
HMGB2 upregulation promotes the progression of hepatocellular carcinoma cells through the activation of ZEB1/vimentin axis
Highlight box
Key findings
• High mobility group box 2 (HMGB2) has the potential as an oncogene which participates in malignant transformation of hepatocellular carcinoma (HCC) via HMGB2-zinc finger E-box binding homeobox 1-vimentin signaling pathway.
What is known and what is new?
• HMGB2 is abnormally expressed in human cancers and closely related with the malignant biological phenotype and poor clinical prognosis. However, the elaborated mechanism of its role in regulating HCC progression is still poorly understood. This study provides a novel molecular mechanism and a new strategy for adjuvant clinical treatment of HCC in the future.
What is the implication, and what should change now?
• This study suggested that HMGB2 may be a key regulatory factor in promoting the malignant progression of HCC. Targeted intervention of key proteins in its signaling pathway may become a novel approach for adjuvant therapy of HCC. The following research requires multi animal models and large cohorts clinical sample validation, as well as screening targeted drugs.
Introduction
Primary liver cancer (PLC) is the fourth leading cause of cancer-related deaths worldwide, with hepatocellular carcinoma (HCC) accounting for the majority of PLC (1). HCC usually occurs in the background of cirrhosis caused by multiple risk factors, including chronic viral infection of hepatitis B virus (HBV) or hepatitis C virus (HCV), alcoholism, exposure to aflatoxin, non-alcoholic steatohepatitis (NASH), and drug-related liver injury (2). With the improvements of surgical skills and the assistance of comprehensive treatment strategies, such as radiotherapy, chemotherapy, and immunotherapy, provide the possibility of prolonging survival time for HCC patients. However, clinical analysis has revealed that the 5-year overall survival rate for patients with HCC remains less than 40% (3). This is explained by common postoperative metastasis and recurrence caused by intrahepatic or extrahepatic invasion and dissemination in a short time period (4,5). Therefore, further investigation of molecular mechanisms of occurrence and metastasis should identify key targets to regulate the survival and invasion of cancer cells, and therefore provide a new strategy for clinical therapy.
High mobility group box 2 (HMGB2), is a highly conserved nucleoprotein which belongs to high mobility group of protein family. Other identified family members include HMGB1 and HMGB3 (6). HMGB2 is widely distributed in mammalian cells and participates in a variety of cellular functions, including erythrocyte production, sperm maturation, intracellular DNA transcription and replication (7). In normal physiological conditions, HMGB2 only exhibits high expression during embryogenesis. Its expression decreases with the maturation of individuals (8). In recent years, researchers have reported that HMGB2 is abnormally expressed in numerous human cancers, including HCC, colorectal cancer and glioma, which is closely related with the malignant biological phenotype and poor clinical prognosis of tumors (9-12). Up to now, the definitive role of HMGB2 in HCC is largely unknown and requires further investigation. Based on the existing research, we speculate that the abnormal expression of HMGB2 is involved in the malignant progression of HCC, but the specific mechanism needs to be further explored.
The purpose of our work is to explore the level of HMGB2 in clinical tissues and cells. Furthermore, its effects on the biological characteristics of HCC and related molecular mechanisms were evaluated. In summary, our study attempts to explore the possibility of HMGB2 as a novel biological target for clinical treatment of HCC. We present this article in accordance with the REMARK and MDAR reporting checklists (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-447/rc).
Methods
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Collection and use of clinical tissues, relevant pathological and follow-up data were approved by Biomedical Ethics Committee of The Third Affiliated Hospital of Navy Military Medical University (No. EHBHKY2020-02-003; Shanghai, China). Written informed consent was received from each patient prior to the surgery.
Patients and specimens
HCC tissues (n=62) were obtained from patients who received radical local hepatectomy in The Third Affiliated Hospital of Navy Military Medical University from February 2013 to January 2014. All cases were confirmed diagnosis of primary HCC by imaging, serological diagnosis and histopathology. All patients did not receive any antineoplastic therapy, such as drug treatment, chemotherapy or radiotherapy before operation and were postoperatively followed up as previously described (13). In brief, the last follow-up was done in April 2019, with a median follow-up time of 41.0 months (range, 5.5–69.0 months). The clinicopathological characteristics of HCC patients were summarized in Table 1. The tissue samples obtained from surgical resection are used for tissue microarray (n=62, for immunohistochemical analysis) and RNA extraction [n=62, for quantitative real-time polymerase chain reaction (qRT-PCR) detection].
Table 1
| Clinicopathologic features | No. of patient | HMGB2 expression | χ2 | P value | ||
|---|---|---|---|---|---|---|
| Low (n=32) | High (n=30) | |||||
| Age (years) | ||||||
| ≤50 | 47 | 26 | 21 | 1.069 | 0.301 | |
| >50 | 15 | 6 | 9 | |||
| Gender | ||||||
| Male | 40 | 22 | 18 | 0.518 | 0.472 | |
| Female | 22 | 10 | 12 | |||
| Etiology | ||||||
| HBV infection | 46 | 26 | 20 | 2.978 | 0.226 | |
| HCV infection | 13 | 4 | 9 | |||
| Alcohol | 3 | 2 | 1 | |||
| Background liver pathology | ||||||
| Normal liver | 3 | 2 | 1 | 0.495 | 0.781 | |
| Chronic hepatitis | 20 | 11 | 9 | |||
| Liver cirrhosis | 39 | 19 | 20 | |||
| Serum AFP (ng/mL) | ||||||
| ≤400 | 45 | 28 | 17 | 7.397 | 0.007 | |
| >400 | 17 | 4 | 13 | |||
| Tumor size (cm) | ||||||
| ≤5 | 41 | 29 | 12 | 17.717 | <0.001 | |
| >5 | 21 | 3 | 18 | |||
| Intrahepatic metastasis | ||||||
| Present | 51 | 30 | 21 | 5.984 | 0.014 | |
| Absent | 11 | 2 | 9 | |||
| Vascular invasion | ||||||
| No | 43 | 27 | 16 | 7.020 | 0.008 | |
| Yes | 19 | 5 | 14 | |||
| Tumor encapsulation | ||||||
| Yes | 24 | 14 | 10 | 0.708 | 0.401 | |
| No | 38 | 18 | 20 | |||
| Early recurrence | ||||||
| No | 33 | 21 | 12 | 4.084 | 0.043 | |
| Yes | 29 | 11 | 18 | |||
| TNM stage | ||||||
| I | 35 | 19 | 16 | 0.230 | 0.632 | |
| II + III | 27 | 13 | 14 | |||
Chi-squared tests and Fisher’s exact tests were employed to analyzed the clinical information. HMGB2, high mobility group box 2; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; TNM, tumor, lymph node and metastasis.
Cell culture
Human HCC cells included MHCC97H, Hep3B, LM3 and Huh7, and immortalized normal liver cell line THLE-2 were used for cell experiments (The Cell Bank of the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences). All cells were cultured as previously described (13). In brief, all the cells were cultured at 37 ℃, 5% CO2 and 95% humidity condition. All experimental cells involved in the study had been identified and confirmed by short tandem repeats (STRs) profiling and mycoplasma testing. HMGB2 small interfering RNAs (siRNAs) and scramble siRNA were artificially synthesized by RiboBio Co., Ltd. (siG000003148; Guangzhou, China). Detailed information of sequence is summarized in Table S1. siRNA transfection was optimized using Lipofectamine 3000 (catalog No. L3000001, Invitrogen; Thermo-Fisher Scientific, Inc., Carlsbad, CA, USA). Recombinant human vimentin protein was obtained from R&D Systems, Inc. (catalog no, 2105-VI-100; Minneapolis, MN, USA).
Cell viability, migration and invasion assays
Cell proliferation activity was evaluated by using cell counting kit-8 assay (CCK-8 assay; catalog No. C0038; Beyotime Institute of Biotechnology, Nantong, China). In short, cells were digested and resuspended as single cell suspension, and then counted and inoculated on 96-well cell culture plates with a density of 5,000 cells per well. The experimental group was divided into three groups: blank control group, scramble control group [transfected with scramble control by lipofectamine 3000 (catalog No. L3000001, Invitrogen; Thermo-Fisher Scientific, Inc.)] and HMGB2 siRNA group [transfected with HMGB2 siRNA by lipofectamine 3000 (catalog No. L3000001, Invitrogen; Thermo-Fisher Scientific, Inc.)]. After routine cultivation for 24, 48 and 72 h, we detected each absorbance at 490 nm by using a spectrophotometer (Multiskan FC, Thermo-Fisher Scientific, Inc.). Cell migration & invasion experiments were carried out on transwell chambers with or without Matrigel-coating (catalog No. 354480, Corning Inc., NY, USA), as previously described (13). After 48 h of transfection, count the number of cells passing through the chambers in different experimental groups. Each experiment was repeated three times.
Immunohistochemistry
Immunohistochemical detection of HCC tissue microarray was conducted as described previously (13). The following antibodies were used: HMGB2 antibody (catalog No. ab133540; dilution: 1:100; Abcam, UK), zinc finger E-box binding homeobox 1 (ZEB1) antibody (catalog No. ab203829; dilution: 1:100; Abcam) and vimentin antibody (catalog No. ab8979; dilution: 1:200; Abcam). Staining evaluation was performed by three investigators. Immunoreactive evaluation was performed as previously described (13). For immunofluorescence detection, HCC cells were inoculated and cultured on cover slides in 6-well culture plates. Following treatment, cell fixation, membrane penetration and primary antibody incubation followed by the appropriate Alexa Fluor 647/Cy3/Alexa Fluor 488-labeled secondary antibodies (catalog nos. A0468, A0516 and A0428; Beyotime Institute of Biotechnology), and co-stained using 4’,6-diamidino-2-phenylindole (DAPI) (catalog No. MBD0015; Sigma-Aldrich: Merck KGaA, St. Louis, USA).
Reverse transcription-quantitative PCR (RT-qPCR)
HCC tissues and cells were dissociated by using TRIzol reagent with the aim to extract the total RNA (catalog No. 15596026; Thermo-Fisher Scientific, Inc.). Reverse transcription and polymerase chain reactions (PCRs) were used to amplify the target genes (StepOnePlus, Applied Biosystems; Thermo-Fisher Scientific, Inc.). The reaction conditions were carried out as conventional procedures. Three replicates were performed with each cDNA sample. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined as control of target genes. The 2−ΔΔCt system was used to analyze the results. The primer sequences of all detected target genes are summarized in Table S2.
Western blot analysis
Proteins were extracted from HCC samples and cells, then separated and visualized as previously described (13). The images were scanned and statistical analyzed using Image J V1.8.0 (National Institute of Health, Bethesda, MD, USA). The following antibodies were used: HMGB2 antibody (catalog No. ab133540; dilution: 1:1,000; Abcam), ZEB1 antibody (catalog No. ab203829; dilution: 1:1,000; Abcam), vimentin antibody (catalog No. ab8979; dilution: 1:2,000; Abcam), p53 antibody (catalog No. ab26; dilution: 1:1,000; Abcam) and vimentin antibody (catalog No. ab137872; dilution: 1:1,000; Abcam). GAPDH was used as internal control (catalog No. ab70699; dilution: 1:6,000; Abcam). The evaluation and statistical analysis of protein bands were carried out by employing Quantity One software (v4.6.6; Bio‑Rad Laboratories, Inc., Hercules, USA).
Detection of cell cycle using flow cytometry
HCC cells were routinely cultured and transfected with different treatment groups. Cells were collected after 48 h of transfection. Cells were fixed with precooled 75% ethanol and incubated at 4 ℃ overnight. Then, they were stained with RNase-containing propidium iodide (PI) solution (catalog No. C1052; Beyotime Institute of Biotechnology) for 30 min in darkness after rinsing with phosphate buffered solution (PBS) for two times. Cell cycle changes were detected by BD flow cytometer (Accuri C6; Franklin Lakes, NJ, USA). The results were analyzed using FlowJo 10.0 software (Tree Star, Inc., Ashland, OR, USA). G0, G0 phase, quiescent period of the cell cycle; G1, G1 phase, the first stage of the cell cycle; G2, G2 phase, mitotic preparation period of the cell cycle; M, M phase, mitotic period of the cell cycle; S, S phase, DNA synthesis stage of the cell cycle.
Statistical analysis
All statistical analysis involved in the study were carried out by SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA). Data is presented as median ± standard error. Student t-test was employed for comparison between groups. Chi-squared tests and Fisher’s exact tests were employed to analyzed the clinical information. Kaplan-Meier method was employed to further analyze prognosis and survival information of HCC patients. Multivariate Cox proportional hazards model was employed to further analyze clinical data. There is a statistically significant difference when P value is less than 0.05.
Results
Nucleoprotein HMGB2 is overexpressed in HCC
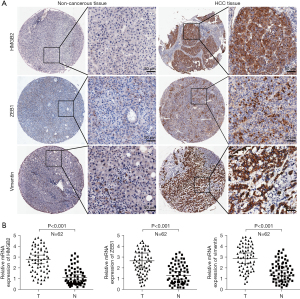
HMGB2 protein expression level of 62 patients was evaluated in a paraffin-embedded tissue microarray. Our findings revealed that the protein expression of HMGB2 was elevated in 30 HCC patients, and predominantly located in the nucleus and cytoplasm (Figure 1A). In para-cancerous liver tissues the protein level of HMGB2 was lower. Similar results could also be found in mRNA expression detection (Figure 1B, P<0.001). This result was also confirmed by western blot analysis with HCC cell lines. HMGB2 expression increased in HCC cells, particularly in MHCC97H and LM3 cells which are generally considered with high metastatic potential HCC cells (Figure 2A, P<0.05), compared to normal liver cells (THLE2). Based on the high metastatic potential characteristics and high expression of HMGB2 in MHCC97H and LM3 cells, we ultimately chose these two cells for subsequent experiments. Our findings suggested ZEB1 and vimentin protein expression were higher in HCC samples accompanied with an increase of HMGB2 and mRNA and protein (Figure 1A,1B, P<0.001). ZEB1 is a transcription factor that promotes metastasis and stem cell characteristics (14). Epithelial mesenchymal transition (EMT) is considered a crucial mechanism of tumor metastasis, and vimentin is one of the most important protein markers related to metastasis in this process (15). These results suggested that HMGB2 was abnormally overexpressed in HCC tissue samples and cell lines. Furthermore, the protein expression of HMGB2, ZEB1 and vimentin were explored in early and advanced stage HCC tissues. It was observed that HMGB2, ZEB1 and vimentin were expressed at higher levels in advanced HCC samples (Figure S1), indicating that these proteins might be related with the malignant development of HCC. HMGB2, a nucleoprotein, can regulate various biological processes by interacting with transcription factors such as p53, p73, lymphoid enhancer-binding factor 1 (LEF1), and Runt-related transcription factor 2 (Runx2) (16-19). Nuclear transcription factor ZEB1 has been reported to facilitate tumor progression in inducing EMT of tumor cells. In addition, the protein level of ZEB1 is positively correlated with vimentin expression (20). Additionally, the present results revealed that HMGB2 could affect the expression of the ZEB1/vimentin axis.
HMGB2 deprivation restricts the proliferation, migration and invasion ability of HCC cells in vitro
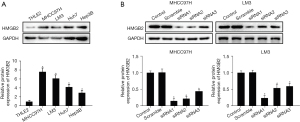
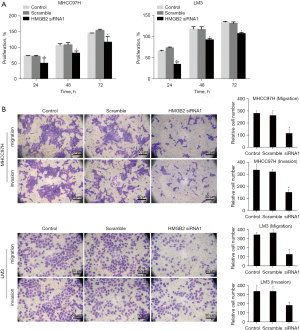
Firstly, the effect of HMGB2-knockdown in MHCC97H and LM3 HCC cells was assessed by western blotting. siRNA1 presented the strongest interference function (Figure 2B, P<0.05). The HCC cells transfected with HMGB2 siRNA had a weaker proliferative capacity compared to the control and scramble groups (Figure 3A, P<0.05). The results of the transwell assay suggested that HMGB2 siRNA1 dramatically inhibited the migratory and invasive potential compared to control and scramble groups (Figure 3B, P<0.05). Notably, the inhibition of proliferation and mobility potential of HCC cells in vitro could be partially restored by recombinant human vimentin protein (10 µM) (Figure S2, P<0.05). Surprisingly, HMGB2 showed no significant effect on the cell cycle (Figure S3). The above findings suggest that decreased expression of HMGB2 could restrict the malignant proliferation, migration, and invasion of HCC cells.
HMGB2 knockdown affects the malignant characteristics of HCC cells via regulating the ZEB1/vimentin axis
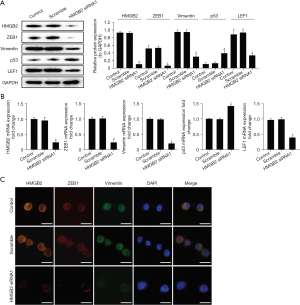
With the aim of evaluating the molecular mechanism of HMGB2 in HCC cells, the relative expression levels of ZEB1/vimentin axis-associated protein were evaluated by western blotting and immunochemical staining. Our present results demonstrated that HMGB2 knockdown in HCC cells decreased ZEB1 and vimentin expression (Figure 4A,4B). As a nucleoprotein, HMGB2 can regulate various biological processes by binding transcription factors, such as p53, p73, LEF1 and Runx2. In our present study, p53 protein level was elevated and LEF1 decreased following the inhibition of HMGB2 expression (Figure 4A,4B, P<0.05). In addition, immunochemical staining demonstrated that ZEB1 and vimentin expression decreased following HMGB2 knockdown in HCC cells (Figure 4C). Overall, HMGB2 overexpression may activate the ZEB1/vimentin axis in HCC.
Correlations of HMGB2 in HCC with clinicopathological indicators
Differences between clinicopathological indicators of HCC subtypes with high or low HMGB2 expression are presented in Table 1. HMGB2-high HCC subtypes were significantly associated with serum α-fetoprotein (AFP) level (P=0.007), tumor size (P<0.001), intrahepatic metastasis (P=0.014), vascular invasion (P=0.008) and early recurrence (P=0.043).
Based on the analysis of multivariate Cox’s proportional hazard models, clinicopathologic features were selected to be further evaluated (Table 2). HMGB2 (P=0.038), vascular invasion (P=0.005) and early recurrence (P=0.001) were independent factors for prognosis.
Table 2
| Term | Risk ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age | 0.532 | 0.198–1.428 | 0.210 |
| Gender | 1.083 | 0.490–2.395 | 0.843 |
| Etiology | 1.446 | 0.854–2.449 | 0.170 |
| Background liver pathology | 2.040 | 0.760–5.475 | 0.157 |
| Serum AFP | 0.372 | 0.136–1.018 | 0.054 |
| Tumor size | 1.322 | 0.395–4.425 | 0.650 |
| Intrahepatic metastasis | 3.412 | 0.759–15.328 | 0.109 |
| Vascular invasion | 3.494 | 1.454–8.396 | 0.005 |
| Tumor encapsulation | 1.703 | 0.682–4.256 | 0.254 |
| Early recurrence | 4.408 | 1.897–10.238 | 0.001 |
| TNM stage | 1.950 | 0.876–4.339 | 0.102 |
| HMGB2 | 2.674 | 1.058–6.763 | 0.038 |
Multivariate Cox proportional hazards model were employed to further analyze clinical data. AFP, α-fetoprotein; TNM, tumor, lymph node and metastasis; HMGB2, high mobility group box 2.
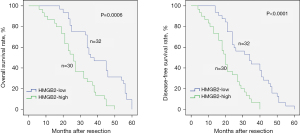
HCC subtypes with high HMGB2 expression predict a poor prognosis
As presented in Figure 5, HCC cases with elevated HMGB2 expression were associated with a lower overall survival rate (P=0.0006) and disease-free survival rate (P<0.0001) compared to HCC patients with decreased HMGB2 expression.
Discussion
HCC is currently the sixth most prevalent malignant cancer and the third most lethal type of tumor worldwide (21-23). Early recurrence and metastasis postoperatively are the most impactful indicators affecting the long-term survival of HCC (24-26). Therefore, identifying novel predictors of recurrence and metastasis is essential for the clinical therapy of HCC.
HMGB2 is a crucial member of HMG protein superfamily, which contains a DNA domain (HMG-box) (27). HMGB2, a nucleoprotein, can regulate various biological processes by interacting with transcription factors such as p53, p73, LEF1, and Runx2. (16-19). In addition, HMGB2 can regulate the development and differentiation of different cells. In recent years, accumulating studies (9-12) have shown that abnormal elevation of HMGB2 can play an important role as a biomarker of tumor progression, metastasis, differentiation and it can also predict the prognosis of patients. However, up to now, the explicit function of HMGB2 in HCC is still a mystery.
Previous studies have demonstrated that HMGB2 is abnormally expressed in human cancers and contributes to the regulation and maintenance of malignant biological phenotype and clinical prognosis of patients (9-12). In this study, we further demonstrated that HMGB2 was overexpressed in HCC samples and cell lines, particularly in advanced HCC tissues compared to para-cancerous liver tissues. Further analysis between clinicopathological data and HMGB2 confirmed that the high expression of HMGB2 was closely related with serum AFP level, tumor size, intrahepatic metastasis, vascular invasion and early recurrence. Therefore, it is speculated that the abnormal overexpression of HMGB2 may participate in multiple stages of malignant progression of HCC.
HMGB2 siRNA was taken to eliminate the expression in MHCC97H and LM3 HCC cells. Our findings suggested that the proliferation and mobility potential of HCC cells were dramatically inhibited in vitro. Although the standard deviation may be caused by some deviation of cell count, the overall trend of the experimental results is consistent, repeatable and reliable. These results confirmed that HMGB2 participates in the regulation of malignant biological characteristics of HCC cells, such as proliferation, migration and invasion.
Mechanistically, we demonstrated that HMGB2 promotes malignant biological behaviors which are dependent on ZEB1-vimentin axis. Vimentin as an intermediate filament protein, is an important marker of the mesenchymal phenotype, which maintains the integrity of the cytoskeleton and promotes the motility of multiple tumor cells (28-30). Tumor cells often exhibit EMT phenotype during metastasis, and Vimentin promotes EMT by altering cell shape and motility characteristics. Proteomics related research reported that vimentin is a metastasis related factor of various malignant tumors, such as prostate cancer and breast cancer, which indicates that it may play an important role in tumor progression and become a potential biomarker of metastasis (31). ZEB1, a master regulator of the EMT, which regulates the expression of vimentin (32), has been reported to facilitate tumor invasion and metastasis by inducing EMT in various tumors. ZEB1 belongs to the E-box binding zinc finger protein family and promotes downstream gene transcription by binding to E-box promoter elements (33). High expression of ZEB1 is also common in highly metastatic epithelial tumor cells (34). The present results showed that HMGB2, ZEB1 and vimentin were overexpressed in a considerable number of HCC tissue samples. Following HMGB2 knockdown by siRNA, ZEB1 and vimentin expression levels decreased simultaneously. Therefore, it was speculated that HMGB2 may bind to the nuclear transcription factor ZEB1 directly or indirectly, affecting the expression and role of vimentin, which impacts on the malignant features of HCC by activating the HMGB2-ZEB1-vimentin axis.
A particularly interesting phenomenon was that HMGB2 particularly highly expressed in advanced HCC samples. It indicated that HMGB2 participated in the malignant development of HCC which could be used as a novel monitoring and evaluation index. The signal transduction in the tumor microenvironment is complex, accompanied by abnormal activation of HMGB2 in the early stage of tumor occurrence. The body may neutralize its expression through some unknown mechanism to maintain the homeostasis. As the disease progresses, when HMGB2 protein accumulates in sufficient, it will break the balance and activate a series of proteins related to invasion and metastasis, ultimately leading to malignant transformation of tumor cells such as invasion and metastasis. Besides, the inhibition of HCC cell proliferation and mobility in vitro could be partially restored by recombinant human vimentin protein. However, the specific mode of interaction and regulation of the HMGB2-ZEB1-vimentin axis needs to be verified by subsequent experiments. In future work, ZEB1 recombinant protein alone or in combination with vimentin is worthy of further study on ZEB1/vimentin signal pathway. HMGB2 as a drug target is also worth paying more attention to investigate in the future. For example, analyze its protein structure through X-ray crystallography for drug design and localization, and finally match drugs with targets. Meanwhile, biochip technology can also be employed to obtain tens of thousands of target information simultaneously, and achieve rapid screening and drug optimization.
Finally, the clinical and prognostic data of HCC patients indicated that elevated HMGB2 could be an independent factor to predict prognosis and survival. It also confirmed the perspective that HMGB2 was involved in tumor progression. Taken together, these data indicate HMGB2 participates in malignant progression of HCC, which expected to become an attractive molecular target for HCC in the future. Our current understanding of HMGB2 in HCC is far from fully explaining its mechanism. Our study uncovered several key observations and mechanisms of action on animal models or larger cohorts of patients which need to be further explored in the future research.
Conclusions
HMGB2 participates in the malignant development of HCC and it is a previously unknown correlation with ZEB1-vimentin axis. We broaden the knowledge of HMGB2 action in HCC progression. Considering the universal role of HMGB2 in promoting tumor cell proliferation and metastasis, combining vimentin inhibitor or ZEB1 inhibitor might improve the anti-tumor effect as a widely applicable new approach in cancer therapy in the future.
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers and editors.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK and MDAR reporting checklists. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-447/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-447/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-447/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-447/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Collection and use of clinical tissues, relevant pathological and follow-up data were approved by Biomedical Ethics Committee of The Third Affiliated Hospital of Navy Military Medical University (No. EHBHKY2020-02-003; Shanghai, China). Written informed consent was received from each patient prior to the surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu B, Shen H, He J, et al. Cytoskeleton remodeling mediated by circRNA-YBX1 phase separation suppresses the metastasis of liver cancer. Proc Natl Acad Sci U S A 2023;120:e2220296120. [Crossref] [PubMed]
- Chen C, Wang Z, Ding Y, et al. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol 2023;14:1133308. [Crossref] [PubMed]
- Xia P, Zhang H, Lu H, et al. METTL5 stabilizes c-Myc by facilitating USP5 translation to reprogram glucose metabolism and promote hepatocellular carcinoma progression. Cancer Commun (Lond) 2023;43:338-64. [Crossref] [PubMed]
- Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015;12:408-24. [Crossref] [PubMed]
- Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology 2018;67:422-35. [Crossref] [PubMed]
- Lee D, Taniguchi N, Sato K, et al. HMGB2 is a novel adipogenic factor that regulates ectopic fat infiltration in skeletal muscles. Sci Rep 2018;8:9601. [Crossref] [PubMed]
- Laurent B, Randrianarison-Huetz V, Maréchal V, et al. High-mobility group protein HMGB2 regulates human erythroid differentiation through trans-activation of GFI1B transcription. Blood 2010;115:687-95. [Crossref] [PubMed]
- Ronfani L, Ferraguti M, Croci L, et al. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development 2001;128:1265-73. [Crossref] [PubMed]
- Han Q, Xu L, Lin W, et al. Long noncoding RNA CRCMSL suppresses tumor invasive and metastasis in colorectal carcinoma through nucleocytoplasmic shuttling of HMGB2. Oncogene 2019;38:3019-32. [Crossref] [PubMed]
- Wu ZB, Cai L, Lin SJ, et al. High-mobility group box 2 is associated with prognosis of glioblastoma by promoting cell viability, invasion, and chemotherapeutic resistance. Neuro Oncol 2013;15:1264-75. [Crossref] [PubMed]
- Li W, Wang Q, Feng Q, et al. Oncogenic KSHV-encoded interferon regulatory factor upregulates HMGB2 and CMPK1 expression to promote cell invasion by disrupting a complex lncRNA-OIP5-AS1/miR-218-5p network. PLoS Pathog 2019;15:e1007578. [Crossref] [PubMed]
- Kwon JH, Kim J, Park JY, et al. Overexpression of high-mobility group box 2 is associated with tumor aggressiveness and prognosis of hepatocellular carcinoma. Clin Cancer Res 2010;16:5511-21. [Crossref] [PubMed]
- Fan M, Shen J, Liu H, et al. Downregulation of PRRX1 via the p53-dependent signaling pathway predicts poor prognosis in hepatocellular carcinoma. Oncol Rep 2017;38:1083-90. [Crossref] [PubMed]
- Lobe C, Vallette M, Arbelaiz A, et al. Zinc Finger E-Box Binding Homeobox 1 Promotes Cholangiocarcinoma Progression Through Tumor Dedifferentiation and Tumor-Stroma Paracrine Signaling. Hepatology 2021;74:3194-212. [Crossref] [PubMed]
- Song Q, Han Z, Wu X, et al. β-Arrestin1 Promotes Colorectal Cancer Metastasis Through GSK-3β/β-Catenin Signaling- Mediated Epithelial-to-Mesenchymal Transition. Front Cell Dev Biol 2021;9:650067. [Crossref] [PubMed]
- Boonyaratanakornkit V, Melvin V, Prendergast P, et al. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol 1998;18:4471-87. [Crossref] [PubMed]
- Stros M, Ozaki T, Bacikova A, et al. HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem 2002;277:7157-64. [Crossref] [PubMed]
- Taniguchi N, Caramés B, Kawakami Y, et al. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via beta-catenin pathway. Proc Natl Acad Sci U S A 2009;106:16817-22. [Crossref] [PubMed]
- Taniguchi N, Caramés B, Hsu E, et al. Expression patterns and function of chromatin protein HMGB2 during mesenchymal stem cell differentiation. J Biol Chem 2011;286:41489-98. [Crossref] [PubMed]
- Zhou YM, Cao L, Li B, et al. Clinicopathological significance of ZEB1 protein in patients with hepatocellular carcinoma. Ann Surg Oncol 2012;19:1700-6. [Crossref] [PubMed]
- Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 2008;7:237-57. [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Shariff MI, Cox IJ, Gomaa AI, et al. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol 2009;3:353-67. [Crossref] [PubMed]
- Yeh CT, Huang YH, Liang KH, et al. Segregation of signaling proteins as prognostic predictors for local recurrence and distant metastasis in hepatocellular carcinoma. Int J Oncol 2014;44:491-504. [Crossref] [PubMed]
- Liu L, Dai Y, Chen J, et al. Maelstrom promotes hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition by way of Akt/GSK-3β/Snail signaling. Hepatology 2014;59:531-43. Erratum in: Hepatology 2016;63:1064. [Crossref] [PubMed]
- Okimoto K, Ogasawara S, Chiba T, et al. Successful resection of intracranial metastasis of hepatocellular carcinoma. Case Rep Gastroenterol 2013;7:182-7. [Crossref] [PubMed]
- Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog 2009;48:571-80. [Crossref] [PubMed]
- Eriksson JE, Dechat T, Grin B, et al. Introducing intermediate filaments: from discovery to disease. J Clin Invest 2009;119:1763-71. [Crossref] [PubMed]
- Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 2010;24:1838-51. [Crossref] [PubMed]
- Berr AL, Wiese K, Dos Santos G, et al. Vimentin is required for tumor progression and metastasis in a mouse model of non-small cell lung cancer. Oncogene 2023;42:2074-87. [Crossref] [PubMed]
- van Beijnum JR, Huijbers EJM, van Loon K, et al. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat Commun 2022;13:2842. [Crossref] [PubMed]
- Vardas V, Politaki E, Pantazaka E, et al. Epithelial-to-mesenchymal transition of tumor cells: cancer progression and metastasis. Int J Dev Biol 2022;66:277-83. [Crossref] [PubMed]
- Caramel J, Ligier M, Puisieux A. Pleiotropic Roles for ZEB1 in Cancer. Cancer Res 2018;78:30-5. [Crossref] [PubMed]
- Li D, Wang J, Zhang M, et al. LncRNA MAGI2-AS3 Is Regulated by BRD4 and Promotes Gastric Cancer Progression via Maintaining ZEB1 Overexpression by Sponging miR-141/200a. Mol Ther Nucleic Acids 2020;19:109-23. [Crossref] [PubMed]


