
Feeding via duodenostomy can reduce intestinal obstruction after radical resection of esophageal cancer better than jejunostomy
Highlight box
Key findings
• Compared to feeding via jejunostomy (FJ), feeding via duodenostomy (FD) reduces postoperative complications (e.g., intestinal obstruction) without prolonging surgical time, affecting postoperative nutritional status, or prolonging length of stay.
What is known and what is new?
• Enteral nutrition is superior to parenteral nutrition in improving the nutritional status of esophageal cancer patients and accelerating postoperative recovery. Therefore, FJ is currently placed during esophagectomy to maintain the postoperative nutrition supply. However, FJ have some serve complications.
• FD can reduce postoperative complication, The method of it is safe, effective and acceptable.
What is the implication, and what should change now?
• FD can reduce intestinal obstruction and it is worthwhile to use in clinic practice.
Introduction
Esophageal cancer (EC) is the seventh most common malignant tumor worldwide and ranks as the sixth leading cause of cancer death (1). China is one of the countries with the highest incidence of EC, and more than half of all EC cases and deaths occur in China. Surgery-based multidisciplinary treatment is the preferred therapeutic strategy for EC, with the 5-year survival rate reaching as high as 55.6% (2). Currently, the recommended surgical procedure for EC is three-field radical esophagectomy.
Most patients with EC present with symptoms of esophageal obstruction, and all experience some degree of malnutrition. Moreover, three-field radical esophagectomy for EC is highly traumatic and has a high incidence of perioperative complications. Thus, nutritional support is crucial in patients treated with this modality to enable smooth postoperative recovery and timely therapy during the follow-up period (3). Based on our experience, we developed a sophisticated nutrition support model and have applied it in our department in recent years. According to most literature and our previous observations, enteral nutrition (EN) is superior to parenteral nutrition (PN) in improving the nutritional status of EC patients and accelerating postoperative recovery (4). Therefore, feeding via jejunostomy (FJ) is currently the preferred mode of nutritional support in our department.
Compared to nasogastric tubes, jejunostomy tubes have better tolerability and a lower dislodgement rate (5). Therefore, they are more suitable for patients who need long-term nutritional support (e.g., patients with anastomotic leak, swallowing disorders, a need for additional adjuvant therapy after surgery) (6). However, jejunostomy tubes also have some disadvantages. Although jejunostomy improves the postoperative nutritional status to a certain extent, there are inevitable complications, such as intestinal obstruction, intestinal torsion, pneumatosis, infection, water-electrolyte disorders, and even intestinal fistula, with intestinal obstruction being the most common and having a significant effect on long-term outcomes (7-10). Although jejunostomy has become a routine “companion” procedure to radical esophagectomy in our department, these complications cannot be completely avoided even if the related surgery is performed by a highly experienced surgeon. In order to further reduce the complications of EN while preserving its advantages, we have modified the traditional jejunostomy and, for the first time in China, have proposed the use of autologous tissue (hepatic round ligament) as a bridge for EN, which is known as feeding via duodenostomy (FD). Key elements of the procedure of FJ is fixate the jejunum to the abdominal wall. The operation changes the anatomical position of the jejunum and can bring about complications such as intestinal obstruction. After the procedure is modified, frequent turnover of the intestinal canal at the nonoperative region can be avoided; meanwhile, with the patients’ own tissue as a bridge, adhesions between the intestinal canal and the abdominal cavity can be avoided, FD greatly reduces the possibility of postoperative intestinal torsion or intestinal adhesions.
In this study, we retrospectively analyzed the clinical data of 154 EC patients undergoing enterostomy after radical esophagectomy in our center from January 1, 2020, to June 30, 2020, to compare the impacts of the two different modes of ostomy. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-667/rc).
Methods
Participants
In this retrospective cohort study, the clinical data of 154 patients with T1 to T3 EC who underwent surgical treatment in our center from January 1, 2020, to June 30, 2020, were collected. Data from a concurrent, nonrandomized control group were also collected. A total of 179 EC patients were admitted during this period, and, after the inclusion and exclusion criteria were applied, 154 patients were ultimately enrolled in this study, including 80 patients in the FD group (using the autologous tissue) and 74 patients in the FJ group (using the traditional ostomy technique). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Fujian Cancer Hospital (No. K2022-016-01). Individual consent for this retrospective analysis was waived.
The inclusion criteria were as follows: (I) all the patients were examined by gastroscopy before surgery, and the lesions were pathologically confirmed as esophageal squamous carcinoma; (II) barium meal, enhanced computed tomography (CT) of the chest and abdomen, chest X-ray, and abdominal ultrasound performed before surgery revealed that the tumor had no obvious external invasion or distant metastasis and the lymph nodes of cervical, mediastinal, and abdominal can be removed; the estimated preoperative stages were T0–3N0–2M0; (III) preoperative electrocardiogram and pulmonary function tests revealed that the patient was able to tolerate anesthesia and surgery; and (IV) the surgical method was subtotal right transthoracic esophagectomy + gastric mobilization + cervical gastroesophageal anastomosis with the use of both thoracoscopy and laparoscopy, with cervicothoracic-abdominal (three-field) lymph node dissection (3-FD) and R0 resection (no tumor cells remaining both visually and microscopically) being achieved as confirmed by postoperative pathology.
The exclusion criteria for patients were the following: (I) with severe cardiac, pulmonary, cerebral, hepatic, or renal comorbidities; (II) undergoing a palliative resection; (III) undergoing a second surgery due to postoperative major complications (without related to the enterostomy procedures); (IV) adenocarcinoma of esophagus; (V) nasal feeding or without feeding tube; (VI) with a previous history of other abdominal surgery; (VII) lost to postoperative follow-up; or (VIII) the surgical method was Ivor Lewis or thoracotomy.
Ostomy and postoperative nutrition
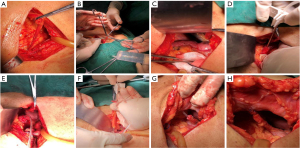
The method of FJ is illustrated in Figure 1. After the Treitz ligament was located intraoperatively, the feeding tube (about 30–35 cm in length) was placed 15–20 cm below the Treitz ligament and fixed with purse-string suture. Subsequently, the feeding tube was led out through the lateral edge of the rectus abdominis muscle on the left abdominal wall, and the jejunum was fixed on the abdominal wall. Finally, the feeding tube was fixed on the skin. EN was started from the first postoperative day (POD 1), and its volume was gradually increased. Fluoroscopy and CT were repeated on POD 7. If there was no abnormality and the general condition was fair, then more than 2,000 mL of EN could be completed. If EN could provide 1,500–2,000 calories, total enteral nutrition (TEN) was offered. The patient was discharged and required to return to the hospital for further examinations 4 weeks postoperatively. TEN was carried out via a jejunostomy tube during this period, and no food was consumed. If no abnormality was found during the postoperative CT and fluoroscopy, the patients could begin to eat food and were followed up until POD 180. During the follow-up period, the patients were followed up 30 days after surgery and then once every 90 days, with the examination items including at least chest CT, gastrointestinal imaging, routine blood test, biochemistry. Patients were communicated with by telephone every 30 days until POD 180. Patients experiencing abdominal pain, vomiting, abdominal distention, and/or cessation of bowel movement during the follow-up period were arranged to receive erect abdominal radiography, gastrointestinal tract imaging, and abdominal CT.
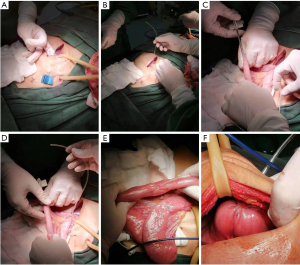
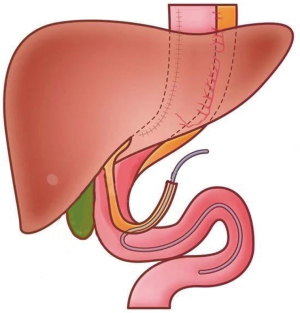
The method of FD is illustrated in Figures 2,3. The hepatic round ligament was dissociated from the abdominal wall, and then the atretic hepatic round ligament was reopened with mosquito forceps. The duodenal bulb was punctured with a puncture needle, and a feeding tube (about 30–35 cm in length) for EN was placed in the direction of the jejunum. The puncture point was sutured with a purse-string suture. A puncture needle was placed into the hepatic round ligament and penetrated it. Through the puncture needle, the feeding tube was drilled out the ligament. The connection site was sutured. A puncture needle was then used to puncture the upper abdomen to bring the feeding tube outwards. The other end of the hepatic round ligament was sutured to the abdominal wall. The hepatic round ligament becomes a bridge between the abdominal wall and the duodenal bulb. EN was started from POD 1, and its volume was gradually increased. Fluoroscopy and CT were repeated on POD 7. If there was no abnormality and the general condition was fair, then more than 2,000 mL of EN could be completed. If EN could provide 1,500–2,000 calories, TEN was offered. The patient was discharged and was required to return to the hospital for further examinations 4 weeks postoperatively. TEN was carried out via duodenostomy tube during this period, and no food was consumed. If no abnormality was found during the postoperative CT and fluoroscopy, the patients could begin to eat food and were followed up until POD 180. During the follow-up period, the patients were followed up 30 days after surgery and then once every 90 days, and the examination items including at least chest CT, gastrointestinal imaging, routine blood test, biochemistry Patients were communicated with by telephone every 30 days until POD 180. Patients experiencing abdominal pain, vomiting, abdominal distention, and/or cessation of bowel movement during the follow-up period were arranged to receive erect abdominal radiography, gastrointestinal tract imaging, and abdominal CT.
Measurements
The operative time was recorded. Albumin and prealbumin levels at the first and fourth postoperative weeks as well as short- and long-term postoperative complications (e.g., intestinal obstruction, intestinal torsion, feeding tube-associated infections, prolapse/rupture of feeding tube, and peritonitis) were also recorded. The operative time was obtained from the anesthesia records, the albumin and prealbumin values were measured by preoperative and postoperative biochemical tests, and the short- and long-term postoperative complications were recorded during postoperative follow-up. Patients with intestinal obstruction or intestinal torsion required further erect abdominal radiography, gastrointestinal tract imaging, and abdominal CT after the appearance of clinical symptoms such as abdominal pain, vomiting, abdominal distension, cessation of defecation, and exhaustion.
Statistical analysis
Sample size calculation
This study primarily focuses on the postoperative complication rate as the main outcome measure. Sample size calculation was conducted using the formula for comparing two sample rates. It is anticipated that the complication rate in the FJ group will be 14%, while in the FD group, it will be 1%. Set the two-sided α=0.05, the power is 80%, and the sample size of each group is 61 calculated by PASS 15 software. Considering the 10% loss to follow-up rate, the total sample size of the test is 68.
Stastistical analysis plan for outcome measurement
Taking into account the impact of confounding factors between the two groups, this study employed the inverse probability of treatment weighting (IPTW) method to control for confounding variables based on ten covariates: gender, age, body mass index (BMI), diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, pathologic type, location of tumor, tumor-node-metastasis (TNM) stage, preoperative therapy. Statistical analysis was conducted on the standardized mean difference (SMD) between the two groups before and after weighting. An SMD value of <0.1 after weighting was considered indicative of good group balance. Weighted analysis was performed on the data after weighting using the survey package. All tests were two-sided, and a P value <0.05 was considered statistically significant. All statistical analysis were performed using R (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
As shown in Figure 4, among 179 patients who had undergone esophagectomy for thoracic EC, 25 were excluded according to the inclusion or exclusion criteria (including postoperative nasal feeding in 3 cases, no postoperative EN in 1 case, non-R0 resection in 4 cases, previous history of other abdominal surgery in 3 cases, postoperative pathological findings of adenocarcinoma in 4 cases, loss to follow-up in 3 cases, intraoperative conversion to thoracotomy in 3 cases, and Ivor-Lewis surgery in 4 cases), and thus 154 patients entered the final analysis. Of these 154 patients, there were 80 in the FD group (using the autologous tissue) and 74 in the FJ group (using the traditional ostomy technique).
The two groups were matched in age, gender, BMI, underlying disease, history of neoadjuvant therapy, pathological type, tumor location, degree of differentiation, and TNM stage (all P values >0.05; Table 1). Taking into account the impact of confounding factors between the two groups, this study employed IPTW method to control for confounding (all SMD value <0.1; Table 2 and Figure 5). Before IPTW, we found the operative time of FD was longer than FJ. However, this difference in ostomy time did not have a significant impact on the overall length of EC surgery (Table 3).
Table 1
| Variable | FD (n=80) | FJ (n=74) | P value | SMD |
|---|---|---|---|---|
| Gender, n (%) | 0.896 | 0.021 | ||
| Female | 37 (46.2) | 35 (47.3) | ||
| Male | 43 (53.8) | 39 (52.7) | ||
| Age, years, median [IQR] | 65.00 [58.75, 72.00] | 62.50 [60.00, 68.00] | 0.612 | 0.042 |
| BMI, kg/m2, mean (SD) | 21.13 (3.31) | 21.24 (3.52) | 0.853 | 0.03 |
| Diabetes mellitus, n (%) | 6 (7.5) | 5 (6.8) | 0.858 | 0.029 |
| Hypertension, n (%) | 12 (15.0) | 14 (18.9) | 0.517 | 0.105 |
| Hyperlipidemia, n (%) | 8 (10.0) | 7 (9.5) | 0.91 | 0.018 |
| Coronary artery disease, n (%) | 2 (2.5) | 2 (2.7) | 0.937 | 0.013 |
| Pathologic type, n (%) | 0.977 | 0.073 | ||
| Well-differentiated | 22 (27.5) | 19 (25.7) | ||
| Moderately differentiated | 33 (41.3) | 33 (44.6) | ||
| Poorly differentiated | 20 (25.0) | 18 (24.3) | ||
| Undifferentiated | 5 (6.2) | 4 (5.4) | ||
| Location of tumor, n (%) | 0.973 | 0.038 | ||
| Upper thoracic esophagus | 16 (20.0) | 14 (18.9) | ||
| Middle thoracic esophagus | 34 (42.5) | 31 (41.9) | ||
| Lower thoracic esophagus | 30 (37.5) | 29 (39.2) | ||
| pStage, n (%) | 0.292 | 0.315 | ||
| Stage 0 (PCR) | 10 (12.5) | 8 (10.8) | ||
| Stage I | 8 (10.0) | 7 (9.5) | ||
| Stage II | 38 (47.5) | 26 (35.1) | ||
| Stage III | 24 (30.0) | 33 (44.6) | ||
| Preoperative therapy, n (%) | 0.766 | 0.118 | ||
| None | 48 (60.0) | 42 (56.8) | ||
| Neoadjuvant chemotherapy | 24 (30.0) | 26 (35.1) | ||
| Neoadjuvant chemoradiotherapy | 8 (10.0) | 6 (8.1) |
FD, feeding via duodenostomy; FJ, feeding via jejunostomy; SMD, standardized mean difference; IQR, interquartile range; BMI, body mass index; SD, standard deviation; PCR, pathologic complete response.
Table 2
| Variable | FD (n=54) | FJ (n=55) | P value | SMD |
|---|---|---|---|---|
| Gender, n (%) | 0.949 | 0.012 | ||
| Female | 25 (46.3) | 26 (47.3) | ||
| Male | 29 (53.7) | 29 (52.7) | ||
| Age, years, median [IQR] | 66.68 [58.48, 72.00] | 62.00 [60.00, 68.00] | 0.327 | 0.016 |
| BMI, kg/m2, mean (SD) | 21.30 (3.49) | 21.34 (3.42) | 0.958 | 0.009 |
| Diabetes mellitus, n (%) | 5 (9.3) | 5 (9.1) | 0.963 | 0.008 |
| Hypertension, n (%) | 10 (18.5) | 9 (16.4) | 0.882 | 0.026 |
| Hyperlipidemia, n (%) | 7 (13.0) | 6 (10.9) | 0.925 | 0.017 |
| Coronary artery disease, n (%) | 2 (3.7) | 2 (3.6) | 0.902 | 0.023 |
| Pathologic type, n (%) | 0.998 | 0.033 | ||
| Well-differentiated | 14 (26.0) | 14 (25.4) | ||
| Moderately differentiated | 21 (38.9) | 21 (38.2) | ||
| Poorly differentiated | 15 (27.8) | 16 (29.1) | ||
| Undifferentiated | 4 (7.3) | 4 (7.3) | ||
| Location of tumor, n (%) | 0.987 | 0.029 | ||
| Upper thoracic esophagus | 10 (18.5) | 9 (16.4) | ||
| Middle thoracic esophagus | 25 (46.3) | 26 (47.3) | ||
| Lower thoracic esophagus | 19 (35.2) | 20 (36.3) | ||
| pStage, n (%) | 0.999 | 0.025 | ||
| Stage 0 (PCR) | 8 (14.8) | 7 (12.7) | ||
| Stage I | 3 (5.6) | 3 (5.5) | ||
| Stage II | 24 (44.4) | 25 (45.5) | ||
| Stage III | 19 (35.2) | 20 (36.3) | ||
| Preoperative therapy | 0.993 | 0.021 | ||
| None | 35 (64.8) | 35 (63.6) | ||
| Neoadjuvant chemotherapy | 13 (24.1) | 14 (25.5) | ||
| Neoadjuvant chemoradiotherapy | 6 (11.1) | 6 (10.9) |
IPTW, inverse probability of treatment weighting; FD, feeding via duodenostomy; FJ, feeding via jejunostomy; SMD, standardized mean difference; IQR, interquartile range; BMI, body mass index; SD, standard deviation; PCR, pathologic complete response.
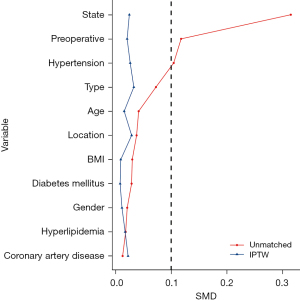
Table 3
| Variable | FD (n=80) | FJ (n=74) | P value |
|---|---|---|---|
| Nutritional markers | |||
| Albumin (g/L) (1 week postoperatively) | 36.80 [35.20, 37.50] | 36.30 [35.15, 37.50] | 0.749 |
| Prealbumin (g/L) (1 week postoperatively) | 178.00 [165.00, 190.00] | 173.00 [169.25, 184.00] | 0.705 |
| Albumin (g/L) (4 weeks postoperatively) | 42.00 [41.00, 44.00] | 41.35 [39.80, 43.25] | 0.043 |
| Prealbumin (g/L) (4 weeks postoperatively) | 225 [214.00, 238.00] | 225.00 [212.00, 237.00] | 0.353 |
| Operative time | |||
| Ostomy (minutes) | 15.5 [13.00, 24.00] | 12.00 [11.00, 13.00] | <0.001 |
| Radical esophagectomy for esophageal cancer (minutes) | 236.5 [212.75, 260.00] | 234.15 [214.00, 244.50] | 0.275 |
| Postoperative hospital stay (days) | 7.00 [7.00, 8.00] | 8.00 [7.00, 8.00] | 0.217 |
Data are presented as median [IQR]. FD, feeding via duodenostomy; FJ, feeding via jejunostomy; IQR, interquartile range.
No intestinal torsion or necrosis was not noted. Complications were recorded in 10 patients in the FJ group, including 2 with proximal intestinal obstruction and 8 with distant intestinal obstruction. No similar complications were observed in the FD group. The only complication reported in the FD group was infection (n=1), which might have been caused by fluid leakage at the puncture site (Table 4). Thus, the difference in complications was statistically significant after IPTW (P=0.017, Table 5).
Table 4
| Variable | FD (n=80) | FJ (n=74) | P value |
|---|---|---|---|
| Overall catheter-related complications | 1 | 10 | 0.003 |
| Complications during catheterization | – | ||
| Mechanical bowel obstruction | 0 | 2 | |
| Mechanical bowel twist | 0 | 0 | |
| Catheter site infection | 1 | 0 | |
| Catheter dislodgement | 0 | 0 | |
| Fracture of the catheter | 0 | 0 | |
| Complications after removal of the catheter | – | ||
| Mechanical bowel obstruction | 0 | 8 | |
| Mechanical bowel twist | 0 | 0 | |
| Catheter site infection | 0 | 0 | |
| Peritonitis | 0 | 0 | |
FD, feeding via duodenostomy; FJ, feeding via jejunostomy.
Table 5
| Variable | FD (n=54) | FJ (n=55) | P value |
|---|---|---|---|
| Albumin (g/L) (1 week postoperatively), median [IQR] | 36.80 [35.20, 37.35] | 36.30 [35.38, 37.38] | 0.792 |
| Prealbumin (g/L) (1 week postoperatively), median [IQR] | 178.00 [165.00, 190.00] | 176.00 [172.00, 189.00] | 0.347 |
| Albumin (g/L) (4 weeks postoperatively), median [IQR] | 42.00 [41.00, 44.00] | 41.00 [39.80, 42.30] | 0.018 |
| Prealbumin (g/L) (4 weeks postoperatively), median [IQR] | 225.00 [214.00, 237.51] | 222.89 [212.00, 235.00] | 0.493 |
| Ostomy (minutes), median [IQR] | 20.00 [13.00, 25.00] | 12.00 [10.00, 13.00] | <0.001 |
| Radical esophagectomy for esophageal cancer (minutes), median [IQR] | 240.00 [215.00, 262.55] | 230.69 [210.00, 242.67] | 0.057 |
| Postoperative hospital stay (days), median [IQR] | 7.00 [7.00, 8.00] | 7.21 [7.00, 8.00] | 0.697 |
| Catheter complication, n (%) | 1 (1.9) | 8 (14.5) | 0.017 |
IPTW, inverse probability of treatment weighting; FD, feeding via duodenostomy; FJ, feeding via jejunostomy; IQR, interquartile range.
All patients received postsurgical EN. About 24 hours after surgery, 500 mL of glucose saline was dripped through the feeding tube. If the patient had no discomfort and the amount of chest drainage was <300 mL, 1 bag of EN solution [4 spoonfuls of Nutrison (Fulda, Germany) + 500 mL of saline] was slowly dripped via the feeding tube 48 hours after surgery. The amount was increased to 4 bags of EN solution (12 spoonfuls of Nutrison + 500 mL of saline) on a daily basis if the patient had no significant discomfort. The amount remained stable in the ensuing days, ensuring that the calorie intake was between 1,500 and 2,000 calories.
After 1 week, the albumin level in the FD group was noninferior to that in the FJ group (36.8 vs. 36.3 g/L; P=0.792), and the prealbumin level also showed no significant difference (178 vs. 176 g/L; P=0.347). Four weeks later, there was significant difference in levels of albumin (42 vs. 41 g/L; P=0.018) but no significant difference prealbumin (225 vs. 222.89 g/L; P=0.493; Table 5). The length of stay (LoS) was similar between the two groups (7 vs. 7.21 days; P=0.697; Table 5). In terms of the time of stoma creation, it was significantly longer in the FD group than in the FJ group (20 vs. 12 minutes; P<0.001; Table 5); however, it did not bring a significant impact on the overall procedure length (240 vs. 230.69 minutes; P=0.057; Table 5).
Discussion
Extended fasting is required after surgery for EC. Therefore, maintaining postoperative nutritional status is particularly important for achieving good prognosis and reducing complications. Most currently available evidence supports EN being superior to PN in these patients (11-14). The main EN modalities after EC surgery include nasoduodenal and nasojejunal tubes and jejunostomy, each of which has its own advantages and disadvantages (15,16), and there is no consensus regarding which enteral modality is preferred. The main complication of nasoduodenal tube placement is the poor tolerance of patients to the tube, and long-term placement often causes nausea or vomiting, dry mouth, sore throat, and foreign body sensation, even leading to problems such as nasal rupture; as a result, patients may remove the nasoduodenal tube themselves (17). The rate of nasoduodenal tube dislodgement reported in the literature ranges from 16% to 36%, with one of the reasons for dislodgement being falling asleep (16,18,19). In contrast, jejunostomy has better patient tolerance, a lower dislodgement rate, and even better efficacy of nutritional support. However, although jejunostomy can avoid the nasopharyngeal discomfort caused by a nasoenteric tube and enable long-term EN, it is an invasive operation that changes the anatomical position of the jejunum and can bring about complications such as tube blockage or prolapse, stoma leakage, abdominal infection, intestinal torsion, intestinal obstruction, or intestinal necrosis, all which may require a second open operation or even be life-threatening. As the jejunostomy-related complications are far more dangerous, with their reported incidence rates ranging from 1.5% to 37% (20), many centers have finally abandoned this procedure. In the study by Torres Júnior et al. (21) the incidence of complications was comparable between the nasoenteric and jejunostomy groups, as no major complication was noted in either group; furthermore, the nasoenteric group required the introduction of parenteral therapy more frequently than did the jejunostomy group (P<0.05), whereas jejunostomy allowed EN for longer periods, especially in patients with complications. Myers et al. (22) summarized the clinical outcomes of 2,022 patients who underwent jejunostomy, among whom 34 (1.5%) had complications, 18 underwent a secondary surgery, and 3 died. Thus, the advantages and disadvantages of jejunostomy are quite clear. In order to reduce the complications associated with jejunostomy, efforts must made to avoid changing the anatomical position of the jejunum, minimize the surgical trauma, and reduce the stoma-related complications. Consequently, the FD method was developed, and in our study, FD dramatically reduced the tube-related complications after surgery.
According to our experience, the most common complications in post jejunostomy patients are intestinal obstruction and nonobstructive abdominal pain associated with jejunostomy. Some patients may still have intestinal obstruction years or even decades after jejunostomy. Many patients may choose to seek medical services in local hospitals, which can lead to an underestimated incidence of jejunostomy-related complications. The main causes of intestinal obstruction or torsion in surgeries could be the following: the bowel is frequently turned over during the search for the Treitz ligament and jejunostomy position, and although the bowel is placed back to its original position as much as possible after the operation, the relative position of the bowel may still be affected to some extent. Moreover, the jejunostomy tube needs to be fixed on the abdominal wall with sutures throughout the procedure and the tube needs to be introduced outwards through the lateral edge of the rectus abdominis muscle on the left side of the abdominal wall. This step artificially adheres the punctured segment of the jejunostomy to the abdominal wall and is probably the primary cause of intestinal obstruction and intestinal torsion (20,23). However, this step is indispensable for preventing extravasation of nutrition solution and digestive fluids. The fixation of the intestinal tube during this process can cause angulation and artificial adhesions, which greatly increases the probability of intestinal obstruction or intestinal torsion. Therefore, some authors have stated that jejunostomy can be eliminated in radical esophagectomy for EC (24). However, in these articles, most participants were patients with adenocarcinoma of the esophagus or tumors in the middle and lower esophagus, which were mostly treated with Ivor-Lewis esophagectomy with intrathoracic esophago-gastric anastomosis. In China, most patients have squamous EC, and three-field radical esophagectomy is more common. In patients with esophageal squamous carcinoma, the lesions are closer to the pharynx, resulting in more severe dysphagia and malnutrition; in addition, postoperative anastomotic leak occurs more frequently, and the jejunostomy tube is placed for a longer time (25). Stoma-related abdominal pain may occur after stoma creation. Although the pain is often subjective without any organic change, it may be caused by changes in the anatomical position of the jejunum or by adhesions or angulation of the jejunum to the abdominal wall. The complications associated with jejunostomy suggest the need for a new EN modality without jejunal attachment to the abdominal wall. In 2009, some Japanese authors had developed a modified approach of inserting an enteral feeding tube through the reconstructed gastric tube using the round ligament of the liver (23). In 2011, another research group successfully applied this technique for placing a feeding tube for retrosternal gastric tube reconstruction after esophagectomy, further demonstrating the effectiveness and safety of this approach (26). Over recent years, several Japanese researchers have continued to develop this technique and have confirmed that ostomy via the hepatic round ligament can effectively alleviate the complications associated with jejunostomy (8,27). Other scholars have used this procedure in patients undergoing gastric tube reconstruction via a posterior trans-sternal approach, with the gastric tube often being chosen as the puncture site. In our center, however, the posterior mediastinum is often selected as the approach for gastric tube reconstruction; due to limitations posed by the length of the hepatic round ligament, the gastric tube cannot be routinely chosen as the puncture site. Therefore, the descending duodenum, which has a more fixed location, is currently used as the conventional ostomy site. After the procedure is modified, frequent turnover of the intestinal canal at the nonoperative region can be avoided; meanwhile, with the patients’ own tissue as a bridge, adhesions between the intestinal canal and the abdominal cavity can be avoided, which greatly reduces the possibility of postoperative intestinal torsion or intestinal adhesions.
In terms of short-term complications, only 1 patient in the FD group experienced ostomy-related abdominal pain. In this patient, the abdominal pain was located near the ostomy site and disappeared after 3 days of fasting, acid control, and suppression of digestive fluid secretion; no notable abnormalities were observed after a second enteral therapy. Based on this patient’s signs and abdominal imaging findings, the pain might have been caused by the leakage of a small number of digestive fluids at the duodenostomy site. Although we covered the duodenostomy tube between the abdominal wall and the digestive tract with the hepatic round ligament, the digestive fluids leaking from the duodenostomy site could have caused peritubular or even abdominal infection if the suturing was performed with insufficient caution or if the hepatic round ligament itself had problems. Fortunately, this event occurred early in the postoperative period and was resolved with nonsurgical management. Moreover, intraoperative exploration of this patient also revealed the presence of liver cirrhosis, which might have affected the quality of the bridge to some extent, considering that cirrhosis may lead to recanalization of the hepatic round ligament. Whether FD is feasible for these types of patients and whether there are other relevant complications warrant further investigations. In this study, the incidence of both short-term and long-term postoperative complications was significantly lower in the FD group than in the FJ group (P=0.017; Table 5), suggesting that FD, to some extent, lowered the risk of complications associated with jejunostomy, a finding which is line with another study (28).
As it is a major factor affecting the occurrence of anastomotic leak (29), we are pursuing means to achieving better nutritional status in the perioperative period to reduce anastomotic leak. Albumin is a marker for nutritional status in the immediate postoperative period, whereas prealbumin is a good indicator of nutritional status in a given phase. In our current study, no significant difference was found in the albumin and prealbumin levels between the FJ group and FD group at 1 and 4 weeks postoperatively, suggesting FD did not diminish nutritional markers while reducing jejunostomy-related complications. The LoS was also comparable between these two groups (7.00 vs. 7.21 days; P=0.697), suggesting FD did not prolong hospital stay.
As a new procedure, FD had longer operative time than did FJ (20 vs. 12 minutes; P<0.001), which might have been due to differences in surgical proficiency. In fact, FJ has long been carried out in our center, while FD is still in its initial stage. Nevertheless, the operative time of FD will gradually decrease when the operation and processes become more efficient. Notably, this difference in ostomy time did not have a significant impact on the overall length of EC surgery (240 vs. 230.69 minutes; P=0.057).
Limitations
This retrospective observational study was conducted at a single center, with small sample size and a limited duration of follow-up. In addition, there may be complications that were not recognized or have not yet arisen. Further studies with larger sample sizes and longer follow-up periods are thus warranted.
Generalizability
FD is relatively easy to perform and learn. Based on our experience, it can effectively reduce the incidence of postoperative intestinal obstruction without affecting the operative time and postoperative nutritional status. Thus, it is superior to the traditional FJ.
Conclusions
FD, to some extent, reduces the incidence of postoperative intestinal obstruction and stoma-associated abdominal pain. The slightly prolonged operative time is still negligible compared to the benefits obtained. In addition, FD does not affect the postoperative nutritional status, nor does it prolong hospital stay.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-667/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-667/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-667/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-667/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Fujian Cancer Hospital (No. K2022-016-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus 2019;16:221-45. [Crossref] [PubMed]
- Omori A, Tsunoda S, Nishigori T, et al. Clinical Benefits of Routine Feeding Jejunostomy Tube Placement in Patients Undergoing Esophagectomy. J Gastrointest Surg 2022;26:733-41. [Crossref] [PubMed]
- Takesue T, Takeuchi H, Ogura M, et al. Erratum to: A Prospective Randomized Trial of Enteral Nutrition After Thoracoscopic Esophagectomy for Esophageal Cancer. Ann Surg Oncol 2016;23:1060-1. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr 2015;34:1-6. [Crossref] [PubMed]
- Zhuang W, Wu H, Liu H, et al. Utility of feeding jejunostomy in patients with esophageal cancer undergoing esophagectomy with a high risk of anastomotic leakage. J Gastrointest Oncol 2021;12:433-45. [Crossref] [PubMed]
- Choi AH, O'Leary MP, Merchant SJ, et al. Complications of Feeding Jejunostomy Tubes in Patients with Gastroesophageal Cancer. J Gastrointest Surg 2017;21:259-65. [Crossref] [PubMed]
- Kawai R, Abe T, Uemura N, et al. Feeding catheter gastrostomy with the round ligament of the liver prevents mechanical bowel obstruction after esophagectomy. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Kingma BF, Turchi MM, Lovera R, et al. Technical notes and outcomes of robot-assisted and laparoscopic jejunostomy placement for tube feeding after esophagectomy. Ann Esophagus 2022;5:21. [Crossref]
- Koterazawa Y, Oshikiri T, Hasegawa H, et al. Routine placement of feeding jejunostomy tube during esophagectomy increases postoperative complications and does not improve postoperative malnutrition. Dis Esophagus 2020;33:doz021. [PubMed]
- Fujita T, Daiko H, Nishimura M. Early enteral nutrition reduces the rate of life-threatening complications after thoracic esophagectomy in patients with esophageal cancer. Eur Surg Res 2012;48:79-84. [Crossref] [PubMed]
- Manba N, Koyama Y, Kosugi S, et al. Is early enteral nutrition initiated within 24 hours better for the postoperative course in esophageal cancer surgery? J Clin Med Res 2014;6:53-8. [PubMed]
- Peng J, Cai J, Niu ZX, et al. Early enteral nutrition compared with parenteral nutrition for esophageal cancer patients after esophagectomy: a meta-analysis. Dis Esophagus 2016;29:333-41. [Crossref] [PubMed]
- Li K, Zeng Z, Zhang Z, et al. Comparisons of nutritional status and complications between patients with and without postoperative feeding jejunostomy tube in gastric cancer: a retrospective study. J Gastrointest Oncol 2023;14:97-109. [Crossref] [PubMed]
- Andersson B, Ansari D, Nordén M, et al. Surgical stress response after colorectal resection. Int Surg 2013;98:292-9. [Crossref] [PubMed]
- Gerritsen A, Besselink MG, Cieslak KP, et al. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg 2012;16:1144-51. [Crossref] [PubMed]
- Prabhakaran S, Doraiswamy VA, Nagaraja V, et al. Nasoenteric tube complications. Scand J Surg 2012;101:147-55. [Crossref] [PubMed]
- Mahadeva S, Malik A, Hilmi I, et al. Transnasal endoscopic placement of nasoenteric feeding tubes: outcomes and limitations in non-critically ill patients. Nutr Clin Pract 2008;23:176-81. [Crossref] [PubMed]
- Metheny NA, Schnelker R, McGinnis J, et al. Indicators of tubesite during feedings. J Neurosci Nurs 2005;37:320-5. [Crossref] [PubMed]
- Han-Geurts IJ, Verhoef C, Tilanus HW. Relaparotomy following complications of feeding jejunostomy in esophageal surgery. Dig Surg 2004;21:192-6. [Crossref] [PubMed]
- Torres Júnior LG, de Vasconcellos Santos FA, Correia MI. Randomized clinical trial: nasoenteric tube or jejunostomy as a route for nutrition after major upper gastrointestinal operations. World J Surg 2014;38:2241-6. [Crossref] [PubMed]
- Myers JG, Page CP, Stewart RM, et al. Complications of needle catheter jejunostomy in 2,022 consecutive applications. Am J Surg 1995;170:547-50; discussion 550-1. [Crossref] [PubMed]
- Sarr MG. Appropriate use, complications and advantages demonstrated in 500 consecutive needle catheter jejunostomies. Br J Surg 1999;86:557-61. [Crossref] [PubMed]
- Kroese TE, Tapias L, Olive JK, et al. Routine intraoperative jejunostomy placement and minimally invasive oesophagectomy: an unnecessary step?†. Eur J Cardiothorac Surg 2019;56:746-53. [Crossref] [PubMed]
- Shen X, Zhuo ZG, Li G, et al. Is the routine placement of a feeding jejunostomy during esophagectomy worthwhile?-a systematic review and meta-analysis. Ann Palliat Med 2021;10:4232-41. [Crossref] [PubMed]
- Watanabe M, Etoh K, Nagai Y, et al. Feeding tube insertion through the round ligament of liver: a safe approach to placing a feeding tube for retrosternal gastric tube reconstruction after esophagectomy. J Am Coll Surg 2011;213:e21-2. [Crossref] [PubMed]
- Otake R, Okamura A, Kanamori J, et al. The Optimal Feeding Enterostomy Creation During Esophagectomy to Reduce the Long-Term Risk of Small Bowel Obstruction. World J Surg 2020;44:3845-51. [Crossref] [PubMed]
- Oya H, Koike M, Iwata N, et al. Feeding duodenostomy decreases the incidence of mechanical obstruction after radical esophageal cancer surgery. World J Surg 2015;39:1105-10. [Crossref] [PubMed]
- Patil PK, Patel SG, Mistry RC, et al. Cancer of the esophagus: esophagogastric anastomotic leak--a retrospective study of predisposing factors. J Surg Oncol 1992;49:163-7. [Crossref] [PubMed]
(English Language Editor: J. Gray)



