Use of positron emission tomography scan response to guide treatment change for locally advanced gastric cancer: the Memorial Sloan Kettering Cancer Center experience
Introduction
Gastric cancer is an aggressive neoplasm and patients with locally advanced disease have a poor prognosis despite curative intent surgery. Adjuvant therapy has been shown to improve survival when added to surgery, with improvements seen for post-operative chemotherapy with radiotherapy (INT-0116) or without (S-1, CLASSIC trials) (1-3). Large randomized trials have also established the benefit of perioperative chemotherapy over surgery alone (4,5). Despite these positive results of additional therapy to curative intent resection, patient outcomes remain poor, despite the additional benefit of systemic therapy.
Response to preoperative chemotherapy of localized gastric and gastroesophageal junction (GEJ) adenocarcinoma, as measured by clinical parameters and pathological response, has been associated with improved patient outcomes and survival following surgery (6). Positron emission tomography (PET) scan has been studied as a tool to assess response to neoadjuvant chemotherapy (7). German investigators showed that an SUV reduction of 35% or greater after induction chemotherapy differentiated patients into two prognostic groups, with the PET responders having significantly better overall survival (OS) than non-responders. Metabolic response was associated with a high histopathologic response rate of 44% compared to 5% in non-responders (P=0.001) (8). The subsequent MUNICON study focusing on GEJ cancers showed PET non-responders who stopped preoperative chemotherapy after two weeks and proceeded directly to surgery continue to have significantly poorer survival, compared to PET responders who completed 3 months of preoperative therapy. To validate the potential prognostic and predictive value of early PET scan imaging during preoperative therapy for esophagogastric cancer, we performed a phase II trial of irinotecan and cisplatin in locally advanced gastric cancer. Early PET response from baseline to day 35 was highly predictive for disease free survival (DFS) (P=0.01) and histopathologic response (P=0.007). Patients continuing the same chemotherapy in the setting of PET no response had significantly worse median DFS of 14.4 months (95% CI, 8.3 months–infinity) compared to >23.3 months (median not reached) for PET responders (9).
The use of novel therapeutics as well as innovative therapeutic strategies is needed to improve survival in patients with resectable gastric cancer. The use of early PET assessment after induction chemotherapy to modify treatment is one such promising strategy. There have been no studies testing whether PET can be used to improve outcomes by changing the chemotherapy regimen in non-responders, allowing otherwise non-responding patients to achieve a significant response to a salvage therapy prior to surgery.
The use of anti-angiogenic therapy is another promising avenue of research in esophagogastric trials. There has been compelling evidence linking tumor growth and metastases with angiogenesis (10,11). In esophagogastric cancer, increased vascular endothelial growth factor (VEGF) levels have been shown to correlate with advanced tumor stage, the presence of nodal and distant metastasis, and poorer survival (12,13). Based on this pre-clinical rationale, bevacizumab, a chimeric murine monoclonal antibody against human VEGF, has been extensively studied in solid tumors. When the current trial was being developed, our group had performed two phase II trials combining bevacizumab with either irinotecan/cisplatin or modified docetaxel, cisplatin, 5-fluorouracil (5-FU) (mDCF) in patients with advanced gastric and GEJ adenocarcinoma with encouraging rates of response, progression free survival (PFS), and OS with acceptable toxicity (14,15). Bevacizumab was being evaluated in the MAGIC 2 trial of perioperative chemotherapy in locally advanced gastric/GEJ cancer, so the feasibility of adding bevacizumab to neoadjuvant therapy was an additional focus of the current trial.
The objective of this phase II study of patients receiving preoperative chemotherapy and bevacizumab for locally advanced, resectable gastric or GEJ cancer is to examine the effectiveness of PET directed early switching to salvage chemotherapy in the non-responding group, as measured by 2-year DFS.
Methods
Eligibility criteria
Eligible patients had histologically proven locally advanced but resectable gastric or GEJ adenocarcinoma (tumor stage T any N+ M0 or T2b–T4N any, M0) and 18-fluorodeoxyglucose-PET (FDG-PET) avid. Staging laparoscopy was recommended. If a laparoscopy was not performed, an endoscopic ultrasound (EUS) was required to confirm locally advanced, but resectable gastric cancer. An FDG avid tumor was defined as primary tumor with an SUV ≥3.5 or a tumor to liver SUV ratio ≥1.5, and felt to be “probably” or “definitely malignant” [i.e., likelihood score of 3 or 4 on a scale from (0–4) by the reference nuclear medicine physician].
Tumors involving the GEJ had to have the bulk of their disease in the stomach (Siewert II or III). All patients were at least 18 years old and candidates for surgical resection. Patients had a Karnofsky performance score (KPS) ≥70% and renal, liver, and bone marrow function that met the following parameters: serum creatinine ≤2.5 mg/dL, urinalysis demonstrating <2+ proteinuria and/or urine protein/creatinine ratio <1.0; total serum bilirubin ≤2× upper limit of normal (ULN), serum AST, ALT, and alkaline phosphatase ≤2.5× ULN, PT (INR) ≤1.5; absolute neutrophil count ≥1,500/mm3, and platelet count ≥100,000/mm3. Informed consent was obtained. The trial was approved by the institutional review board at Memorial Sloan Kettering Cancer Center. The trial was registered at clinicaltrials.gov (NCT00737438).
Patients were excluded if they had baseline blood pressure >150/100 mmHg despite adequate medical management, significant co-morbidities including cardiac disease, history of abdominal fistula or perforation, serious non-healing wound or ulcer, peripheral vascular disease or stroke, or significant hearing loss.
Pretreatment evaluation and PET scan
Patients underwent a FDG-PET/CT scan before the initiation of preoperative chemotherapy (baseline PET) and after cycle 1 of therapy (follow-up PET). Patients fasted for 4–6 hours before PET imaging to ensure euglycemic glucose metabolism. Blood glucose levels were measured before each PET scan and if glucose ≤200 mg/dL, FDG was injected. Approximately 60 minutes post-injection, PET/CT images were acquired. Low dose CT was used for attenuation correction and anatomic localization. First, PET images of the stomach region (1 or 2 bed positions) were acquired for 6–10 minutes (min) per bed position (body weight ≤70 kg: 6 min; 71–90 kg: 8 min; >90 kg: 10 min per bed position). The purpose of this longer acquisition was to maximize signal to noise ratio in the stomach region. Then a PET scan of the torso (mid skull to upper thigh) was obtained at 3 min per bed position. Images were reconstructed using standard clinical parameters. Baseline and follow-up scans were obtained on the same machines and same time (±10 min) after injection. Images were reviewed independently by a nuclear medicine physician and entered into separate data sheets.
Treatment
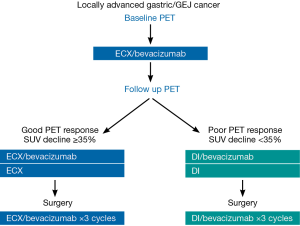
All patients received preoperative therapy with bevacizumab 15 mg/kg, epirubicin 50 mg/m2, cisplatin 60 mg/m2 on day 1 and capecitabine 625 mg/m2 bid orally on days 2–21 (ECX). Each cycle of therapy was 21 days. Near the completion of cycle 1 of therapy (during week 3, days 18–21), patients underwent the follow-up PET scan.
Patients with a metabolic response (i.e., ≥35% reduction in FDG uptake at the primary tumor on the follow-up scan compared to the baseline FDG uptake) continued ECX for 2 additional cycles (cycle 2 and 3). Bevacizumab was administered with cycle 2 but not cycle 3. There was a planned 10-week time interval (70 days) between the last bevacizumab treatment and surgery.
Patients who had a poor PET response (i.e., <35% FDG reduction on the follow-up PET scan compared with baseline) were switched to a salvage regimen of docetaxel 30 mg/m2 and irinotecan 50 mg/mg2 administered on day 1 and day 8 of a 21 day cycle for 2 cycles (DI). Patients received bevacizumab 15 mg/kg for the first cycle of salvage docetaxel/irinotecan only. There was again a planned 10-week time interval between the last bevacizumab treatment and surgery. The treatment schema is shown in Figure 1.
Post-operatively, patients continued to receive the treatment they last received (ECX/bevacizumab or salvage DI/bevacizumab) for three additional cycles.
Patients who were not cisplatin candidates (i.e., creatinine clearance 40–60/cc, older age, marginal performance status, etc.) were allowed to receive oxaliplatin 130 mg/m2 on day 1 every 21 days. Patients who were not able to receive capecitabine (i.e., insurance restriction, unable to swallow) were allowed to receive infusional 5-FU instead of capecitabine after discussion with the principal investigator. 5-FU was administered at 200 mg/m2 day × 21 days (held for 48 hours prior to follow-up PET scan).
Surgery
Following completion of induction chemotherapy, patients underwent repeat staging evaluation. All patients without evidence of metastatic disease then proceeded to surgery. The surgical procedure performed included radical subtotal or total gastrectomy with at least a D1 lymph node dissection. A D2 dissection was recommended.
Pathologic response
Evaluation of pathologic response was performed by a pathologist. Response assessment was based on examination of multiple microscopic sections, and areas of tumor treatment effect scored on a percent histological response scale (0–100%), which is correlated to the Mandard regression score (16). Pathologic response was classified as follows: pathologic complete response (pCR) regression score 1 (100% treatment effect); pathologic partial response (pPR) regression score 2 (90% treatment effect); no response (pNR) regression scores 3 to 5 (0–80% treatment effect).
Statistical consideration
The primary endpoint of the study was to examine the effectiveness of PET directed early switching to salvage chemotherapy as measured by 2-year DFS in the PET non-responder population. We estimated the 2-year DFS, from the time of resection, for the non-responding group to be 30%. We expected a ratio of PET responders versus non-responders of 1:1. Assumptions were based on the Lordick (6) and Ott (5) trials and our previous data (10). With a planned enrollment of 60, we expected 30 patients (50%) to proceed to salvage chemotherapy. Using an exact single stage design, with 30 patients in the non-responding group, we can differentiate 2-year DFS from 30% to 53% with type I error rates of 5% and 81% power. If 14 of the 30 patients in the salvage group are alive and disease free at 2-year, then this combination and treatment algorithm will be considered reasonable. DFS and OS curves were generated using the Kaplan-Meier method and compared by the log-rank test. Fisher’s exact test and Wilcoxon rank sum test were used to evaluate baseline and post-treatment characteristics and toxicity.
Results
Patient characteristics
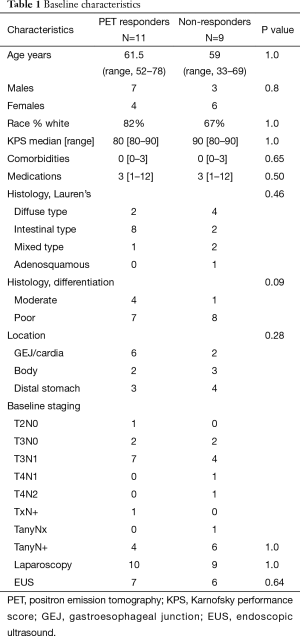
Between August 2008 and November 2011, 23 of the planned 60 patients were enrolled before the study closed for poor accrual. Twenty of the patients are evaluable (1 patient was excluded because the primary tumor was not FDG avid, 1 taken off study for toxicity after the first cycle of chemotherapy and 1 patient withdrew consent prior to initiating treatment). Of the 20 evaluable patients, there were 14 males (64%), 8 females (39%) with a median age 62 (range, 33–78) years old, and median KPS of 90. Ten (50%) had intestinal histology, 6 (30%) diffuse type, and 4 (20%) mixed type. Eight (40%) of primary tumors were located in the GEJ with 12 (60%) in the body or distal stomach. Patients in the study underwent extensive staging: 13/20 (65%) received endoscopic ultrasound and 19/20 (95%) underwent staging laparoscopy with baseline T and N stage shown in Table 1.
Full table
Metabolic response
PET metabolic response was assessed after the first cycle of ECX/bevacizumab: 11 (55%) patients were PET responders and 9 (45%) were PET non-responders. No significant differences were noted in the baseline characteristics of responders versus non-responders with regard to age, sex, race, performance status, staging evaluation, location of primary tumor (Table 1). The two groups were equivalent in regards to stage II disease (T2–3N0) and node positive patients (P=1.0). There was no difference in the tumor location (P=0.28) or histology (P=0.46) between the groups; however there was a trend towards more poorly differentiated tumors in the non-responders (P=0.09).
The tumors of PET responder patients had a significantly higher median baseline SUV of 11.8 (interquartile range, 10.4) versus 5.5 (interquartile range, 6) for PET non-responders. All of the PET responder patients had a SUV decline ≥35% (range, 35–100% decline from baseline). In the non-responders, the SUV decline ranged from 8–30% and 3/9 (33%) patients had an increase in SUV compared to baseline.
Surgery
Of the 20 patients, 19 (95%) underwent surgical resection: 14 had gastrectomies and 5 underwent Ivor-Lewis esophagectomies. One patient had progressive disease prior to surgery. Of the 19 resected patients, 17 (89%) had R0 resections, 2 (11%) patients had R1 resections (Table 2). R0 resections could be performed in 10 of 11 PET responders (91%) versus 7 of 9 non-responder patients (88%). D2 lymph node dissections were performed in 18/19 (95%) of resected patients with a median number of 23 lymph nodes sampled (range, 15–43), no difference seen in the two groups. One patient in the non-responder group died from post-operative complications.

Full table
Pathologic response
In the PET responder group, 1 patient achieved a pCR and 3 patients had a partial pathologic response. The SUV decrease was not statistically different in patients achieving complete pathologic response compared to those with partial response (P=0.2). No pathologic response was noted in the non-responder group. Clinical downstaging was seen in 5 (45%) patients in the PET responder group but in only 1 (11%) non-responder. Pathologic and clinical downstaging responses are shown in Table 2.
Therapy completion/toxicity
In the PET responders, 8 (73%) patients received any post-operative chemotherapy and, of those, 6 (55%) completed all planned treatment. Of the non-responder patients, 5/9 (55%) completed all post-operative chemotherapy. Reasons for not completing post-operative treatment were: R1 resection, post-operative death, prolonged post-operative recovery, and chemotherapy toxicity.
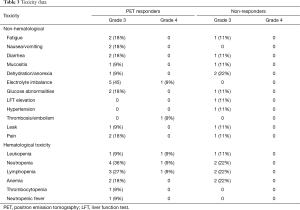
Toxicity was assessed for the 20 evaluable patients (Table 3). Significant chemotherapy related toxicity included grade 3 fatigue (15%), diarrhea (15%), dehydration/anorexia (15%), electrolyte imbalance (25%), neutropenia (30%), lymphopenia (25%), and anemia (20%). One patient developed febrile neutropenia (5%). Grade 4 thromboembolism was seen in 1 patient (5%) and 2 patients developed a gastrointestinal leak (10%). Bevacizumab-related toxicity included grade 3 hypertension in 1 patient (5%). There was no significant difference in all grade 3 toxicity (P=0.07) or grade 4 toxicity (P=0.09) between the responder and non-responder groups.
Full table
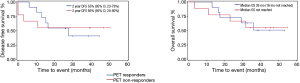
The median follow up was 38.2 months (range, 26 to 50.7 months). Analysis of all 20 evaluable patients showed a median DFS of 27.9 months [95% confidence interval (CI), 10.3 months–not estimable]. The median OS was 36 months (95% CI, 17.2 months–not reached). In the PET responder group, the 2-year DFS was 55% (95% CI, 23–78%) compared with 56% in the non-responder group (95% CI, 20–80%) as shown in Figure 2. There was no significant difference in DFS between the two groups (P=0.93).
Discussion
Current adjuvant therapy strategies in gastric cancer, including perioperative chemotherapy and post-operative chemotherapy with or without radiotherapy, achieved only a modest improvement in survival in gastric cancer. The majority of patients with this disease treated in the West still die of recurrent disease, and the need to identify new agents and treatment strategies is a priority.
In our prospective pilot trial, neoadjuvant chemotherapy was switched to a non-cross resistant regimen in metabolic non-responding patients in an attempt to improve the 2-year DFS rate in patients with gastric and GEJ cancer. Response to preoperative chemotherapy for patients with localized gastric and GEJ cancer has been recognized as a strong prognostic factor. Several studies have demonstrated the ability of FDG-PET to assess response to preoperative treatment in esophagogastric cancer and indicate that early PET scan metabolic response is associated with improved outcomes (8,17). Ott et al. found that metabolic responders had a pathologic response rate of 44% and 3-year survival of 70% compared a response rate of 5% and 3-year survival of 35% in non-responders who continued the same chemotherapy (4). In the MUNICON trial, patients who did not achieve an early PET response discontinued chemotherapy and underwent surgical resection. Despite this change, non-responders had worse median relapse free survival (14.1 vs. 29.7 months, P=0.002) and OS (25.8 months versus median not reached, P=0.015) compared to PET responders. We included bevacizumab based on our promising phase II data in advanced disease, and the use of this agent in an ongoing neoadjuvant randomized trial (18).
In comparison to prior studies showing a poor outcome in PET non-responders continuing neoadjuvant therapy, our current pilot study results suggest an improvement in the 2-year DFS in the PET non-responding group when these patients were changed to a potentially non cross-resistant chemotherapy. The 2-year DFS was 55% (95% CI, 30–85%) in the PET responders compared with 56% in the non-responders (95% CI, 20–80%). Median OS was 36 months in the PET responders and not yet reached in non-responders, with no significant difference in median OS (log rank test P=0.95).
The contribution of bevacizumab in this trial is difficult to interpret. There was no excessive toxicity seen with the addition of bevacizumab to the neoadjuvant regimen. The phase III global study of bevacizumab on the AVAGAST trial failed to show a survival benefit when bevacizumab is added to first line chemotherapy, despite improvements in response rates and PFS (19). The MAGIC 2 trial combining bevacizumab with neoadjuvant chemotherapy in esophagogastric cancer is ongoing, with preliminary data indicating no adverse toxicity impact on therapy or surgical outcomes (20).
We clearly acknowledge the limitations of a study with only 20 patients. However, we do feel that this study provides exploratory data on the use of PET scan to help identify potential early treatment failures and guide treatment in gastric cancer. The results suggest that PET non-responding patients, who have had historically worse outcomes, can change regimens and may be able to achieve survival comparable to those with an initial PET response. The PET non-responder group had a greater percentage of patients with diffuse type and poorly differentiated cases, and overall had lower baseline SUV levels. PET scan may be less sensitive in gastric adenocarcinoma with these features. Toxicity was similar between the two groups, suggesting change in chemotherapy is feasible.
Conclusions
In our study, patients with suboptimal PET response to induction chemotherapy were changed to an alternative regimen prior to surgical resection. The results from this trial are hypothesis-generating and clearly need more evaluation to see if this strategy changes outcomes. These preliminary results have helped engender the basis for an alliance/CALGB multicenter randomized trial using PET scan response to direct therapy preoperative in locally advanced gastric cancer (NCT02485834) (21).
Acknowledgements
This study was sponsored by Genentech.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center and written informed consent was obtained from all patients.
References
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2006;24:4692-8. [Crossref] [PubMed]
- Wu AJ, Goodman KA. Positron emission tomography imaging for gastroesophageal junction tumors. Semin Radiat Oncol 2013;23:10-5. [Crossref] [PubMed]
- Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003;21:4604-10. [Crossref] [PubMed]
- Shah MA, Yeung H, Coit D, et al. A phase II study of preoperative chemotherapy with irinotecan (CPT) and cisplatin (CIS) for gastric cancer (NCI 5917): FDG-PET/CT predicts patient outcome. J Clin Oncol 2007;25:abstr 4502.
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27-31. [Crossref] [PubMed]
- Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med 1991;324:1-8. [Crossref] [PubMed]
- Kleespies A, Guba M, Jauch KW, et al. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol 2004;87:95-104. [Crossref] [PubMed]
- Saad RS, El-Gohary Y, Memari E, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol 2005;36:955-61. [Crossref] [PubMed]
- Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006;24:5201-6. [Crossref] [PubMed]
- Shah MA, Jhawer M, Ilson DH, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 2011;29:868-74. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- zum Büschenfelde CM, Herrmann K, Schuster T, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 2011;52:1189-96. [Crossref] [PubMed]
- Cunningham D. Chemotherapy with or without bevacizumab or lapatinib to treat operable oesophagogastric cancer. Clinical trials.gov identifier: NCT00450203. Available online: https://clinicaltrials.gov/ct2/show/NCT00450203
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Okines AF, Langley RE, Thompson LC, et al. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: a safety report. Ann Oncol 2013;24:702-9. [Crossref] [PubMed]
- Alliance for Clinical Trials in Oncology FDG-PET Directed Treatment in Improving Response in Patients With Locally Advanced Stomach or Gastroesophageal Junction Cancer. NLM Identifier: NCT02485834. Bethesda (MD): National Library of Medicine (US). 2000 [Cited 2016 Feb 27]. Available online: https://clinicaltrials.gov/ct2/show/NCT02485834