
In vitro study of miRNA-369-3p targeting TCF4 regulating the malignant biological behavior of colon cancer cells
Highlight box
Key findings
• miR-369-3p inhibits the proliferation, invasion and oxidative stress of colorectal carcinoma (CRC) cells by targeting transcription factor 4.
What is known and what is new?
• miR-369-3p plays a tumor suppressor role in several cancers.
• miR-369-3p was downregulated in CRC tissue and cell lines and miR-369-3p may be a target for the diagnosis and treatment of colon cancer.
What is the implication, and what should change now?
• This might provide light in the miRNA field and targets for the treatment of CRC.
Introduction
Colorectal carcinoma (CRC) is a common gastrointestinal malignant tumor and the fourth leading cause of cancer-related deaths (1). In the past 10 years, despite the great progress made in diagnosis and treatment, the 5-year survival rate of colon cancer patients is still only 50% to 65% (2). Currently, chemotherapy is still one of the key methods for the treatment of colorectal cancer (3). In recent years, with the emergence of new chemotherapy drugs, targeted drugs and the formulation of chemotherapy regimens, the treatment of colorectal cancer has made great progress, but there are still a large number of patients with poor efficacy and even recurrence or metastasis after treatment (4). Researchers have identified many oncogenes and oncogenes by exploring the biological mechanisms of colon cancer. Studies have shown that alterations in the expression of certain oncogenes or oncogenes suppressors may also contribute to the development of colon cancer. A better understanding of the mechanisms of colon cancer onset, progression, migration and recurrence, and the exploration of new molecular markers for colon cancer will contribute to the early diagnosis and treatment of colon cancer.
MicroRNAs (miRNAs) are a class of endogenous small non-coding RNAs that can lead to protein degradation by directly binding to target mRNAs (5). A study has shown that miRNAs play an important role in promoting or inhibiting tumor cell proliferation, invasion and drug resistance by regulating oncogenes or tumor suppressor genes (6). A study has shown that miR-369-3p plays a tumor suppressor role in several cancers. For example, miR-369-3p inhibits the viability and motility of hepatocellular carcinoma (HCC) cells by binding to paired Box 6 (7). miR-369-3p overexpression inhibits cell proliferation and migration of endometrioid adenocarcinoma (8). Another study showed that miR-369-3p inhibits cell proliferation and induces apoptosis in thyroid cancer (9). Dong et al. (10) reported that miR-369-3p played an anticancer role in gastric cancer cells by regulating jun proto-oncogene and v-akt mouse thymoma virus oncogene homolog 1. Hao et al. (11) found that inhibition of miR-369-3p sensitizes cisplatin (DDP) to the inhibitory effect of lung cancer cell invasion in the presence of DDP treatment. Interestingly, Ogawa et al. found that miRNA-369-3p induced epigenetic reprogramming and inhibited the malignant phenotype of human colon cancer cells (12). However, the regulatory mechanism of miR-369-3p in human CRC has not yet been elucidated. Therefore, this study focuses on the effects of miR-369-3p on the proliferation, invasion and oxidative stress of CRC cells to confirm that miR-369-3p may be a target for the diagnosis and treatment of colon cancer. We present this article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-628/rc).
Methods
CRC tissue samples
Twenty pairs of CRC tissues and normal tissues were collected from patients diagnosed in our hospital from January 2021 to September 2022. All patients who provided samples were confirmed by pathological examination and did not receive radiotherapy and chemotherapy before surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from each patient, and all the experiments were approved by the Ethics Committee of Wuwei Hospital of Traditional Chinese Medicine (No. 2021011). All tissue samples were maintained in liquid nitrogen.
Cell culture
Human CRC cell lines SW480 (cat. no. BFN60800644), HT-29 (cat. no. BFN60800646), HCT-116 (cat. no. BFN60800649) and SW620 (cat. no. BFN60800643) (Bluefbio, China) and human normal colonic epithelial cell CCD-841CoN (cat. BFN60804016) (Bluefbio, China) were cultured in DMEM (Gibco, USA) containing 10% FBS (Gibco, USA), 100 U/mL lyophilic streptomycin and streptomycin (Gibco, USA) in an incubator with 5% CO2, and saturated humidity at 37 ℃.
Cell transfection
The miR-369-3p mimics, negative control miRNA mimics (mimic-NC), TCF4-overexpressing vectors (pcDNA-TCF4) and corresponding empty vectors (pcDNA3.1) were all purchased from RiboBio Co., Ltd. (RioBio, China). The cells were randomly divided into the control, mimic, pcDNA-TCF4 and mimic + pcDNA-TCF4 groups. The cells were incubated into 6-well plates and transfected at a density of 50–70%. Lipofectamine 2000 (Invitrogen, USA) was used to transiently transfect the above plasmids in groups, and the transfection procedure was in accordance with the manufacturer’s instructions. Approximately 48 h after transfection, cells were harvested and used for subsequent assays.
RNA extraction and real time quantitative PCR (RT-qPCR)
RNA was extracted from cells using TRIzol (Invitrogen, USA). RT-qPCR was performed on a 7900HT RT-PCR system (Applied Biosystems, USA). Complementary DNA (cDNA) was synthesized using the TaqMan MicroRNA RT Reagent Kit (Takara, Japan). The expression of miR-369-3p and transcription factor 4 (TCF4) was analyzed with SYBR-Green II (Takara, Japan). U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as endogenous controls for miR-369-3p and TCF4 mRNA expression, respectively. The primer sequences were as follows: U6 forward, 5'‑TGCGGGTGCTCGCTTCGCAGC‑3', and reverse, 5'‑CCAGTGCAGGGTCCGAGGT‑3'; GAPDH forward, 5'‑CGGAGTCAACGGATTTGGTCGTAT‑3' and reverse, 5'‑AGCCTTCTCCATGGTGGT GAAGAC‑3'; miR-369-3p forward, 5'-TGACCTAAGGGACTCCCACAA-3', reverse, 5'-TAGCAATATTGCACAGAAGGC-3'; TCF4 forward, 5'-CCTGGCTATGCAGGAATGTT-3', reverse, 5'-CAGGAGGCGTACAGGAAGAG-3'. Three independent experiments were performed in triplicate.
Western blotting
Total proteins from cells were isolated with radioimmunoprecipitation assay (RIPA) buffer (Sobarbio, China). Protein concentrations were measured using a BCA Protein Assay Kit (Beyotime, China). Equal amounts of protein (30 µg/lane) were fractionated using a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinyl fluoride (PVDF) membranes (Sobarbio, China). Then, the membranes were completely blocked with 5% nonfat milk in Tris-buffered saline with Tween 20 (TBST) for 2 h at room temperature. Membranes were incubated at 4 ℃ overnight with the following primary antibodies: TCF4 (1/10,000; cat. no. ab217668; Abcam, UK), Ki67 (1/5,000; cat. no. ab92742; Abcam, UK), PCNA (1/1,000; cat. no. ab92552; Abcam, UK), vascular endothelial growth factor (VEGF) (1 µg/mL; cat. no. ab46154; Abcam, UK), E-cadherin (1/500; cat. no. ab40772; Abcam, UK) and GAPDH (1/500–1/10,000; cat. no. ab8245; Abcam, UK). Subsequently, the membranes were further developed with goat anti-rabbit horseradish peroxidase (HRP) conjugated secondary antibody for 1 h at room temperature. Protein expression levels were visualized with enhanced chemiluminescence (ECL) detection solution. Three independent experiments were performed.
Labeled immunofluorescence assay using BrdU antibody
The cells were inoculated into 96-well plates and cultured to a cell density of 50–70%. After adding 20 µL BrdU Solution (MultiSciences, China) for 4 h, the cells were fixed with 4% paraformaldehyde for 20 min, permeated with 0.2% Triton X-100 for 10 min, and incubated with BrdU antibody (ab6326, Abam, USA) overnight at 4 ℃. Finally, the cells were covered with Prolong Gold Antifade Reagent with DAPI (#9071, Cell Signaling Technology, USA) and photographed with an IX73 fluorescence microscope (Olympus, Japan). Three independent experiments were performed in triplicate.
Flow cytometry analysis
The transfected SW480 cells were harvested and re-suspended to detect the ability of apoptosis. Subsequently, cells were stained with 5 µL Annexin V-FITC (Sigma-Aldrich, USA) and 10 µL propidium iodide (PI) (Sigma, USA) in the dark for 15 minutes. Flow cytometry (Beckman, USA) was used to evaluate the results.
Transwell assay
The dissolved Matrigel (Corning, USA) was mixed with serum-free medium (1:8 dilution) and evenly spread to the bottom of the Transwell chamber (upper chamber surface). The Matrigel was solidified in a 37 ℃ incubator for approximately 2 h. 1×105 cells were cultured in the serum-free medium, and the cells were uniformly cultivated on the upper of the Transwell chamber. Then, the Transwell chamber was placed into the pore plate with 10% fetal bovine serum (FBS) medium for culture. After 24 h of cell culture, the Transwell chamber was removed, and the cells in the chamber and the residual Matrigel were wiped with cotton swabs and washed with PBS 3 times. The cells that passed through the bottom of the chamber were fixed with paraformaldehyde, stained with crystal violet (Solarbio, China, cat. no. G1063), counted under the microscope and analyzed for drawing. Images of three random fields were collected to calculate the number of invading cells.
Detection of superoxide dismutase (SOD) and LDH activities and malondialdehyde (MDA) content
The SOD activity detection kit, MDA content detection kit, and lactate dehydrogenase (LDH) activity detection kit were all purchased from Solarbio Co., Ltd. (Solarbio, China). The cells were treated and tested according to the kit manufacturer’s instructions. The absorbance was measured at 450 nm. Three independent experiments were performed in triplicate.
Statistical analysis
All data were analyzed by GraphPad Prism 8.0 software (GraphPad Software, Inc., La Jolla, CA, USA), and the data are expressed as the mean ± standard deviation. All experiments were repeated three times. The Kolmogorov-Smirnov and Shapiro-Wilk method normality tests were used to determine whether the data conformed to a normal distribution. If the data were normally distributed, an analysis of variance (ANOVA) was performed, followed by a post hoc Tukey test for multiple comparisons. Bilateral P<0.05 was considered statistically significant.
Results
The expression of miR-369-3p was downregulated in CRC cell lines
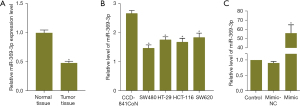
We examined the expression of miR-369-3p in CRC tissues and normal tissues, normal osteoblasts (CCD-841CoN) and in a variety of CRC cell lines (SW480, HT-29, HCT-116 and SW620). The results showed that the levels of miR-369-3p were downregulated in CRC tissues and all CRC cell lines compared with normal tissues and CCD-841CoN cells (Figure 1A,1B). The lowest expression of miR-369-3p was detected in SW480 cells. Then, SW480 cells were selected for subsequent study. To reveal the biological function of miR-369-3p in CRC cells, gain-of-function assays were carried out by transfecting SW480 cells with miR-369-3p mimic. According to the qRT-PCR assay, the miR-369-3p mimic obviously increased the level of miR-369-3p compared with normal cells (Figure 1C). This indicated that the transfection was successful.
miR-369-3p targets the TCF4 gene
A putative target of miR-369-3p was predicted to be TCF4 by TargetScan. The binding sites between miR-369-3p were the 58–64 and 3769–3776 positions of TCF4 mRNA 3'UTR (Figure 2A). To confirm that TCF4 was a direct target gene of miR-369-3p, SW480 cells were cotransfected with wild type (wt) or mutation (mut) TCF4-3'UTR vectors and miR-369-3p mimics. The dual luciferase assay data demonstrated that the overexpression of miR-369-3p significantly reduced the luciferase activity of TCF4 wt but did not change the luciferase activity of TCF4 mut (Figure 2B,2C). RT-qPCR results indicated that upregulation of miR-369-3p reduced the mRNA levels of TCF4 in SW480 cells (Figure 2D). To determine that the effect of miR-369-3p on CRC cells was mediated through TCF4, we transfected SW480 cells with pcDNA-TCF4 vectors for rescue assays. The mRNA and protein levels of TCF4 in SW480 cells were upregulated after overexpression of TCF4 (Figure 2E,2F).

miR-369-3p targeting TCF4 inhibits CRC cell proliferation
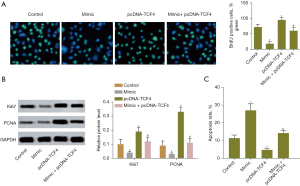
Next, the effect of miR-369-3p and/or TCF4 overexpression on cell proliferation was assessed by BrdU assay. Overexpression of miR-369-3p decreased the number of BrdU-positive cells, while TCF4 upregulation increased the number of BrdU-positive cells compared with normal cells. The BrdU-positive cells were reduced in cells cotransfected with miR-369-3p mimic and pcDNA-TCF4 compared with cells separately transfected with pcDNA-TCF4 (Figure 3A). Moreover, the expression of Ki67 and PCNA associated with proliferation was determined by western blotting. The results indicated that high levels of miR-369-3p downregulated the protein expression of Ki67 and PCNA. TCF4 overexpression led to the upregulation of the levels of Ki67 and PCNA. miR-369-3p counteracted the enhanced expression of Ki67 and PCNA by TCF4 (Figure 3B). Overexpression of miR-369-3p increased the apoptosis rate of SW480 cell, while TCF4 upregulation decreased the apoptosis rate compared with control group. The apoptosis rate were induced in cells cotransfected with miR-369-3p mimic and pcDNA-TCF4 compared with cells separately transfected with pcDNA-TCF4 (Figure 3C). The above experiments demonstrated that miR-369-3p could target TCF4 to inhibit CRC cell proliferation and promote apoptosis.
miR-369-3p targeting TCF4 inhibits CRC cell invasion
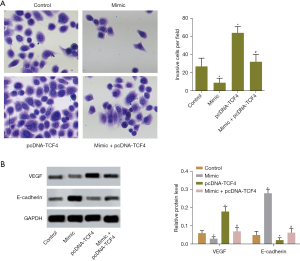
To investigate the effect of miR-369-3p on the invasive ability of CRC cells, a Transwell assay was performed. According to the results, upregulation of miR-369-3p reduced the number of invasive cells and overexpression of TCF4 increased the number of invasive cells. miR-369-3p neutralized the effect of overexpressed TCF4 (Figure 4A). Furthermore, the results of western blotting suggested that the levels of invasion-related proteins such as VEGF and E-cadherin in cells transfected with miR-369-3p mimic were lower than those in normal cells. The expression of VEGF and E-cadherin protein was upregulated in cells transfected with pcDNA-TCF4 (Figure 4B). MiR-369-3p could reverse the strengthening of TCF4 on cell invasion. These data indicated that miR-369-3p could inhibit CRC cell invasion by binding to TCF4 mRNA and downregulating mRNA and protein expression.
miR-369-3p targeting of TCF4 attenuates the oxidative stress of CRC cells
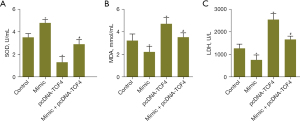
In addition, we also measured the levels of SOD, MDA and LDH related to the oxidative stress system. Overexpression of miR-369-3p increased the activity of SOD while reducing the content of MDA and activity of LDH. Upregulated TCF4 decreased the activity of SOD while increasing the level of MDA and activity of LDH. MiR-369-3p counteracted the influence of TCF4 on SOD, MDA and LDH (Figure 5A-5C). These results demonstrated that miR-369-3p binding to TCF4 could alleviate the oxidative stress of CRC cells.
Discussion
A study has confirmed that the increase in morbidity and mortality of patients with colon cancer is related to changes in the interaction of lifestyle, diet, age, obesity, genetic and environmental factors (exposure to carcinogens and smoking, etc.) (13). Due to the high incidence of colon cancer and poor prognosis, colon cancer is a major public health problem and clinical challenge worldwide (14). According to reports, many signal transduction pathways and molecules are related to the occurrence and development of colon cancer (15,16). However, the molecular mechanism of colon carcinogenesis remains largely unknown. Therefore, it is necessary to study the therapeutic targets of colon cancer more deeply.
A large number of studies have shown that miR-369-3p plays a tumor suppressor role in a variety of tumors (11,17,18). Compared with the normal control group, the expression of miR-369-3p was downregulated in HCC tissues and cell lines. Overexpression of miR-369-3p inhibited the proliferation, migration and invasion of HCC cells (19). miR-369-3p inhibits the proliferation and promotes the apoptosis of papillary thyroid carcinoma (PTC) cells by downregulating the expression of Tetraspanin 13 (TSPAN 13) (20). miR-369-3p is downregulated in HCC. Overexpressed miR-369-3p inhibited cell viability and motility in HCC by targeting PAX6 (7). In this research, we found that miR-369-3p was downregulated in CRC tissue and cell lines.
Cumulative studies have shown that multiple miRNAs can regulate CRC cells by targeting target genes. It was reported that overexpression of miRNA-143 inhibited colon cancer cell proliferation by suppressing glucose uptake (21). The data from Zeng et al. indicated that miR-378 inhibited the proliferation, migration and invasion of colon cancer cells by targeting SDAD1 (22). Another study showed that miR-185 inhibited colon cancer cell proliferation and invasion by targeting Wnt1 (23). Li et al. (24) also demonstrated that miR-195 suppressed colon cancer proliferation and metastasis by targeting WNT3A. Similarly, the target gene of miR-369-3p in CRC cells was predicted and confirmed to be TCF4 by bioinformatics software and experiments in the present study.
TCF4 is the key Wnt signaling molecule that can interact with β-catenin (25). In some cell types, TCF4 gradually becomes an important regulator of epithelial-mesenchymal transformation (EMT) and plays an important role in embryonic development, tissue repair and cancer metastasis. Various convergent lines of evidence support the role of TCF4 as an EMT regulator in different cells (26). Liu et al. demonstrated that TCF4 might play a tumorigenic role in epithelial ovarian cancer and is a useful independent prognostic indicator (27). A study from Lee et al. indicated that TCF4 induced enzalutamide resistance via neuroendocrine differentiation in prostate cancer (28). Another study has shown that miRNA-495 targeting TCF4 inhibits the activation of the Wnt/β-Catenin pathway and hinders the progression of non-small cell lung cancer (29). In our study, TCF4 played a cancer-promoting role in CRC cells by inducing proliferation and invasion.
In addition, we also studied the effect of the miR-369-3p/TCF4 axis on oxidative stress in colorectal cancer cells. The normal function of mitochondria plays a key role in cell proliferation and apoptosis, and reactive oxygen species (ROS) are normal byproducts of mitochondrial metabolism and protein folding (30). Excessive ROS are eliminated by the antioxidant system in normal cells, while cancer cells seem to benefit from increased levels of ROS. Higher ROS in cancer cells may activate proliferation and survival pathways (31). Similar to normal cells, antioxidant proteins such as SOD, catalase, and nuclear factor erythroid 2-related factor 2 (Nrf2) can inhibit excessive ROS levels. Cancer cells also show increased levels of antioxidant proteins to eliminate ROS (32). Our research indicated that overexpression of TCF4 accelerated oxidative stress, while miR-369-3p reversed the effect of TCF4 on oxidative stress in CRC cells by binding to TCF4 mRNA. However, this study has not been confirmed by in vivo experiments, which was a limitation of this study. Therefore, the next study should be conducted in vivo to verify the inhibitory effect of miR-369-3p on CRC.
Conclusions
In conclusion, miR-369-3p could inhibit human CRC cell proliferation and invasion and attenuate oxidative stress by targeting TCF4. This might provide light in the miRNA field and targets for the treatment of CRC.
Acknowledgments
Funding: This work was supported by Municipal Science and Technology Program Project of Wiwu City (No. WW180229).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-628/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-628/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-628/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-628/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from each patient, and all the experiments were approved by the Ethics Committee of Wuwei Hospital of Traditional Chinese Medicine (No. 2021011).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo Y, Zhou Y, Gu X, et al. Tripartite motif 52 (TRIM52) promotes proliferation, migration, and regulation of colon cancer cells associated with the NF-κB signaling pathway. J Gastrointest Oncol 2022;13:1097-111. [Crossref] [PubMed]
- Jiang J, Zhu F, Zhang H, et al. Luteolin suppresses the growth of colon cancer cells by inhibiting the IL-6/STAT3 signaling pathway. J Gastrointest Oncol 2022;13:1722-32. [Crossref] [PubMed]
- Wu C. Systemic Therapy for Colon Cancer. Surg Oncol Clin N Am 2018;27:235-42. [Crossref] [PubMed]
- Wang Z, Hu T, Jin C, et al. The anti-tumor effect of miR-539-3p on colon cancer via regulating cell viability, motility, and nude mouse tumorigenicity with CDK14 inhibition. J Gastrointest Oncol 2020;11:899-910. [Crossref] [PubMed]
- Zhai X, Wu Y, Zhang D, et al. MiR-6838-5p facilitates the proliferation and invasion of renal cell carcinoma cells through inhibiting the DMTF1/ARF-p53 axis. J Bioenerg Biomembr 2021;53:191-202. [Crossref] [PubMed]
- Ye K, Xu C, Hui T. MiR-34b inhibits the proliferation and promotes apoptosis in colon cancer cells by targeting Wnt/β-catenin signaling pathway. Biosci Rep 2019;39:BSR20191799. [Crossref] [PubMed]
- Xu Q, Liu K. MiR-369-3p inhibits tumorigenesis of hepatocellular carcinoma by binding to PAX6. J Biol Regul Homeost Agents 2020;34:917-26. [PubMed]
- Liu P, Ma C, Wu Q, et al. MiR-369-3p participates in endometrioid adenocarcinoma via the regulation of autophagy. Cancer Cell Int 2019;19:178. [Crossref] [PubMed]
- Yi S, Liu L, Chen Z. lncRNA EGFEM1P promotes thyroid cancer progression by acting as an miR-369-3p sponge and upregulating TCF4. Oncol Lett 2022;24:456. [Crossref] [PubMed]
- Dong L, Zhang Z, Xu J, et al. Consistency analysis of microRNA-arm expression reveals microRNA-369-5p/3p as tumor suppressors in gastric cancer. Mol Oncol 2019;13:1605-20. [Crossref] [PubMed]
- Hao GJ, Ding YH, Wen H, et al. Attenuation of deregulated miR-369-3p expression sensitizes non-small cell lung cancer cells to cisplatin via modulation of the nucleotide sugar transporter SLC35F5. Biochem Biophys Res Commun 2017;488:501-8. [Crossref] [PubMed]
- Ogawa H, Wu X, Kawamoto K, et al. MicroRNAs Induce Epigenetic Reprogramming and Suppress Malignant Phenotypes of Human Colon Cancer Cells. PLoS One 2015;10:e0127119. [Crossref] [PubMed]
- Zhang X, Wang H, Yu M, et al. Inhibition of autophagy by 3-methyladenine promotes migration and invasion of colon cancer cells through epithelial mesenchymal transformation. Transl Cancer Res 2022;11:2834-42. [Crossref] [PubMed]
- Liska D, Stocchi L, Karagkounis G, et al. Incidence, Patterns, and Predictors of Locoregional Recurrence in Colon Cancer. Ann Surg Oncol 2017;24:1093-9. [Crossref] [PubMed]
- Liu B, Xu T, Xu X, et al. Biglycan promotes the chemotherapy resistance of colon cancer by activating NF-κB signal transduction. Mol Cell Biochem 2018;449:285-94. [Crossref] [PubMed]
- Zhou P, Wang C, Hu Z, et al. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer 2017;17:813. [Crossref] [PubMed]
- Zou Y, Yao S, Chen X, et al. LncRNA OIP5-AS1 regulates radioresistance by targeting DYRK1A through miR-369-3p in colorectal cancer cells. Eur J Cell Biol 2018;97:369-78. [Crossref] [PubMed]
- Wo Q, Zhang D, Hu L, et al. Long noncoding RNA SOX2-OT facilitates prostate cancer cell proliferation and migration via miR-369-3p/CFL2 axis. Biochem Biophys Res Commun 2019;520:586-93. [Crossref] [PubMed]
- Chen C, Zong Y, Tang J, et al. miR-369-3p serves as prognostic factor and regulates cancer progression of hepatocellular carcinoma. Per Med 2021;18:375-88. [Crossref] [PubMed]
- Li P, Dong M, Wang Z. Downregulation of TSPAN13 by miR-369-3p inhibits cell proliferation in papillary thyroid cancer (PTC). Bosn J Basic Med Sci 2019;19:146-54. [PubMed]
- Zhao J, Chen Y, Liu F, et al. Overexpression of miRNA-143 Inhibits Colon Cancer Cell Proliferation by Inhibiting Glucose Uptake. Arch Med Res 2018;49:497-503. [Crossref] [PubMed]
- Zeng M, Zhu L, Li L, et al. miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell Mol Biol Lett 2017;22:12. [Crossref] [PubMed]
- Zhang W, Sun Z, Su L, et al. miRNA-185 serves as a prognostic factor and suppresses migration and invasion through Wnt1 in colon cancer. Eur J Pharmacol 2018;825:75-84. [Crossref] [PubMed]
- Li B, Wang S, Wang S. MiR-195 suppresses colon cancer proliferation and metastasis by targeting WNT3A. Mol Genet Genomics 2018;293:1245-53. [Crossref] [PubMed]
- Aono S, Hatanaka A, Hatanaka A, et al. β-Catenin/TCF4 Complex-Mediated Induction of the NRF3 (NFE2L3) Gene in Cancer Cells. Int J Mol Sci 2019;20:3344. [Crossref] [PubMed]
- Fang XQ, Lee M, Lim WJ, et al. PGC1α Cooperates with FOXA1 to Regulate Epithelial Mesenchymal Transition through the TCF4-TWIST1. Int J Mol Sci 2022;23:8247. [Crossref] [PubMed]
- Liu L, Zeng Z, Yi J, et al. Expression and clinical significance of transcription factor 4 (TCF4) in epithelial ovarian cancer. Cancer Biomark 2019;24:213-21. [Crossref] [PubMed]
- Lee GT, Rosenfeld JA, Kim WT, et al. TCF4 induces enzalutamide resistance via neuroendocrine differentiation in prostate cancer. PLoS One 2019;14:e0213488. [Crossref] [PubMed]
- Zheng HE, Wang G, Song J, et al. MicroRNA-495 inhibits the progression of non-small-cell lung cancer by targeting TCF4 and inactivating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci 2018;22:7750-9. [PubMed]
- Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des 2018;24:4771-8. [Crossref] [PubMed]
- Chen X, Zhao Y, Luo W, et al. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020;10:10290-308. [Crossref] [PubMed]
- Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell 2020;38:167-97. [Crossref] [PubMed]


