
Prognostic impact of portal area inflammation on intrahepatic cholangiocarcinoma patients without lymph node metastasis
Highlight box
Key findings
• Portal area inflammation (PAI) influences the prognosis of intrahepatic cholangiocarcinoma (ICC) patients after surgery.
What is known and what is new?
• Inflammatory cell infiltration in tumors will significantly affect the prognosis of patients.
• Impact of PAI on the prognosis of ICC patients without lymph node metastasis.
What is the implication, and what should change now?
• It is strongly recommended to pay attention to the inflammatory status of the portal area in ICC patients and increase the frequency of postoperative follow-up to improve the prognosis of ICC patients after curative resection.
Introduction
Cholangiocarcinoma ranks second in incidence among primary malignant hepatic tumors, after hepatocellular carcinoma. Intrahepatic cholangiocarcinoma (ICC) is a type of cholangiocarcinoma located proximal to the secondary bile ducts. The early diagnosis of ICC is difficult, the treatment effect is far from satisfactory, and the prognosis is poor (1). In the past several decades, the incidence of ICC had increased annually, raising concerns (2). Radical resection is the only curative treatment available for ICC. Nevertheless, many patients are at an advanced stage of the tumor when they are first diagnosed, and only approximately 30% of patients are considered for radical resection (3). Even if some patients are fortunate enough to undergo radical resection, the chance of recurrence remains high (4). There are many reasons for the high degree of malignancy, low cure rate, and rapid progression of ICC, and inflammation could be a key factor (5).
Tumorigenesis involves not only the proliferation of cancer cells. Rather, tumors are complex tissues composed of cells of different origins. Normal cells that form the tumor stroma are also important factors in promoting tumorigenesis and metastasis (6). Some previous studies have shown that the occurrence and progression of liver cancer are also associated with inflammation, and some potential therapeutic targets affect the prognosis of liver cancer by affecting inflammation (7-9). At the early stage of tumor formation, inflammation can create a favorable environment for tumorigenesis. In advanced stages, inflammatory responses can also promote tumor spread and metastasis (10). Previous studies have shown that infiltration of large numbers of inflammatory cells in patients with breast or kidney cancer is indicative of poor prognoses (11,12). In lung cancer, inflammatory cell infiltration often indicates that the cancer is in an advanced stage (13). A study has found that inflammatory cells are characteristically distributed in cholangiocarcinoma, and that pro-tumorigenic inflammation can suppress anti-tumor immunity, making the tumor microenvironment more favorable for tumor growth (14).
The portal area, an important basic structure in the liver, refers to the connective tissue area between adjacent hepatic lobules. This area contains interlobular veins, interlobular arteries, and interlobular bile ducts (15). Portal area inflammation (PAI) refers to inflammation in the portal area, which can affect the prognosis of some liver diseases (16).
Many previous studies have reported that inflammatory cell infiltration in tumors will significantly affect the prognosis of patients (11-13,17). However, few studies have investigated the influence of inflammation in the peritumoral normal tissues on tumors. Specifically, the impact of PAI on the prognosis of ICC patients without lymph node metastasis (LNM) is not known. Thus, the present study aimed to determine the impact of PAI on the prognosis of ICC patients after surgery. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1143/rc).
Methods
Study cohort and data collection
This study retrospectively analyzed ICC patients (n=297) who underwent curative-intent resection (R0/R1) at the Eastern Hepatobiliary Surgery Hospital, Shanghai, between January 2011 and December 2015. Only patients with histologically confirmed ICC without lymph node or distant metastasis were included. Specific inclusion and exclusion criteria were as follows: (I) only patients whose pathological examination met the criteria of curative-intent resection (R0/R1) were included, and patients who underwent palliative surgery or whose pathological results fail to meet the criteria were excluded. (II) Patients with pathological findings of lymph node or distant metastasis were excluded. (III) Patients with accidental death, such as car accidents, were excluded. This study screened 297 eligible patients from a total of 701 patients.
Standard demographic, clinical laboratory, and clinicopathological data were collected. Experienced pathologists determined the presence or absence of PAI based on standard pathological criteria. PAI was defined as the presence of inflammatory cell infiltration in more than 50% of the portal area under microscope during biopsy (Figure 1). The incidence of PAI was 43.1% (n=128). Other clinicopathological data, including tumor characteristics, specifically, tumor diameter, tumor number, histological grade, presence of vascular/nerve invasion, and data on concomitant liver disease, such as steatosis and cirrhosis, were also included. These factors were evaluated based on the final histological examination. Notably, the included patients underwent routine lymph node dissection during the operation, and no LNM was found.
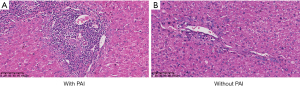
Follow-up and patient outcomes
After surgery, the patients were routinely examined using abdominal ultrasonography, computed tomography, or magnetic resonance imaging to determine the postoperative liver status and whether tumor recurrence had occurred. Overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up and was the primary endpoint of the study. The secondary endpoint was recurrence-free survival (RFS), which represents the period between the date of surgery and tumor recurrence. All investigators involved in the follow-up had received special training before the follow-up, and the follow-up adopted a unified and standardized survey method to reduce the bias.
Statistical analysis
Except OS and RFS, all variables in this study are categorical variables. Continuous variables, such as tumor markers, were converted into categorical variables for presentation. Categorical variables are expressed as numbers and percentages and were compared using the Chi-squared (χ2) test or Fisher’s exact test. The OS and RFS curves were analyzed using the Kaplan-Meier method, and the median survival time was estimated using the log-rank test. Independent factors associated with RFS and OS were determined using Cox regression models. Hazard ratios (HRs) with corresponding 95% confidence intervals (95% CIs) were also estimated using Cox regression models. In Cox regression analysis, multivariate analysis was performed with variables yielding P<0.05 in univariate analysis. P<0.05 (two-tailed) was considered statistically significant. Statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, New York, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Eastern Hepatobiliary Surgery Hospital (No. EHBHKY2016-01-018). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Results
Baseline characteristics
Among the 297 patients with ICC enrolled in this study, approximately three-quarters were 65 years or older and most were male (n=198, 66.7%). The overall incidence of PAI was 43.1% (n=128). Patients with PAI were more likely to have adverse clinical or tumor characteristics, including liver cirrhosis, high carbohydrate antigen 19-9 levels (CA19-9) (>40 U/mL), high carcinoembryonic antigen levels (CEA >5 ng/mL), larger tumor size (diameter >5 cm), and worse American Joint Committee on Cancer (AJCC) T stage (T3–4 stage) (Table 1). Notably, there was no significant difference in the incidence of nerve invasion or vascular invasion between patients with and without PAI. Subgroup analysis of factors that differed at baseline are presented below.
Table 1
| Characteristics | Without PAI, n=169 | With PAI, n=128 | P value |
|---|---|---|---|
| OS (months) | 32.79 (16.64) | 25.20 (17.09) | <0.001 |
| RFS (months) | 24.36 (17.20) | 17.33 (15.62) | <0.001 |
| Age (years) | 0.739 | ||
| <65 | 132 (78.1) | 97 (75.8) | |
| ≥65 | 37 (21.9) | 31 (24.2) | |
| Gender | 0.341 | ||
| Male | 117 (69.2) | 81 (63.3) | |
| Female | 52 (30.8) | 47 (36.7) | |
| Smoking | 0.514 | ||
| Yes | 56 (33.1) | 37 (28.9) | |
| No | 113 (66.9) | 91 (71.1) | |
| Drinking | 0.358 | ||
| Yes | 36 (21.3) | 34 (26.6) | |
| No | 133 (78.7) | 94 (73.4) | |
| Diabetes | 0.691 | ||
| Yes | 25 (14.8) | 16 (12.5) | |
| No | 144 (85.2) | 112 (87.5) | |
| Hypertension | 0.89 | ||
| Yes | 36 (21.3) | 29 (22.7) | |
| No | 133 (78.7) | 99 (77.3) | |
| Liver cirrhosis | 0.003 | ||
| Yes | 48 (28.4) | 59 (46.1) | |
| No | 121 (71.6) | 69 (53.9) | |
| AFP (ng/mL) | 0.275 | ||
| ≤400 | 165 (97.6) | 121 (94.5) | |
| >400 | 4 (2.4) | 7 (5.5) | |
| CA19-9 (U/mL) | 0.006 | ||
| ≤40 | 89 (52.7) | 46 (35.9) | |
| >40 | 80 (47.3) | 82 (64.1) | |
| CEA (ng/mL) | 0.016 | ||
| ≤5 | 132 (78.1) | 83 (64.8) | |
| >5 | 37 (21.9) | 45 (35.2) | |
| HBV | 0.176 | ||
| Yes | 70 (41.4) | 64 (50.0) | |
| No | 99 (58.6) | 64 (50.0) | |
| HCV | 0.169 | ||
| Yes | 12 (7.1) | 16 (12.5) | |
| No | 157 (92.9) | 112 (87.5) | |
| Tumor size (cm) | 0.041 | ||
| ≤5 | 90 (53.3) | 52 (40.6) | |
| >5 | 79 (46.7) | 76 (59.4) | |
| Multiple tumors | 0.59 | ||
| Yes | 59 (34.9) | 40 (31.2) | |
| No | 110 (65.1) | 88 (68.8) | |
| Stone history | 0.993 | ||
| Yes | 12 (7.1) | 10 (7.8) | |
| No | 157 (92.9) | 118 (92.2) | |
| Nerve invasion | 0.837 | ||
| Yes | 10 (5.9) | 6 (4.7) | |
| No | 159 (94.1) | 122 (95.3) | |
| Vascular invasion | 0.692 | ||
| Yes | 36 (21.3) | 24 (18.8) | |
| No | 133 (78.7) | 104 (81.2) | |
| AJCC T stage | <0.001 | ||
| T1–2 | 146 (86.4) | 87 (68.0) | |
| T3–4 | 23 (13.6) | 41 (32.0) | |
| Steatosis | 0.908 | ||
| Yes | 15 (8.9) | 10 (7.8) | |
| No | 154 (91.1) | 118 (92.2) | |
| PAI | <0.001 | ||
| Yes | 0 (0.0) | 128 (100.0) | |
| No | 169 (100.0) | 0 (0.0) | |
| Schistosome | 0.469 | ||
| Yes | 11 (6.5) | 5 (3.9) | |
| No | 158 (93.5) | 123 (96.1) | |
| Poor differentiation | 0.165 | ||
| Yes | 23 (13.6) | 10 (7.8) | |
| No | 146 (86.4) | 118 (92.2) | |
Data are represented as mean (SD) or number (%). PAI, portal area inflammation; OS, overall survival; RFS, recurrence-free survival; AFP, alpha fetoprotein; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer.
Impact of PAI on RFS and OS in overall cohort
The Kaplan-Meier method was used to evaluate the prognostic value of PAI in postoperative patients with ICC. The median follow-up time of these patients was 28.97 months, and approximately three-fifths of the patients (n=184, 61.9%) died during follow-up. The 1-, 2-, and 3-year OS rates were 81.5%, 59.9%, and 35.0%, respectively. Compared to patients without PAI, both OS and RFS of patients with PAI were worse (median OS, 33.37 months without PAI versus 21.87 months with PAI, P<0.001) (Figure 2A) (median RFS, 21.60 months without PAI versus 12.33 months with PAI, P<0.001) (Figure 2B). Univariate and multivariate analyses of prognostic factors in postoperative patients with ICC were performed using a Cox regression model. On multivariate analysis of OS and RFS, PAI (HR 1.60; 95% CI: 1.18–2.17, P=0.003) (Table 2), tumor diameter >5 cm, vascular invasion, and AJCC T3–4 stage were independent prognostic factors for OS. PAI (HR 1.40; 95% CI: 1.06–1.85, P=0.019) (Table 3), tumor diameter >5 cm, and AJCC T3–4 stage were independent prognostic factors for RFS. PAI is significantly associated with worse OS and RFS.
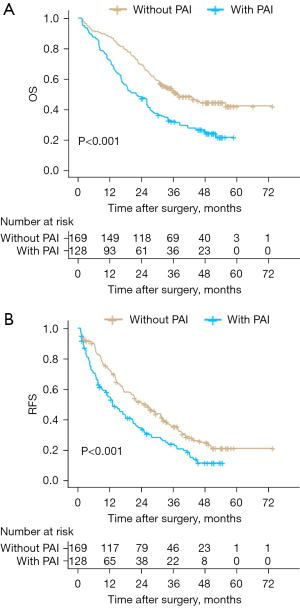
Table 2
| Characteristics | Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Old age | 1.12 | 0.80–1.57 | 0.525 | ||||
| Male gender | 0.81 | 0.60–1.09 | 0.169 | ||||
| Smoking | 0.95 | 0.70–1.30 | 0.768 | ||||
| Drinking | 1.19 | 0.86–1.66 | 0.297 | ||||
| Diabetes | 1.28 | 0.85–1.93 | 0.237 | ||||
| Hypertension | 1.09 | 0.78–1.54 | 0.611 | ||||
| Liver cirrhosis | 1.22 | 0.91–1.64 | 0.193 | ||||
| AFP >400 ng/mL | 1.28 | 0.63–2.61 | 0.492 | ||||
| CA19-9 >40 U/mL | 1.21 | 0.90–1.62 | 0.205 | ||||
| CEA >5 ng/mL | 1.36 | 0.99–1.86 | 0.056 | ||||
| HBV(+) | 1.24 | 0.93–1.66 | 0.144 | ||||
| HCV(+) | 1.16 | 0.72–1.86 | 0.543 | ||||
| Tumor size, cm | |||||||
| Diameter ≤5 | Reference | Reference | |||||
| Diameter >5 | 1.80 | 1.34–2.42 | <0.001 | 1.58 | 1.16–2.15 | 0.004 | |
| Multiple tumors | 1.26 | 0.94–1.71 | 0.126 | ||||
| Stone history | 1.09 | 0.62–1.91 | 0.768 | ||||
| Nerve invasion | 1.27 | 0.69–2.34 | 0.444 | ||||
| Vascular invasion | 1.71 | 1.22–2.41 | 0.002 | 1.87 | 1.31–2.67 | 0.001 | |
| AJCC T stage | |||||||
| T1–2 | Reference | Reference | |||||
| T3–4 | 1.81 | 1.30–2.52 | <0.001 | 1.47 | 1.01–2.12 | 0.042 | |
| Liver steatosis | 1.19 | 0.72–1.96 | 0.492 | ||||
| PAI | 1.87 | 1.40–2.50 | <0.001 | 1.60 | 1.18–2.17 | 0.003 | |
| Liver schistosome | 0.75 | 0.37–1.52 | 0.424 | ||||
| Poor differentiation | 1.04 | 0.65–1.65 | 0.876 | ||||
HR, hazard ratio; CI, confidence interval; AFP, alpha fetoprotein; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer; PAI, portal area inflammation.
Table 3
| Characteristics | Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Old age | 1.07 | 0.78–1.47 | 0.674 | ||||
| Male gender | 0.97 | 0.73–1.29 | 0.818 | ||||
| Smoking | 0.89 | 0.67–1.19 | 0.431 | ||||
| Drinking | 0.92 | 0.67–1.26 | 0.588 | ||||
| Diabetes | 1.11 | 0.75–1.63 | 0.610 | ||||
| Hypertension | 1.22 | 0.89–1.66 | 0.217 | ||||
| Liver cirrhosis | 1.16 | 0.88–1.53 | 0.281 | ||||
| AFP >400 ng/mL | 1.74 | 0.89–3.39 | 0.107 | ||||
| CA19-9 >40 U/mL | 1.14 | 0.87–1.49 | 0.333 | ||||
| CEA >5 ng/mL | 1.33 | 1.00–1.79 | 0.054 | ||||
| HBV(+) | 1.12 | 0.86–1.46 | 0.402 | ||||
| HCV(+) | 1.00 | 0.62–1.60 | 0.987 | ||||
| Tumor size, cm | |||||||
| Diameter ≤5 | Reference | Reference | |||||
| Diameter >5 | 1.60 | 1.23–2.10 | 0.001 | 1.43 | 1.08–1.89 | 0.012 | |
| Multiple tumors | 1.19 | 0.90–1.58 | 0.228 | ||||
| Stone history | 0.99 | 0.58–1.67 | 0.961 | ||||
| Nerve invasion | 1.19 | 0.66–2.13 | 0.560 | ||||
| Vascular invasion | 1.15 | 0.83–1.60 | 0.396 | ||||
| AJCC T stage | |||||||
| T1–2 | Reference | Reference | |||||
| T3–4 | 1.80 | 1.31–2.48 | <0.001 | 1.45 | 1.03–2.04 | 0.031 | |
| Liver steatosis | 1.44 | 0.92–2.27 | 0.112 | ||||
| PAI | 1.58 | 1.21–2.06 | 0.001 | 1.40 | 1.06–1.85 | 0.019 | |
| Liver schistosome | 0.64 | 0.33–1.26 | 0.197 | ||||
| Poor differentiation | 0.97 | 0.64–1.49 | 0.902 | ||||
HR, hazard ratio; CI, confidence interval; AFP, alpha fetoprotein; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer; PAI, portal area inflammation.
Impact of PAI on RFS and OS in patients with early-stage ICC
Among the 297 patients, 233 (78.5%) had AJCC T1–2 stage and 64 (21.5%) had AJCC T3–4 stage cancer. Among patients with AJCC T1–2 stage, it is found that, compared to patients without PAI, those with PAI had relatively worse OS (median OS, 18.73 months with PAI versus 33.97 months without PAI, P<0.001) and RFS (median RFS, 12.33 months with PAI versus 25.00 months without PAI, P=0.004). In contrast, among patients with AJCC T3–4 stage, no significant difference was found in OS (median OS, 26.13 months with PAI versus 22.50 months without PAI, P=0.230) or RFS (median RFS, 12.50 months with PAI versus 7.90 months without PAI, P=0.850) between those with or without PAI (Figure 3A,3B). In addition, Cox univariate and multivariate analyses were performed for patients with stage T1–T2. PAI remained a prognostic factor for OS (HR 1.73; 95% CI: 1.22–2.44, P=0.002) and RFS (HR 1.56; 95% CI: 1.15–2.13, P=0.005) (Tables S1,S2).
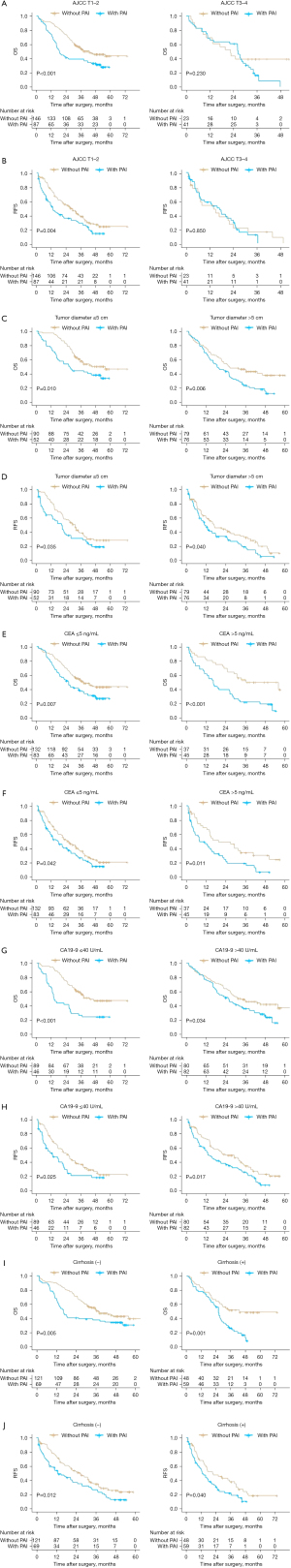
Impact of PAI on RFS and OS in patients with differing tumors sizes
Among patients with tumor diameter ≤5 cm, those with PAI had worse OS (median OS, 26.40 months with PAI versus 34.90 months without PAI, P=0.010) and RFS (median RFS, 16.97 months with PAI versus 28.03 months without PAI, P=0.035) than those without PAI. Similarly, among patients with a tumor diameter >5 cm, the OS (median OS, 18.80 months with PAI versus 26.17 months without PAI, P=0.006) and RFS (median RFS, 10.83 months with PAI versus 13.93 months without PAI, P=0.040) (Figure 3C,3D) of patients with PAI were also worse than those of without PAI. In both these subgroups, PAI had statistically significant effects on the RFS and OS of the patients (P<0.05).
Impact of PAI on RFS and OS in patients with different levels of tumor markers
Among patients with CEA ≤5 ng/mL, patients with PAI had worse OS (median OS, 24.30 months with PAI versus 32.87 months without PAI, P=0.007) and RFS (median RFS, 13.10 versus 21.70 months without PAI, P=0.042) than those without PAI. Similarly, among patients whose CEA >5 ng/mL, OS (median OS, 16.30 months with PAI versus 33.37 months without PAI, P<0.001) and RFS (median RFS, 7.67 months with PAI versus 16.03 months without PAI, P=0.011) of PAI patients were also worse than those of patients without PAI (Figure 3E,3F). Among patients whose CA19-9 ≤40 U/mL, patients with PAI had worse OS (median OS, 13.90 months with PAI versus 33.77 months without PAI, P<0.001) and RFS (median RFS, 9.37 months with PAI versus 21.97 months without PAI, P=0.025) than those without PAI. Similarly, among patients with CA19-9 >40 U/mL, the OS (median OS, 24.03 months with PAI versus 31.37 months without PAI, P=0.034) and RFS (median RFS, 12.50 months with PAI versus 20.23 months without PAI, P=0.017) of patients with PAI were also worse than those of patients without PAI (Figure 3G,3H).
Impact of PAI on RFS and OS in patients with and without liver cirrhosis
The presence of liver cirrhosis did not modify the effect of PAI on prognosis. Among patients without liver cirrhosis, patients with PAI had worse OS (median OS, with PAI 16.30 versus without PAI 32.87 months, P=0.005) and RFS (median RFS, with PAI 11.00 versus without PAI 21.90 months, P=0.012) than those without PAI. Similarly, among patients with liver cirrhosis, the OS (median OS, with PAI 26.10 versus without PAI 33.40 months, P=0.001) and RFS (median RFS, with PAI 12.50 versus without PAI 17.10 months, P=0.040) of patients with PAI were also worse than those of patients without PAI (Figure 3I,3J).
Discussion
There are many causes of chronic inflammation, including microbial infection and autoimmune diseases (18). The role of inflammation in carcinogenesis is now widely accepted, and the inflammatory microenvironment is an important component of tumors (19,20). In such microenvironment, inflammation gives rise to the survival and proliferation of cancer cells and stimulates angiogenesis and tumor metastasis (21). However, alongside investigating inflammation within the tumor microenvironment, it is necessary to study the influence of inflammation within normal peritumoral tissues on the tumor itself. For example, the prognosis of lung cancer patients with non-tumor-associated inflammation of the lungs is worse (22), in addition, liver inflammation is a key pathophysiological mechanism that promotes liver fibrosis (23), which is the main prognostic determinant of liver related death (24). Considering the distinctive anatomical structure of the intrahepatic bile duct, the portal area is often the structure adjacent to the ICC; therefore, it is plausible that its inflammatory status will affect the prognosis of ICC patients. According to the analysis of clinical data, PAI influences the prognosis of ICC patients after surgery. Analyzing the causes of this phenomenon, it is suggest that, first of all, inflammation in the portal area leads to poor function of residual liver after surgery, which increases the probability of postoperative liver failure, thus causing poor survival prognosis of patients (25,26). In addition, the presence of inflammation in the portal area means that the inflammatory factors in the liver, such as tumor necrosis factor and interleukin, are at a high level, which is often positively related to tumor recurrence (27,28).
Furthermore, subgroup analysis of the factors with significant differences in distribution at the baseline were conducted. In the subgroup analyses of tumor size, liver cirrhosis, and tumor markers, such as CA19-9 and CEA, PAI maintained a statistically significant impact on OS and RFS. In contrast, the prognosis of early-stage patients (AJCC T1–2) was significantly related to PAI, but the prognosis of advanced-stage (AJCC T3–4) patients was not significantly related to PAI. It is considered that this may be because the prognosis of patients with T3–4 stage is already poor. Therefore, the adverse impact of PAI on the prognosis of patients at T3–4 cannot be seen. Further research could consider whether PAI itself could distinguish the prognosis of patients at T3–4, but due to an insufficient sample size, such results were not observed in this study.
To date, few studies have investigated the effect of inflammation of the portal area on the prognosis of ICC patients after curative-intent resection. However, based on the clinical data analysis, PAI had a statistically significant effect on OS and RFS. Through multivariate analysis, PAI was found to be an independent risk factor for ICC, particularly for the prognosis of patients with early-stage ICC. This poses a challenge to treatment.
Precision therapy has been advocated for cholangiocarcinoma. New treatment strategies, such as therapy targeting the fibroblast growth factor receptor/isocitrate dehydrogenase (FGFR/IDH) pathways among others, as well as immunotherapy, are emerging. However, to date, the effects of ICC drug treatment have not been satisfactory (29). Gemcitabine combined with cisplatin is currently the first-line treatment for patients with advanced-stage cholangiocarcinoma. Nevertheless, even when using this first-line medication, the median survival of patients is still less than one year (30). Emerging treatments, such as immunotherapy, may require further long-term research. Results provide further insights into alternative therapeutic approaches using existing drugs to improve the efficacy of treatment in ICC patients.
The tumor microenvironment and PAI provide ideal targets for anti-tumor therapy. Non-tumor cells have higher genetic stability than tumor cells. This reduces the problem of acquired resistance to chemotherapy drugs (31). Therefore, the use of safe, inexpensive, non-selective anti-inflammatory drugs, such as aspirin, may reduce the short-term recurrence rate in patients with a history of adenoma or cancer (32).
According to the findings, PAI was associated with worse prognosis in patients with ICC. Therefore, PAI should also be considered as a possible indication for adjuvant therapy. Therapeutic strategies to limit tumor-associated inflammation have been successful in preclinical experimental models (33). This encouraging result could provide new directions for cancer combination therapy aimed at reducing cancer-promoting inflammation and for maximizing anti-tumor treatment efficacy.
The current study has some limitations. First, serological inflammatory indicators were not analyzed in this study, which may have allowed systemic assessment of the relationship between inflammatory status and ICC prognosis. Second, none of the patients included in the study had LNM; thus, for ICC patients with LNM, the impact of PAI on prognosis needs to be further explored. Lastly, this was a retrospective study from a single center. A multicenter prospective study is therefore warranted.
Conclusions
This study revealed that PAI is an important independent risk factor for recurrence and OS in ICC patients without LNM after curative-intent resection. Additionally, patients without PAI have a better prognosis than those with PAI. It is strongly recommended to pay attention to the inflammatory status of the portal area in ICC patients and increase the frequency of postoperative follow-up to improve the prognosis of ICC patients after curative resection.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81970453, 82270634, and 81772529) and the Shanghai Science and Technology Innovation Action Plan project (No. 20XD1405100).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1143/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1143/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1143/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1143/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594-9. [Crossref] [PubMed]
- Kelley RK, Bridgewater J, Gores GJ, et al. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol 2020;72:353-63. [Crossref] [PubMed]
- Zhao DY, Lim KH. Current biologics for treatment of biliary tract cancers. J Gastrointest Oncol 2017;8:430-40. [Crossref] [PubMed]
- Zhang Y, Shi SM, Yang H, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer 2019;10:494-503. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis 2019;39:26-42. [Crossref] [PubMed]
- Mitra A, Yan J, Xia X, et al. IL6-mediated inflammatory loop reprograms normal to epithelial-mesenchymal transition(+) metastatic cancer stem cells in preneoplastic liver of transforming growth factor beta-deficient β2-spectrin(+/-) mice. Hepatology 2017;65:1222-36. [Crossref] [PubMed]
- Kuang DM, Peng C, Zhao Q, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 2010;51:154-64. [Crossref] [PubMed]
- Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603-16. [Crossref] [PubMed]
- Lim B, Woodward WA, Wang X, et al. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat Rev Cancer 2018;18:485-99. [Crossref] [PubMed]
- Soyupek S, Tulunay O, Armağan A, et al. Clinical importance of intratumoral and normal renal parenchymal inflammatory cell infiltration in renal cell carcinoma. Scand J Urol Nephrol 2007;41:387-91. [Crossref] [PubMed]
- Banat GA, Tretyn A, Pullamsetti SS, et al. Immune and Inflammatory Cell Composition of Human Lung Cancer Stroma. PLoS One 2015;10:e0139073. [Crossref] [PubMed]
- Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665-74. [Crossref] [PubMed]
- Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology 1998;28:323-31. [Crossref] [PubMed]
- Mann JP, De Vito R, Mosca A, et al. Portal inflammation is independently associated with fibrosis and metabolic syndrome in pediatric nonalcoholic fatty liver disease. Hepatology 2016;63:745-53. [Crossref] [PubMed]
- Li N, Zhou ZS, Shen Y, et al. Inhibition of the sterol regulatory element-binding protein pathway suppresses hepatocellular carcinoma by repressing inflammation in mice. Hepatology 2017;65:1936-47. [Crossref] [PubMed]
- Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 2020;119:199-245. [Crossref] [PubMed]
- Bakiri L, Hamacher R, Graña O, et al. Liver carcinogenesis by FOS-dependent inflammation and cholesterol dysregulation. J Exp Med 2017;214:1387-409. [Crossref] [PubMed]
- Ringelhan M, Pfister D, O'Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222-32. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer 2019;19:9-31. [Crossref] [PubMed]
- Schuster S, Cabrera D, Arrese M, et al. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol 2018;15:349-64. [Crossref] [PubMed]
- Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265-73. [Crossref] [PubMed]
- Laursen TL, Siggaard CB, Kazankov K, et al. Time-dependent improvement of liver inflammation, fibrosis and metabolic liver function after successful direct-acting antiviral therapy of chronic hepatitis C. J Viral Hepat 2020;27:28-35. [Crossref] [PubMed]
- Liang Z, Yu M, Liu Z, et al. Inflammation Affects Liver Function and the Metabolism of Voriconazole to Voriconazole-N-Oxide in Adult and Elderly Patients. Front Pharmacol 2022;13:835871. [Crossref] [PubMed]
- Cai H, Zhang Y, Meng F, et al. Preoperative Serum IL6, IL8, and TNF-α May Predict the Recurrence of Hepatocellular Cancer. Gastroenterol Res Pract 2019;2019:6160783. [Crossref] [PubMed]
- Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci 2014;21:754-60. [Crossref] [PubMed]
- Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603-13. [Crossref] [PubMed]
- Marelli G, Sica A, Vannucci L, et al. Inflammation as target in cancer therapy. Curr Opin Pharmacol 2017;35:57-65. [Crossref] [PubMed]


