
Latin American young patients with gastric adenocarcinoma: worst prognosis and outcomes
Highlight box
Key findings
• The incidence of gastric cancer in young patients increased from 2% to 18%.
• Young female patients reported a high percentage of diffuse-type adenocarcinoma, signet ring cells, poorly differentiated tumors, and metastatic disease, with lower overall survival (OS) rates than man.
What is known and what is new?
• Young gastric cancer diagnosis has clinicopathological features correlated with a bad prognosis.
• This is one of the pioneer studies correlating age with gender and the prognostic factors in Latin-American population. Female patients reported more rates of metastatic disease, diffuse-type, signet-ring cells, poorly differentiated tumors, and worse rates of OS than male patients.
What is the implication, and what should change now?
• It is fundamental to increase knowledge in first-contact physicians to promote early diagnosis of gastric adenocarcinoma in young population.
• Screening campaigns at early-age are needed, and must include the awareness of the disease and the modifiable risk factors related.
Introduction
Gastric cancer is the 6th most common cancer and the 4th most frequent cancer related to death worldwide (1). In Mexico, gastric cancer is the 6th cause of cancer-related death (1). Incidence rates are twice as high in men, predominantly diagnosed in older age groups with a median age of 68 years old in USA, and 58 in Mexico (1,2).
Studies have indicated an increasing incidence of GC in the young population over the past decade (3,4). Young patients (aged 40 years or younger) diagnosed with GC incidence have increased from 4.6% to 6.2% out of the total cases in the USA (4), and up to 6% in Mexican population (2). GC in young patients, has been described to have several clinicopathological features that differ from elderly patients, including a higher female to male ratio, more diffuse type, poorly differentiated carcinoma, familial susceptibility of tumors, advanced stage of the disease and an increased familial cancer aggregation, as described in China, Korea, and Mexico (2,3,5,6). These clinicopathological features have been associated to an “aggressive growth pattern” which may lead to a different prognosis between young and elderly patient (5,7,8).
Recent studies, have demonstrated poor survival outcomes for young patients when compared to middle-age patients (35–64 years) and elderly patients (65–74 years) (9). Likewise, Wong et al. and Li et al. described that young population with GC showed a three-year OS of 6.9% (10,11). Other studies have also demonstrated that young populations with GC have lower progression free survival rates (P=0.012) (12), and higher cancer related mortality (P=0.048) (13), which can be explained by tumor location, ethnicity, tumor size, surgery, tumor, node, metastasis (TNM) stage, clinical stage of disease, poorly differentiated, signet cell type, and female gender prevalence (9,10,12-14).
On the other hand, studies revealed that younger patients showed better overall survival (OS) rates; 62.1% versus 28.1% for elderly patients ranging different races and TNM stage (14-16), Additionally several meta-analyses have associated young age with a better prognosis, favored by a good performance status, surgery and adjuvant chemotherapy (12,15,16). Moreover, other studies found no differences in survival rates between young and elderly patients (17,18). Therefore, the role of age in prognosis remains controversial (14) and many questions regarding carcinogenesis, treatment, prognosis and prevention remain unexplained (19,20), representing a challenge for physicians and research (19). For this reason, the present study aims to describe the prevalence, clinic-pathological features, and prognosis of young Latin American patients with gastric adenocarcinoma (GC). We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-259/rc).
Methods
This study is a retrospective, observational study that included the registry of patients treated at the National Cancer Institute from 2004 to 2020. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study has been approved by the Ethics and Clinical Research Committee of the National Cancer Institute, Mexico as a retrospective study (No. 2021/046), without risks for patients since data was obtained from clinical records. Therefore, the approval of informed consent is not necessary.
Study size
In the present study a non-probabilistic consecutive sampling was used.
Inclusion/exclusion criteria
Inclusion criteria: patients diagnosed with GC, treated at the National Cancer Institute, Mexico, between January 2004 and December 2020. Exclusion criteria: loss of follow up, and abandonment, or patients with missing information.
Clinical data collection
Clinical data included age, gender, tumor size, TNM stage (defined by the eighth American Joint Committee on Cancer TNM system), surgical procedure, lymphadenectomy, histological differentiation, chemotherapy, radiotherapy, and at least 3-month follow-up status.
Statistical analysis
For all analyses, patients were classified into two groups: young (≤40 years) and elderly (>41 years). Statistical analysis was performed using SPSS v. 24 software. Continuous variables were expressed as mean ± standard deviation values or as median and range (minimum and maximum) values according to their distribution (normal vs. not normal). Categorical variables were expressed as percentages. Statistical comparisons among groups were performed using the t-test when data were normally distributed; otherwise, the Mann-Whitney U test was performed. Statistical significance differences were assessed when P was bilaterally <0.05.
OS analysis
OS was defined as the time from GC diagnosis to death by any cause. OS rates were calculated using the Kaplan-Meier method, and different variables were compared using the log-rank test. Statistical significance differences were assessed when P was bilaterally <0.05.
Results
Clinicopathological features
A total of 2,543 patients met the inclusion criteria. Only 15% (n=380) corresponded to young patients (≤40 years), and the remaining 85% corresponded to elderly patients. Nevertheless, the number of cases among the young population has been increasing over the years, from 6% in 2004 to 15% by 2020 (Figure 1). Besides, young females have shown an increased incidence by year with only 3% in 2004 up to 17% by 2020, compared to young males whose incidence have decreased from 15% to 9% between over the same period (Table 1). Among patients' clinical characteristics, young populations where predominantly female (54%), with histological classification of diffuse type adenocarcinoma (68%), signet ring cell component, which was identified in 72% of the patients, and poor differentiation in 90% of the cases. Compared to elderly patients who were mostly male (n=1,232), in male population, the diffuse type was observed in 43% of the cases, signet ring cell component in 50%, and poor differentiation in 76% (Table 1).
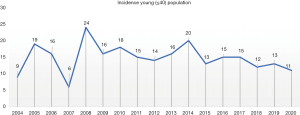
Table 1
| Characteristics | CS I–III | CS LA unresectable | CS metastatic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (≤40 years) | Elderly patient (>41 years) | P | Young (≤40 years) | Elderly patient (>41 years) | P | Young (≤40 years) | Elderly patient (>41 years) | P | |||
| Gender | |||||||||||
| Men | 12 (50%) | 125 (52%) | 1.000 | 39 (70%) | 309 (60%) | 0.153 | 124 (41%) | 798 (57%) | <0.001** | ||
| Woman | 12 (50%) | 117 (48%) | 17 (30%) | 209 (40%) | 176 (59%) | 606 (43%) | |||||
| Diffuse | |||||||||||
| Negative | 4 (17%) | 118 (49%) | 0.002** | 27 (48%) | 325 (63%) | 0.043** | 91 (30%) | 718 (51%) | <0.001** | ||
| Diffuse | 20 (83%) | 124 (51%) | 29 (52%) | 193 (37%) | 209 (70%) | 686 (49%) | |||||
| Grade | |||||||||||
| Well | 0 | 8 (3%) | 2 (4%) | 28 (5%) | 1 (0%) | 30 (2%) | |||||
| Moderated | 1 (4%) | 47 (19%) | 6 (11%) | 127 (25%) | 0.032** | 15 (5%) | 226 (16%) | <0.001** | |||
| Poor | 23 (96%) | 176 (73%) | 0.181 | 45 (80%) | 353 (68%) | 275 (92%) | 1,107 (79%) | ||||
| ND | 0 | 11 (5%) | 3 (5%) | 10 (2%) | 9 (3%) | 36 (3%) | |||||
| Signet ring cells | |||||||||||
| No | 3 (12%) | 116 (48%) | 0.001** | 22 (39%) | 285 (55%) | 0.018** | 71 (24%) | 635 (45%) | <0.001** | ||
| Yes | 21 (88%) | 126 (52%) | 34 (61%) | 233 (45%) | 229 (76%) | 769 (55%) | |||||
| HER2 | |||||||||||
| Negative | 22 (92%) | 181 (75%) | 0.078 | 52 (93%) | 426 (82%) | 0.057 | 261 (87%) | 1,005 (72%) | <0.001** | ||
| HER2 | 2 (8%) | 61 (25%) | 4 (7%) | 92 (18%) | 39 (13%) | 399 (28%) | |||||
| Surgery | |||||||||||
| Absent | 0 | 2 (1%) | 1.00 | 33 (59%) | 397 (77%) | 0.005** | 223 (74%) | 1,054 (75%) | 0.826 | ||
| Surgery | 24 (100%) | 240 (99%) | 23 (41%) | 119 (23%) | 77 (26%) | 350 (25%) | |||||
| Chemotherapy | |||||||||||
| Absent | 1 (4%) | 67 (28%) | 0.012** | 11 (20%) | 211 (41%) | 0.002** | 83 (28%) | 551 (39%) | <0.001** | ||
| Chemotherapy | 23 (96%) | 175 (72%) | 45 (80%) | 307 (59%) | 217 (72%) | 853 (61%) | |||||
Most young patients were diagnosed with metastatic disease (79%). Besides statistical differences were found between the clinicopathological characteristics and age. **, statistical significance. CS, clinical stage; LA, locally Advanced disease (unresectable); ND, undetermined.
By November 2010, the determination of Her2neu (performed by immunohistochemistry) was incorporated into the histological report. Her2 determination was available for 53 young patients, 45 of them were negative, and the remaining 8 positive. Contrarily, Her2 determination in elderly patients was available in 669 cases, 508 of them were negative, 91 Her2 3(+), 54 Her2 1(+), and 16 Her2 2(+).
The extent of the disease at the time of evaluation by Medical Oncology Department was based on operative and histological findings (in patients who underwent initial surgical exploration, either with staging or therapeutic purpose). In patients who did not undergo surgery, data was collected from clinical examination, CT scans and upper gastrointestinal endoscopy studies. Young patients’ most frequent clinical stage at diagnosis was metastatic (79%), followed by locally advanced disease (LA-unresectable) (15%), and early stages I–III (6%). Accordingly for elderly patients, 65% were diagnosed at metastatic disease, 24% LA-unresectable and the remaining 11% at early stages I–III (Table 1).
OS analysis
For OS analysis patients were subdivided into young and elderly. The First analysis included clinical stage, young patients diagnosed at stage I–III had a median OS of 33 months, versus elderly patients who had a median OS of 233 months (P=0.001) (Figure 2A). Likewise, young patients diagnosed at LA-unresectable stage of the disease showed a median OS of 20 versus 25 months for elderly patients (P=0.055) (Figure 2B). Regarding metastatic disease, young patients had a median OS of 8 months versus elderly patients who had a median OS of 13 months (P=0.001) (Figure 2C).

A second analysis was performed for mainly for advanced disease, comparing the 2 groups mentioned above (young vs. elderly) and sex, regarding advanced stages of the disease (LA-unresectable versus metastatic). Among patients with LA-unresectable disease, young female patients had a median OS of 8 months versus male patients, who had a median OS of 24 months (Figure 3A). Moreover, elderly female patients had a median OS of 20 versus 27 months for male patients (P=0.039) (Figure 3B). Likewise, amid patients with metastatic disease, young female patients had a median OS of 5 versus 11 months for male patients (P=0.001) (Figure 4A), moreover, elderly patients reported a median OS of 13 months for both female and male patients (P=0.994) (Figure 4B). Table 2 illustrates the univariate and multivariate analysis, variables such as female-gender, metastatic stage, diffuse type adenocarcinoma, poor differentiation and signet ring cell component remained predictors of OS in young population.
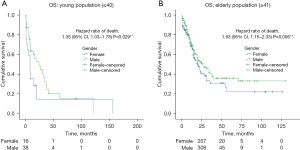
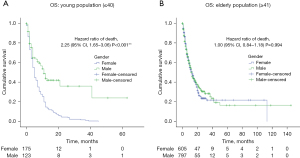
Table 2
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | ||
| Age | <0.001** | 1.98 (1.72–2.28) | <0.001** | 1.88 (1.62–2.19) | |
| Gender | 0.420 | 1.05 (0.929–1.19) | – | – | |
| Clinical stage | <0.001** | 4.69 (3.70–5.96) | <0.001** | 2.03 (1.71–2.41) | |
| Grade | 0.620 | 1.05 (0.829–1.20) | – | – | |
| Signet ring cells | <0.001** | 1.45 (1.27–1.64) | <0.001** | 1.44 (1.22–1.69) | |
| Her2 | 0.024** | 1.58 (1.06–2.36) | <0.017** | 1.63 (1.09–2.44) | |
| Surgery | <0.001** | 0.34 (0.29–0.39) | <0.001** | 0.46 (0.40–0.54) | |
| Chemotherapy | <0.001** | 0.43 (0.37–0.49) | <0.001** | 0.33 (0.29–0.39) | |
Illustrates the univariate and multivariate analysis, variables such as female-gender, metastatic stage, diffuse type adenocarcinoma, poor differentiation and signet ring cell component remained predictors of overall survival in young population. **, statistical significance. HR, hazard ratio; CI, confidence interval.
Discussion
This study describes the incidence and mortality of Latin patients diagnosed with GC.This type of cancer is the second most common neoplasm within gastrointestinal pathologies in our hospital and one of the leading cancer diagnoses and mortality in our country and the Latin Hispanic population (21,22). Even though the incidence and mortality of GC is mostly in patients aged 50 years or more, in the past 30 years, the diagnosis of GC has increased in younger adults (5,23,24). In the present study the incidence of GC in younger population increased from 2% up to 18% between 2004 and 2019.
In developed countries, incidence of GC is more predominant in male population with a ratio of 2.5:1 (men: women) (19,25). Nevertheless, in Latin and Asian population, this ratio decreases significantly to 2:1 (men: women) (3,25,26). In the present study, GC diagnosis is slightly more frequent in women with a ratio of 1:1.1 (men: women). Chen et al., reported that ratio distribution is modified according primary tumor location, such as distal location (2:1) and proximal (5:1) male: female (12). In our analysis the ratio was 1:1.2 (male: female) in distal gastric cancer and 2.5:1 (male: female) in esophagogastric junction.
Around 90–95% of gastric tumors are adenocarcinomas; according to Lauren’s classification, subtypes are divided into two: intestinal and diffuse type (27). Intestinal type is more common in older African-American men, while the diffuse type is similar between men and women (28,29). These three components such as diffuse type, signet ring cells, and a poorly differentiation have been described as poor prognosis features amid GC population (21).
The incidence of young age at the time of diagnosis has increased over the past decade, defined as “early onset of GC”, these populations have demonstrated to highlight the prevalence of these 3 features of bad prognosis, besides higher metastatic risk (19,21,30). Besides, the early onset of gastric cancer has also been associated with hereditary diffuse gastric cancer, which is an autosomal dominant cancer syndrome caused by the inactivation of germline mutations in E-cadherin, tumor suppressor gene (CDH1), and less frequently variants in CTNNA1. In this study, young patients reported to have a high percentage of diffuse type adenocarcinoma (70%), signet ring cells (72%) and poorly differentiated (87%). Nevertheless, the association with germline mutations have not been described in our population. On the other hand, when comparing among sex, these three poor prognostic features were higher in the female population with statistical significance, which may be one of the causes young female patients reported worse outcomes when compared with male patients. Along with these three poor prognostic factors, the Her2neu receptor has been described as an additional factor with implications for worse outcome of GC patients (31,32). The “ToGA study” reported a 22% prevalence of this receptor in the Mexican population, nevertheless, in our study this receptor was positive (Her2neu +++) in only 10% of the cases. This is mainly due the inclusion criteria differences in both studies (32,33).
The extent of the disease at the time of diagnosis is the main prognostic factor (19). Most GC patients are diagnosed at advanced stages of the disease (metastatic), as demonstrated in studies from different populations, such as Japanese (25%), Hispanic (45%), American (40%), and Chinese (30%). These findings are similar to the Mexican population included in the present study, where 75% of the patients were diagnosed at stage IV (metastatic disease). In addition, young population presented more metastatic disease (80%) than the elderly (66%). In Latin American population, there are several factors that may be leading to this delay in the diagnosis of GC: absence of awareness campaigns in the prevention and early detection among young population, lack of knowledge or update about GC disease, symptoms and screening in first contact-non oncologist physicians and easy access to drugs against gastritis, which only delays diagnosis at GC onset (4,12,19,34).
This information shows that young female patients are predominantly diagnosed at metastatic disease stage (59%), with tumor location at stomach (70%), histological classification of diffuse type (68%), signet ring cell component (72%); and poor differentiation (90%). All clinical features related to a bad prognosis of OS and outcome in other studies worldwide (14,24,35). Thus, we performed a sub-analysis regarding clinical stage of the disease. Patients with metastatic disease, showed statistical differences of median OS when comparing age and gender. Young females had a lower OS and a higher risk of death compared to young man. On the other hand, elderly males and females had similar median OS. Among the group of LA-disease, differences between gender and age where also observed, young females had lower OS and higher risk of death compared to young males with statistical differences between the groups. Also, when analyzing elderly patients, women had higher risk of death compared to elderly man. Our findings are in agreement with several publications, where female patients have worse OS than males, regardless of age, due to the greater presence of poor prognostic factors such as histology and clinical stage as mentioned before (4,21,30,36).
In addition, at the multivariate analysis, age (≤40 years), gender (female), clinical stage (IV), and primary tumor location (gastric) remained predictors of worst prognosis and OS. These variables have also been correlated among other studies including different population such as Chinese (9,35), Korean (3), American (29,34), and European (37,38), concluding that young female patients are commonly diagnosed at a metastatic stage of the disease and have worst outcome and higher risk of death.
This study has strengths and limitations; despite our findings, this study has some limitations, such its retrospective nature, the lack of associations with hereditary disease, probable E-cadherin and CDH1 gene mutations (12,39), and/or hormonal components, which have already been implicated in hereditary diffuse gastric cancer (32). It is also possible that many young patients are part of a hereditary-familial component of gastric adenocarcinoma in Mexico. On the other hand, this study has several strengths, including a new population described, the age described, and the fundamental differences demonstrated regarding the identification worse prognosis clinical factors among young female patients.
Conclusions
This is one of the pioneer studies correlating age, gender, and clinical stage in the Latin American population, supporting the idea that a global effort is required to improve awareness of the disease, prevention, as long as, early diagnosis, through screening campaigns in young populations. Besides educational programs in first contact physicians are fundamental to increase the knowledge of the disease and improve an early detection in young populations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-259/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-259/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-259/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-259/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study has been approved by the Ethics and Clinical Research Committee of the National Cancer Institute, Mexico as a retrospective study (No. 2021/046), without risks for patients since data was obtained from clinical records. Therefore, the approval of informed consent is not necessary.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Calderillo-Ruiz1 G, Takahashi A, Herrera M, et al. Gastric cancer in young Latin women: bad prognostic factors and outcome. Ann Oncol 2019;30:IV39.
- Wu SL, Zhang Y, Fu Y, Li J, Wang JS. Gastric cancer incidence, mortality and burden in adolescents and young adults: a time-trend analysis and comparison among China, South Korea, Japan and the USA. BMJ Open 2022;12:e061038. [Crossref] [PubMed]
- Moore A, Hikri E, Goshen-Lago T, et al. Young-onset gastric cancer and Epstein-Barr Virus (EBV) - a major player in the pathogenesis? BMC Cancer 2020;20:34. [Crossref] [PubMed]
- Jiang Y, Xie J, Huang W, et al. Chemotherapy Use and Survival Among Young and Middle-Aged Patients With Gastric Cancer. Clin Transl Gastroenterol 2020;11:e00253. [Crossref] [PubMed]
- Shah SC, Nunez H, Chiu S, et al. Low baseline awareness of gastric cancer risk factors amongst at-risk multiracial/ethnic populations in New York City: results of a targeted, culturally sensitive pilot gastric cancer community outreach program. Ethn Health 2020;25:189-205. [Crossref] [PubMed]
- Huang J, Ngai A, Lok V, et al. IDDF2021-ABS-0188 Worldwide incidence and lifestyle risk factors of gastric cancer among young adults: a global study. Gut 2021;70:A141-2.
- Jiang Y, Huang W, Xie J, et al. Young age increases risk for lymph node positivity in gastric cancer: A Chinese multi-institutional database and US SEER database study. J Cancer 2020;11:678-85. [Crossref] [PubMed]
- Afify AY. Incidence trends of gastric cancer among adolescents and young adults. J Gastroenterol Hepatol 2023;38:337. [Crossref] [PubMed]
- Wong MCS, Huang J, Chan PSF, et al. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw Open 2021;4:e2118457. [Crossref] [PubMed]
- Li H, Zhang H, Zhang H, et al. Survival of gastric cancer in China from 2000 to 2022: A nationwide systematic review of hospital-based studies. J Glob Health 2022;12:11014. [Crossref] [PubMed]
- Chen GL, Huang Y, Zhang W, et al. Three-Tier Prognostic Index in Young Adults With Advanced Gastric Cancer. Front Oncol 2021;11:667655. [Crossref] [PubMed]
- Kim M, Seo AN. Molecular Pathology of Gastric Cancer. J Gastric Cancer 2022;22:273-305. [Crossref] [PubMed]
- Cheng L, Chen S, Wu W, et al. Gastric cancer in young patients: a separate entity with aggressive features and poor prognosis. J Cancer Res Clin Oncol 2020;146:2937-47. [Crossref] [PubMed]
- Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine 2022;47:101404. [Crossref] [PubMed]
- Ueda Y, Shiraishi N, Fujishima H, et al. Technical and oncological safety of laparoscopic gastrectomy for gastric cancer in elderly patients ≥ 80 years old. BMC Geriatr 2022;22:475. [Crossref] [PubMed]
- Liao F, Guo X, Lu X, et al. A validated survival nomogram for early-onset diffuse gastric cancer. Aging (Albany NY) 2020;12:13160-71. [Crossref] [PubMed]
- Sandeep B, Huang X, Li Y, et al. Gastric Carcinoma in Young Patients and Its Clinicopathological Characteristics and Prognosis. Gastroenterol Res Pract 2020;2020:7378215. [Crossref] [PubMed]
- Li J. Gastric Cancer in Young Adults: A Different Clinical Entity from Carcinogenesis to Prognosis. Gastroenterol Res Pract 2020;2020:9512707. [Crossref] [PubMed]
- Li Y, Feng A, Zheng S, et al. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control 2022;29:10732748221099227. [Crossref] [PubMed]
- Icaza-Chávez ME, Tanimoto MA, Huerta-Iga FM, et al. The Mexican consensus on the detection and treatment of early gastric cancer. Rev Gastroenterol Mex 2020;85:69-85. (Engl Ed). [PubMed]
- Torres-Roman JS, Alvarez CS, Guerra-Canchari P, et al. Sex and age differences in mortality trends of gastric cancer among Hispanic/Latino populations in the United States, Latin America, and the Caribbean. Lancet Reg Health Am 2022;16:100376. [Crossref] [PubMed]
- Oh J, Abboud Y, Burch M, et al. Rising Incidence of Non-Cardia Gastric Cancer among Young Women in the United States, 2000-2018: A Time-Trend Analysis Using the USCS Database. Cancers (Basel) 2023;15:2283. [Crossref] [PubMed]
- Wong EYT, Goh WL, Hong J, Lam YCJ, Ng M, Poon EYL. 1468P Characteristics and prognosis of gastric cancer (GC) in adolescents and young adults (AYA) in Singapore. Ann Oncol 2020;31:S918. [Crossref]
- Zhou QP, Ge YH, Liu CY. Comparison of metastasis between early-onset and late-onset gastric signet ring cell carcinoma. BMC Gastroenterol 2020;20:380. [Crossref] [PubMed]
- Ma Z, Liu X, Paul ME, et al. Comparative investigation of early-onset gastric cancer. Oncol Lett 2021;21:374. [Crossref] [PubMed]
- Li B, Chen T, Liang D, et al. Comparison of clinical and pathological features between early-stage gastric-type and intestinal-type differentiated adenocarcinoma: a retrospective study. BMC Gastroenterol 2023;23:92. [Crossref] [PubMed]
- Setia N, Wang CX, Lager A, et al. Morphologic and molecular analysis of early-onset gastric cancer. Cancer 2021;127:103-14. [Crossref] [PubMed]
- Bergquist JR, Leiting JL, Habermann EB, et al. Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery 2019;166:547-55. [Crossref] [PubMed]
- Shi W, Wang Y, Xu C, et al. Multilevel proteomic analyses reveal molecular diversity between diffuse-type and intestinal-type gastric cancer. Nat Commun 2023;14:835. [Crossref] [PubMed]
- Rha SY, Chung HC. Breakthroughs in the Systemic Treatment of HER2-Positive Advanced/Metastatic Gastric Cancer: From Singlet Chemotherapy to Triple Combination. J Gastric Cancer. 2023;23:224-49. [Crossref] [PubMed]
- Xu Q, Xu X, Tang H, et al. Exploring potential molecular resistance and clonal evolution in advanced HER2-positive gastric cancer under trastuzumab therapy. Oncogenesis 2023;12:21. [Crossref] [PubMed]
- Holowatyj AN, Viskochil R, Ose D, et al. Diabetes, Body Fatness, and Insulin Prescription Among Adolescents and Young Adults with Cancer. J Adolesc Young Adult Oncol 2021;10:217-25. [Crossref] [PubMed]
- Tavakkoli A, Pruitt SL, Hoang AQ, et al. Ethnic Disparities in Early-Onset Gastric Cancer: A Population-Based Study in Texas and California. Cancer Epidemiol Biomarkers Prev 2022;31:1710-9. [Crossref] [PubMed]
- Puhr HC, Karner A, Taghizadeh H, et al. Clinical characteristics and comparison of the outcome in young versus older patients with upper gastrointestinal carcinoma. J Cancer Res Clin Oncol 2020;146:3313-22. [Crossref] [PubMed]
- Li L, Lau KS, Ramanathan V, et al. Ileostomy creation in colorectal cancer surgery: risk of acute kidney injury and chronic kidney disease. J Surg Res 2017;210:204-12. [Crossref] [PubMed]
- Machlowska J, Kapusta P, Baj J, et al. High-Throughput Sequencing of Gastric Cancer Patients: Unravelling Genetic Predispositions Towards an Early-Onset Subtype. Cancers (Basel) 2020;12:1981. [Crossref] [PubMed]
- Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, et al. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand J Gastroenterol 2020;55:62-6. [Crossref] [PubMed]
- García-Ruvalcaba A, Vázquez-Ibarra KC, Magaña-Torres MT, et al. Low expression of E-Cadherin and CDH1 variants associated with diffuse gastric cancer. Rev Invest Clin 2023;75:037-044.


