-malnutrition group (P=0.035). Multivariate analyses showed that GLIM-malnutrition [odds ratio (OR): 1.753, 95% confidence interval (CI): 1.100–2.795, P=0.018] and hypoalbuminemia (OR: 2.450, 95% CI: 1.089–5.512, P=0.030) were independent risk factors for post-operative complications (Table 3).
Table 2
| Complications | Total (n=385) | Non-malnutrition (n=267) | GLIM-malnutrition (n=118) | P value |
|---|---|---|---|---|
| Total complicationsa | 113 (29.4) | 68 (25.5) | 45 (38.1) | 0.012* |
| Surgical complications | 66 (17.1) | 43 (16.1) | 23 (19.5) | 0.416 |
| Wound infection | 17 (4.4) | 10 (3.7) | 7 (5.9) | 0.336 |
| Intra-abdominal infection | 16 (4.2) | 9 (3.4) | 7 (5.9) | 0.246 |
| Anastomotic leakage | 12 (3.1) | 11 (4.1) | 1 (0.8) | 0.088 |
| Intra-abdominal hemorrhage | 6 (1.6) | 4 (1.5) | 2 (1.7) | 0.886 |
| Intestinal obstruction | 6 (1.6) | 5 (1.9) | 1 (0.8) | 0.454 |
| Wound rupture | 4 (1.0) | 3 (1.1) | 1 (0.8) | 0.805 |
| Gastrointestinal dysfunction | 2 (0.5) | 0 (0.0) | 2 (1.7) | 0.033 |
| Seroperitoneum | 2 (0.5) | 1 (0.4) | 1 (0.8) | 0.552 |
| Gastroplegia | 1 (0.3) | 0 (0.0) | 1 (0.8) | 0.132 |
| Medical complications | 47 (12.2) | 25 (9.4) | 22 (18.6) | 0.010* |
| Pneumonia | 17 (4.4) | 9 (3.4) | 8 (6.8) | 0.133 |
| Venous thrombosis | 12 (3.1) | 5 (1.9) | 7 (5.9) | 0.035* |
| Pleural effusion | 4 (1.0) | 1 (0.4) | 3 (2.5) | 0.053 |
| Heart failure | 4 (1.0) | 2 (0.7) | 2 (1.7) | 0.399 |
| Urinary infection | 3 (0.8) | 2 (0.7) | 1 (0.8) | 0.919 |
| Pulmonary embolism | 2 (0.5) | 2 (0.7) | 0 (0.0) | 0.346 |
| Sepsis | 2 (0.5) | 1 (0.4) | 1 (0.8) | 0.552 |
| Septic shock | 1 (0.3) | 1 (0.4) | 0 (0.0) | 0.506 |
| Acute kidney injury | 1 (0.3) | 1 (0.4) | 0 (0.0) | 0.506 |
| Death | 1 (0.3) | 1 (0.4) | 0 (0.0) | 0.506 |
| Severe complicationsb | 30 (7.8) | 22 (8.2) | 8 (6.8) | 0.622 |
Data are presented as n (%). *, statistically significant. a, complications classified as grade II and above. b, complications classified as grade III and above. GLIM, Global Leadership Initiative on Malnutrition.
Table 3
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| GLIM-malnutrition | |||||
| Yes vs. no | 1.804 (1.136, 2.864) | 0.012* | 1.753 (1.100, 2.795) | 0.018* | |
| Age, years | |||||
| ≥80 vs. <80 | 1.361 (0.808, 2.294) | 0.247 | |||
| Gender | |||||
| Male vs. female | 1.183 (0.753, 1.859) | 0.465 | |||
| Low BMI | |||||
| Yes vs. no | 0.907 (0.501, 1.643) | 0.748 | |||
| Low HGS | |||||
| Yes vs. no | 1.513 (0.872, 2.625) | 0.141 | |||
| Anemia | |||||
| Yes vs. no | 1.397 (0.898, 2.171) | 0.138 | |||
| Hypoalbuminemia | |||||
| Yes vs. no | 2.590 (1.161, 5.780) | 0.020* | 2.450 (1.089, 5.512) | 0.030* | |
| CCI | |||||
| ≥2 vs. <2 | 1.293 (0.753, 2.221) | 0.352 | |||
| ASA grade | |||||
| III vs. I, II | 1.286 (0.721, 2.294) | 0.394 | |||
| Previous abdominal surgery | |||||
| Yes vs. no | 1.200 (0.766, 1.880) | 0.427 | |||
| Tumor location | |||||
| Colon vs. rectum | 0.983 (0.624, 1.548) | 0.940 | |||
| Differentiation of tumor | |||||
| Low vs. median or high | 1.537 (0.906, 2.608) | 0.111 | |||
| TNM stage | |||||
| III vs. I, II | 1.092 (0.698, 1.709) | 0.700 | |||
| Laparoscopic surgery | |||||
| Yes vs. no | 0.723 (0.463, 1.129) | 0.153 | |||
*, statistically significant. GLIM, Global Leadership Initiative on Malnutrition; OR, odds ratio; CI, confidence interval; BMI, body mass index; HGS, handgrip strength; CCI, Charlson Comorbidity Index; ASA, American Society of Anesthesiologists; TNM, tumor-node-metastasis.
Relationship between GLIM-malnutrition and long-term prognosis
K-M survival analysis showed that patients with GLIM-malnutrition had worse OS than patients with non-malnutrition, but there was no significant difference in DFS between the two groups (Figure 2). In multivariate Cox regression analyses, GLIM-malnutrition (OR: 2.145, 95% CI: 1.234–3.728, P=0.007), low BMI (OR: 2.218, 95% CI: 1.236–3.980, P=0.008), low HGS (OR: 2.044, 95% CI: 1.122–3.722, P=0.020), hypoalbuminemia (OR: 3.077, 95% CI: 1.408–6.724, P=0.005), and low tumor differentiation (OR: 1.795, 95% CI: 1.029–3.129, P=0.039) and high TNM stage (OR: 3.482, 95% CI: 2.061–5.882, P<0.001) were independent risk factors for OS in elderly patients with CRC, and ASA grade (OR: 0.492, 95% CI: 0.224–1.081, P=0.077) was an independent protective factor (Table 4).
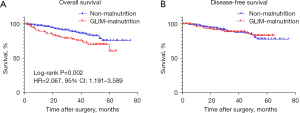
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| GLIM-malnutrition | |||||
| Yes vs. no | 2.223 (1.277, 3.870) | 0.005* | 2.145 (1.234, 3.728) | 0.007* | |
| Age, years | |||||
| ≥80 vs. <80 | 2.286 (0.735, 7.105) | 0.153 | |||
| Gender | |||||
| Male vs. female | 1.436 (0.825, 2.499) | 0.201 | |||
| Low BMI | |||||
| Yes vs. no | 2.039 (1.056, 3.935) | 0.034* | 2.218 (1.236, 3.980) | 0.008* | |
| Low HGS | |||||
| Yes vs. no | 1.865 (1.010, 3.445) | 0.047* | 2.044 (1.122, 3.722) | 0.020* | |
| Anemia | |||||
| Yes vs. no | 1.039 (0.606, 1.783) | 0.888 | |||
| Hypoalbuminemia | |||||
| Yes vs. no | 2.872 (1.277, 6.463) | 0.011* | 3.077 (1.408, 6.724) | 0.005* | |
| CCI | |||||
| ≥2 vs. <2 | 1.268 (0.617, 2.603) | 0.518 | |||
| ASA grade | |||||
| III vs. I, II | 0.470 (0.200, 1.104) | 0.083 | 0.492 (0.224, 1.081) | 0.077 | |
| Previous abdominal surgery | |||||
| Yes vs. no | 0.806 (0.467, 1.395) | 0.553 | |||
| Tumor location | |||||
| Colon vs. rectum | 1.192 (0.701, 2.029) | 0.516 | |||
| Differentiation of tumor | |||||
| Low vs. median or high | 1.881 (1.0722, 3.2999) | 0.028* | 1.795 (1.029, 3.129) | 0.039* | |
| TNM stage | |||||
| III vs. I, II | 3.379 (1.987, 5.746) | <0.001* | 3.482 (2.061, 5.882) | <0.001* | |
| Laparoscopic surgery | |||||
| Yes vs. no | 0.660 (0.380, 1.146) | 0.140 | |||
*, statistically significant. GLIM, Global Leadership Initiative on Malnutrition; OR, odds ratio; CI, confidence interval; BMI, body mass index; HGS, handgrip strength; CCI, Charlson Comorbidity Index; ASA, American Society of Anesthesiologists; TNM, tumor-node-metastasis.
Discussion
Patients with CRC often have reduced food intake, increased energy expenditure, vomiting, intestinal obstruction, and anemia. Due to decreased physiological reserve, elderly patients with CRC are at increased risk of malnutrition.
In previous studies, the incidence of malnutrition in elderly cancer patients assessed by various diagnostic indicators was approximately 27–41.9% (12-14). Song et al. reported that the incidence of malnutrition in CRC patients of all ages who were assessed by the GLIM criteria was 23.6% (15). Similarly, our study showed that 30.9% of elderly patients who underwent CRC surgery were diagnosed with malnutrition according to the GLIM criteria.
At present, many nutritional assessment indicators have been shown to be closely related to the occurrence of post-operative complications in tumor patients, such as PG-SGA score (16), Mini-nutrition assessment MNA (17), albumin (18), and sarcopenia (19). As a newer diagnostic indicator of malnutrition, GLIM-malnutrition is also associated with increased post-operative complications in patients with different types of malignancies (20-22). In this study, we found that GLIM-malnutrition (OR: 1.753, 95% CI: 1.100–2.795, P=0.018) was an independent risk factor for increased post-operative complications in elderly colorectal patients through univariate and multivariate regression analyses. Patients in the GLIM-malnutrition group had a higher incidence of venous thrombosis than those without malnutrition (P=0.035). This finding correlates with Achen et al., who reported that malnutrition is strongly associated with venous thrombosis in patients with malignancy (23). Moreover, the recent PREHAB trial (24) has demonstrated optimized post-operative recovery and fewer severe and medical post-operative complications in patients with CRC that underwent a multimodal prehabilitation program before surgery compared with standard care. The above results suggest that early diagnosis and early intervention of malnutrition are of great significance in reducing post-operative complications in patients with malignancy.
The K-M analysis showed that in older CRC patients, the GLIM-malnutrition group had lower OS than the non-malnutrition group, but there was no significant difference in DFS. Univariate and multivariate COX regression analyses revealed that GLIM-malnutrition (OR: 2.144, 95% CI: 1.234–3.728, P=0.007) was an independent risk factor for OS in older CRC patients. Likewise, GLIM-malnutrition was reported to be an independent risk factor for OS in patients with gastric cancer (25). Orell et al. pointed out that among head and neck cancer patients, patients with GLIM-malnutrition had a shorter median survival time than well-nourished patients (57 vs. 72 months) (26). However, Song et al. showed that GLIM-malnutrition was significantly related to DFS in colorectal patients across all age groups (15). We speculate that this may be related to the different characteristics of the patient populations utilized in the studies. Song et al. reported that patients with GLIM-malnutrition had a higher TNM stage of tumor, a higher proportion of previous abdominal surgery and a lower proportion of laparoscopic surgery than those without malnutrition (15). In contrast, our study showed that malnutrition in elderly CRC patients was not associated with these clinical baseline indicators. In addition, our study shows that hypoalbuminemia is also an independent risk factor for post-operative complications and OS in older CRC patients, which correlates with other data available in the literature (27,28).
Nutritional diagnosis and treatment encompasses nutritional screening, assessment, and intervention. The GLIM criteria integrates nutritional screening and assessment, which sets it apart from other nutritional screening tools. The selection of nutritional risk screening tools and muscle mass assessment tools in the GLIM criteria does not incur significant costs. NRS 2002 is a common choice as a nutritional risk screening tool (11,29). However, the choice of muscle mass assessment tool remains controversial. Different studies have used various methods of assessing muscle mass, such as bioelectrical impedance analysis (30), computed tomography (CT) (13) or hand grip strength (31). A multi-center clinical study showed that hand grip strength is a reasonable surrogate for muscle mass as one of the constituents in the diagnostic criteria of GLIM in patients with gastrointestinal cancer (32). Grip strength was chosen to assess muscle mass in this study as it does not require additional testing equipment and is therefore cost-effective. However, our research still has some limitations. First, this is a single-center retrospective study that included only 385 elderly CRC patients. Second, the measurement of muscle mass in the GLIM criteria remains controversial. In this study, we only used grip strength as a measurement. Further research is needed to compare different methods of muscle mass measurement. Third, we assumed that all CRC patients meet the definition for ‘disease burden’ according to the GLIM criteria, which while reasonable, may not be rigorous enough. This problem remains to be explored by future studies.
Conclusions
Our study shows that the GLIM criteria can accurately evaluate the nutritional status of elderly patients with CRC. GLIM-malnutrition is significantly associated with post-operative complications in elderly patients with CRC. At the same time, GLIM-malnutrition is an independent risk factor for OS in elderly patients with CRC. Therefore, using the GLIM criteria to provide early assessment of the nutritional status of elderly patients with CRC can allow corresponding nutritional interventions to be implemented, thereby reducing post-operative complications and improving survival outcomes.
Acknowledgments
Funding: This work was supported by the Health Commission of Zhejiang (Grant No. 2023RC203).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-543/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-543/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-543/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-543/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (ethical no. 2014063) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Martínez-Escribano C, Arteaga Moreno F, Pérez-López M, et al. Malnutrition and Increased Risk of Adverse Outcomes in Elderly Patients Undergoing Elective Colorectal Cancer Surgery: A Case-Control Study Nested in a Cohort. Nutrients 2022;14:207. [Crossref] [PubMed]
- Zhang Z, Wan Z, Zhu Y, et al. Predictive validity of the GLIM criteria in treatment outcomes in cancer patients with radiotherapy. Clin Nutr 2022;41:855-61. [Crossref] [PubMed]
- Baitar A, Kenis C, Decoster L, et al. The prognostic value of 3 commonly measured blood parameters and geriatric assessment to predict overall survival in addition to clinical information in older patients with cancer. Cancer 2018;124:3764-75. [Crossref] [PubMed]
- Qiu Y, You J, Wang K, et al. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: A randomized control trial. Nutrition 2020;69:110558. [Crossref] [PubMed]
- Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr 2021;40:2898-913. [Crossref] [PubMed]
- Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1-9. [Crossref] [PubMed]
- Xu LB, Shi MM, Huang ZX, et al. Impact of malnutrition diagnosed using Global Leadership Initiative on Malnutrition criteria on clinical outcomes of patients with gastric cancer. JPEN J Parenter Enteral Nutr 2022;46:385-94. [Crossref] [PubMed]
- Wang P, Chen X, Liu Q, et al. Good performance of the Global Leadership Initiative on Malnutrition criteria for diagnosing and classifying malnutrition in people with esophageal cancer undergoing esophagectomy. Nutrition 2021;91-92:111420. [Crossref] [PubMed]
- Przekop Z, Szostak-Węgierek D, Milewska M, et al. Efficacy of the Nutritional Risk Index, Geriatric Nutritional Risk Index, BMI, and GLIM-Defined Malnutrition in Predicting Survival of Patients with Head and Neck Cancer Patients Qualified for Home Enteral Nutrition. Nutrients 2022;14:1268. [Crossref] [PubMed]
- Zhang X, Tang M, Zhang Q, et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin Nutr 2021;40:1224-32. [Crossref] [PubMed]
- Edwards BJ, Zhang X, Sun M, et al. Overall survival in older patients with cancer. BMJ Support Palliat Care 2020;10:25-35. [Crossref] [PubMed]
- Huang DD, Yu DY, Song HN, et al. The relationship between the GLIM-defined malnutrition, body composition and functional parameters, and clinical outcomes in elderly patients undergoing radical gastrectomy for gastric cancer. Eur J Surg Oncol 2021;47:2323-31. [Crossref] [PubMed]
- Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218-26. [Crossref] [PubMed]
- Song HN, Wang WB, Luo X, et al. Effect of GLIM-defined malnutrition on postoperative clinical outcomes in patients with colorectal cancer. Jpn J Clin Oncol 2022;52:466-74. [Crossref] [PubMed]
- Tsai YT, Lai CH, Huang TH, et al. Association of malnutrition with postoperative complication risk after curative surgery for oral cancer: Observational study. Medicine (Baltimore) 2020;99:e23860. [Crossref] [PubMed]
- Ścisło L, Bodys-Cupak I, Walewska E, et al. Nutritional Status Indicators as Predictors of Postoperative Complications in the Elderly with Gastrointestinal Cancer. Int J Environ Res Public Health 2022;19:13453. [Crossref] [PubMed]
- Kathiresan AS, Brookfield KF, Schuman SI, et al. Malnutrition as a predictor of poor postoperative outcomes in gynecologic cancer patients. Arch Gynecol Obstet 2011;284:445-51. [Crossref] [PubMed]
- Şengül Ayçiçek G, Erol T, Ünsal P, et al. Impact of frailty and ultrasonography-based sarcopenia on the development of postoperative complications in gastrointestinal cancer patients. Turk J Med Sci 2021;51:1261-6. [Crossref] [PubMed]
- Huang DD, Yu DY, Wang WB, et al. Global leadership initiative in malnutrition (GLIM) criteria using hand-grip strength adequately predicts postoperative complications and long-term survival in patients underwent radical gastrectomy for gastric cancer. Eur J Clin Nutr 2022;76:1323-31. [Crossref] [PubMed]
- Gao X, Liu H, Zhang L, et al. The application value of preoperative fat-free mass index within Global Leadership Initiative on Malnutrition-defined malnutrition criteria for postoperative outcomes in patients with esophagogastric cancer. Nutrition 2022;102:111748. [Crossref] [PubMed]
- Yin L, Cheng N, Chen P, et al. Association of Malnutrition, as Defined by the PG-SGA, ESPEN 2015, and GLIM Criteria, With Complications in Esophageal Cancer Patients After Esophagectomy. Front Nutr 2021;8:632546. [Crossref] [PubMed]
- Achen G, Dolivet E, Turck M, et al. Incidence and impact of venous thrombosis in the diagnosis and therapeutic management of ovarian cancer. Gynecol Obstet Fertil Senol 2020;48:506-13. [PubMed]
- Molenaar CJL, Minnella EM, Coca-Martinez M, et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg 2023;158:572-81. [Crossref] [PubMed]
- Cai W, Yang H, Zheng J, et al. Global leaders malnutrition initiative-defined malnutrition affects long-term survival of different subgroups of patients with gastric cancer: A propensity score-matched analysis. Front Nutr 2022;9:995295. [Crossref] [PubMed]
- Orell HK, Pohju AK, Osterlund P, et al. GLIM in diagnosing malnutrition and predicting outcome in ambulatory patients with head and neck cancer. Front Nutr 2022;9:1030619. [Crossref] [PubMed]
- Kang B, Zhao ZQ, Liu XY, et al. Effect of hypoalbuminemia on short-term outcomes after colorectal cancer surgery: A propensity score matching analysis. Front Nutr 2022;9:925086. [Crossref] [PubMed]
- Almasaudi AS, Dolan RD, Edwards CA, et al. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers (Basel) 2020;12:1986. [Crossref] [PubMed]
- De Groot LM, Lee G, Ackerie A, et al. Malnutrition Screening and Assessment in the Cancer Care Ambulatory Setting: Mortality Predictability and Validity of the Patient-Generated Subjective Global Assessment Short form (PG-SGA SF) and the GLIM Criteria. Nutrients 2020;12:2287. [Crossref] [PubMed]
- Henriksen C, Paur I, Pedersen A, et al. Agreement between GLIM and PG-SGA for diagnosis of malnutrition depends on the screening tool used in GLIM. Clin Nutr 2022;41:329-36. [Crossref] [PubMed]
- Yin L, Lin X, Zhao Z, et al. Is hand grip strength a necessary supportive index in the phenotypic criteria of the GLIM-based diagnosis of malnutrition in patients with cancer? Support Care Cancer 2021;29:4001-13. [Crossref] [PubMed]
- Zhou LP, Yu DY, Ma BW, et al. Feasibility of substituting handgrip strength for muscle mass as a constituent standard in the Global Leadership Initiative on Malnutrition for diagnosing malnutrition in patients with gastrointestinal cancers. Nutrition 2021;84:111044. [Crossref] [PubMed]



