
Comparison of transarterial bland embolization and drug-eluting beads transarterial chemoembolization for very early and early hepatocellular carcinoma not amenable for surgery or ablation: a single center retrospective data analysis
Highlight box
Key findings
• Transarterial bland embolization (TAE) is equally safe and effective as drug-eluting beads transarterial chemoembolization (DEB-TACE) in very early and early-stage hepatocellular carcinoma (HCC).
What is known and what is new?
• According to current guidelines, TACE is recommended for the treatment of HCC.
• This study showed no difference in outcome, local response and toxicity of TAE compared with DEB-TACE in very early and early HCC.
What is the implication, and what should change now?
• TAE is safe and might be equally effective as DEB-TACE for very early and early HCC.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related mortality with increasing incidence worldwide. Current guidelines recommend transarterial chemoembolization (TACE) as accepted therapy for HCC classified as Barcelona Clinic of Liver Cancer (BCLC) intermediate stage B and as an alternative treatment option for patients with very early and early HCC (BCLC 0 and A) not amenable for resection, ablation or transplantation (1-4). For latter patients, TACE is the accepted embolization procedure, performed either with lipiodol or with drug-eluting beads (DEB) plus chemotherapeutic agents, despite very little evidence in the literature (5,6). So far, only two studies investigated the feasibility of doxorubicin-eluting bead TACE (DEB-TACE) in patients with very early and early HCC (7,8). No data for bland transarterial embolization (TAE) in patients with very early and early HCC is available. In addition, the superiority of DEB-TACE compared with TAE is still under debate. While TAE delivers its anti-tumoral activity only by vascular occlusion, DEB-TACE combines it with the effects of chemotherapy (9).
Up to date, only few studies addressed the non-inferiority of TAE compared with DEB-TACE in patients with HCC (10,11). Even though no study specifically compared DEB-TACE with TAE in very early or early-stage HCC, a previous published study investigated up to 24% of patients with BCLC A indicating that early HCCs are routinely treated by embolization techniques (11).
Overall survival (OS) is the benchmark of research and treatment choice for patients with HCC (12).
We therefore aimed to analyze the outcome, local response and toxicity of TAE compared with DEB-TACE in our own retrospective and monocentric patient cohort of very early and early HCC not amenable for resection or ablation. We present this article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-261/rc).
Methods
Study design
All patients with early or very early HCC receiving either DEB-TACE or TAE at the Bern University Hospital (Department of Visceral Surgery and Medicine, Interdisciplinary Center of vascular Interventions, and Radiology) in Switzerland between December 2009 and December 2019 were retrospectively enrolled. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Regional Ethics Review Board of Bern, Switzerland (No. KEK-Nr.2018-00416). All patients signed a general informed consent.
Study population
Patients were included with either histologically proven HCC or HCC diagnosis based on radiological non-invasive criteria according to the European Association of the Study of the Liver (EASL) guidelines (3). Each patient was presented and discussed individually at the institutional multidisciplinary tumor board. Embolization was recommended as treatment whenever the tumor was deemed unresectable and/or unablatable including patients awaiting liver transplantation (LT).
In this study we compare chemoembolization performed by DEB-TACE. Therefore, all interventions using lipiodol or ethanol were excluded. Further exclusion criteria were poor quality of pre- and postinterventional imagining without sufficient visibility of the lesions/treatment effect. Furthermore, patients receiving both kind of embolization, or patients with simultaneous systemic therapy were excluded.
Demographic and clinical data were retrieved from patient’s medical records. During the study period, 147 patients were treated with DEB-TACE or TAE, respectively.
Angiogram and embolization technique
DEB-TACE was the standard embolization technique at our hospital between 2009 and 2014 and was performed using doxorubicin as the chemotherapeutic agent, applicated by DEB (DC beads, Boston Scientific®, USA; Table S1). The loaded bead size was between 300–700 µm. For small and large supplying arteries, bead sizes of 100–300 and 500–700 µm were applied, respectively. Per embolization, a dose of 25–150 mg doxorubicin was administered. Since 2014, TAE became the standard embolization technique at our institution using microparticles or microspheres with a size of 100 µm up to 700 µm (Embozene, Varian®, USA; Hydropearls, Terumo®, Japan; PVA foam embolization particles, Cook®, USA; Table S1).
All embolizations were guided by 2D angiography and, if necessary and available, cone-beam computed tomography (CT) control. First overview angiographies of the coeliac trunk and the superior mesenteric artery were performed using 4–5 Charrière catheters as chosen by interventional radiologists’ preference. The embolization position was defined and the diagnostic catheter or a microcatheter was placed as desired. After angiography control of the correct catheter position the embolization was performed. The procedure was finished by a final angiogram from a central position.
For the TAE approach, the embolization was done as superselective as possible, meaning embolization in a subsegmental position as close to the tumor as possible. In general, for TAE the smallest possible particle size was used to achieve distal/intratumoral vessel occlusion, as this was thought to produce more necrosis. Overall goal was to use 100 µm microspheres, if considered safe by the interventionalist, judged by the angiography and previous imaging. If considered unsafe, larger spheres or PVA were used. For DEB-TACE, on the other hand, a more central embolization position was usually accepted. DC beads were used in all cases for DEB-TACE with a standard size of 300–500 or 500–700 µm. Endpoint of the embolization was the lack of tumor blush with obtained antegrade perfusion for DEB-TACE and complete stasis of the superselective tumor feeder for TAE, respectively.
Intervention time was considered as time between the first angiography and the final one.
Radiological analysis
Diagnosis of HCC was defined by typical behavior on liver imaging by multiphase contrast enhanced CT or multi-parametric contrast enhanced MRI (13). Radiological data were extracted from imaging files in the picture archiving and communication system (PACS) of the clinic. For this study, all hepatic lesions in all patients were retrospectively re-analyzed and re-confirmed by two radiologists with a subspecialisation in abdominal and liver imaging (Mertineit N and Maurer MH). All intra-procedural images/angiographies were retrospectively analyzed by a subspecialised interventional radiologist with training and experience in TAE and DEB-TACE (Mertineit N). Imaging follow-up was performed 1–3 months after the embolization and then 3-monthly by either magnetic resonance imaging (MRI), CT or contrast enhanced ultrasound (CEUS; since 2016 CEUS was additionally performed 2 weeks after TAE as an early assessment of treatment response). To evaluate treatment efficiency of the embolization, modified Response Evaluation Criteria in Solid Tumors (mRECIST) was used (Table 1) (14,15).
Table 1
| Categories | Description |
|---|---|
| CR | No sign of remaining viable tumor tissue with contrast enhancement |
| PR | Reduction of the sum of diameters of embolized target lesions ≥30% |
| SD | Neither response nor progression |
| PD | Increase of ≥20% of the sum of the diameters of the embolized lesions |
mRECIST, modified Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Outcome and variables
Local recurrence (LR) was defined as the presence of a detectable tumor after an initial complete response (CR). In contrast, new lesions within the liver other than the embolized tumor were considered as overall progression (OP).
Patients showing a partial response (PR) underwent re-TACE or re-TAE, if no contraindication was present. Cases were re-discussed in the interdisciplinary tumor board, if no CR was present after 2 TACE or TAE. All hepatic lesions were followed until recurrence or progressive disease (PD) and re-treatments like additional TACE or TAE, surgery or additional interventions were documented and were censored for the follow-up analysis. Further sub-group analyses according to the size of DC beads and the doses of doxorubicin within the DEB-TACE group as well as the comparison of size of microparticles in the TAE group in respect to local response were performed and showed no difference (see Table S2). Patient records were analyzed for post-interventional complications such as the commonly reported post-embolization syndrome (nausea, vomiting and abdominal pain) and constitutional syndrome (deterioration of the general condition) (16). At the beginning of the study period, embolizations were performed as outpatient procedures, what changed over time due to the changes in the reimbursement policy requiring a minimum 2-night hospital stay in order to not be deficient. Patients were admitted the day before the intervention and discharged one day after the intervention when pain-free and in an adequate general condition.
Statistical analysis
For statistical analysis using SPSS (version 25), t-test and Mann-Whitney U test was applied for continuous variables with normal and not normal distribution, respectively. For nominal variables, depending on the size, Chi-square test (total n>120) and Fisher’s exact test (total n<120) was performed, respectively. Survival data was evaluated by Kaplan-Meier curve. For the survival analysis, death or end of the study period was considered as the end of follow-up. The threshold for statistical significance was P≤0.05. Propensity score matching was not achievable due to size of patient groups.
Results
Clinical data
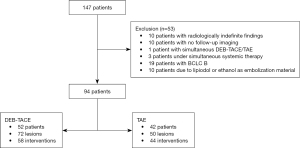
Fifty-three (36.1%) patients were excluded due to the defined exclusion criteria. For the final analyzes, 94 patients (52 DEB-TACE and 42 TAE) were included undergoing a total of 102 interventions of 122 HCC lesions (Figure 1).
Patient characteristics are summarized in Table 2. There was a predominance of male patients in both groups with no difference of the median age between the groups. At the time of intervention, the Model for End-stage Liver Disease (MELD), Child-Pugh classification and albumin-bilirubin (ALBI) score were not significantly different between DEB-TACE and TAE patients in both groups (Table 2). Patients treated with TAE had a more marked thrombocytopenia (DEB-TACE 120×109/L, TAE 85×109/L; Mann-Whitney U test, P=0.003; Table 2). In total, 22 patients had previously been treated for other HCC lesions, 11 (21.2%) patients in the DEB-TACE group and 10 (23.8%) patients in the TAE group, respectively (P=0.81, Fisher’s exact test; Table 2). Totally, 73 (77.7%) patients were treatment naïve, 41 (78.8%) patients in the TACE group and 32 (76.2%) in the TAE group, respectively (Table 2). Mean follow-up time was 39.0 months, with 42.4 months (range, 3–132 months) in the DEB-TACE group and 34.7 months (range, 1–102 months) in the TAE group (P=0.35).
Table 2
| Characteristics | DEB-TACE (n=52) | TAE (n=42) | P value |
|---|---|---|---|
| Gender (male), n (%) | 48 (92.3) | 37 (88.1) | 0.51 |
| Age (years), median [range] | 63 [45–84] | 65.5 [45–85] | 0.30 |
| MELD, median [range] | 9 [6–22] | 9 [6–22] | 0.38 |
| BCLC, n (%) | 0.77 | ||
| 0 | 8 (15.4) | 5 (11.9) | |
| A | 44 (84.6) | 37 (88.1) | |
| Child-Pugh classification, n (%) | 0.48 | ||
| A | 36 (69.2) | 25 (59.5) | |
| B | 16 (30.8) | 16 (38.1) | |
| C | 0 | 1 (2.4) | |
| Albumin-bilirubin score, n (%) | 0.36 | ||
| 1 | 9 (17.3) | 8 (19.0) | |
| 2 | 39 (75.0) | 27 (64.3) | |
| 3 | 4 (7.7) | 7 (16.7) | |
| Thrombocytes (×109/L), median [range] | 120 [60–319] | 85 [22–241] | 0.003 |
| AFP (kU/L), median [range] | 10.9 [1.3–9,207.7] | 6.1 [1.0–9,263.0] | 0.78 |
| Etiology of liver disease, n (%) | 0.357 | ||
| HCV | 8 (15.4) | 13 (31.0) | |
| HBV | 6 (11.5) | 3 (7.1) | |
| HCV + HBV | 0 | 1 (2.4) | |
| Alcohol | 18 (34.6) | 11 (26.2) | |
| NASH | 6 (11.5) | 2 (4.8) | |
| ASH/NASH | 3 (5.8) | 5 (11.9) | |
| Combination† | 3 (5.78) | 3 (7.1) | |
| Other‡ | 8 (15.4) | 4 (9.5) | |
| Embolization as first therapy, n (%) | 0.81 | ||
| Yes | 41 (78.8) | 32 (76.2) | |
| No | 11 (21.2) | 10 (23.8) | |
| Previous therapy, n (%) | |||
| Surgery | 6 (11.5) | 7 (16.7) | |
| Surgery + locoregional therapy | 2 (3.8) | 1 (2.4) | |
| Locoregional therapy | 2 (3.8) | 2 (4.7) | |
| Radiotherapy | 1 (1.9) | 0 | |
Mann-Whitney U test for continuous variables with not normal distribution. Fisher’s exact for nominal variables. †, combination: DEB-TACE: HBV/ASH [1], HCV/ASH [1], HBV/NASH [1]; TACE: HCV/ASH [1], HCV/NASH [2]. ‡, other: DEB-TACE: hemochromatosis [2], Alagille syndrome [1], idiopathic [3], primary biliary cholangitis [2]; TAE: autoimmune [1], idiopathic [1], hemochromatosis [2]. MELD, Model for End-stage Liver Disease; BCLC, Barcelona Clinic of Liver Cancer, AFP, alpha-fetoprotein; HCV, hepatitis C virus; HBV, hepatitis B virus; NASH, non-alcoholic steatohepatitis; ASH, alcoholic steatohepatitis.
Intervention-specific data
During the study period, 123 lesions were treated during 102 interventions (Table 2). In the DEB-TACE group 72 lesions were treated in 58 interventions and in the TAE-group 50 lesions in 44 interventions. While the mean number of performed embolizations was not different between the two groups [mean 1.44 (range, 1–3) for DEB-TACE group and mean 1.2 (range, 1–3) for TAE group; P=0.08; Mann-Whitney U test], the intervention time was 42.6 minutes in the DEB-TACE compared to 55.9 minutes in the TAE group (Mann-Whitney U test, P=0.08). Median tumor size at the time of intervention was 28.0 mm overall, 23.0 mm (range, 6–64 mm) in the DEB-TACE-group and 27.5 mm (range, 11–100 mm) in the TAE group (P=0.12; Student’s t-test).
In the DEB-TACE group, 1 lesion (1.4%) was embolized with DC beads 100–300 µm, 30 lesions (41.7%) with DC beads 300–500 µm, 1 (1.4%) with DC beads 500–700 µm and 36 lesions (50.0%) with DC beads 300–500 and 500–700 µm. The data for embolization materials were not available for 4 lesions (5.5%). Applied doxorubicin dose varied from 20 to 150 mg per embolization (median: 95.0 mg). Applied dose of chemotherapeutic agents could not be elicited for 8 (11.1%) lesions. For TAE different types of embolization agents were used including microparticles (n=35; 70%) and DC beads (n=15; 30.0%; see Table S1).
There were no significant differences in terms of adverse events. Side effects occurred after 11.8% of all interventions, showing no differences between the groups (P=0.35; Fisher’s exact test, Table 3). According to the Society of Interventional Radiology (SIR) classification of adverse events, one patient treated by TAE suffered a grade D category, namely septic shock of unknown origin and needed intensive care (17). Other complications included postembolic syndrome, constitutional symptoms or liver decompensation (Table 3).
Table 3
| Variables | DEB-TACE | TAE | P value |
|---|---|---|---|
| Treated tumors | 72 | 50 | |
| Tumor size (mm), median [range] | 23 [6–64] | 27.5 [11–100] | 0.12 |
| Number of interventions | 58 | 44 | |
| Intervention time | |||
| Available number | 52 | 42 | |
| Median [range], min | 42.6 [21.3–116.5] | 55.9 [12.5–223.4] | 0.08 |
| Side effects, n (%) | 5 (8.6) | 7 (15.9) | 0.35 |
| Postembolic syndrome | 2 (3.4) | 3 (6.8) | |
| Constitutional symptoms | 1 (1.8) | 3 (6.8) | |
| Liver decompensation | 2 (3.4) | 0 | |
| Septic shock | 0 | 1 (2.3) | |
| Hospitalization duration (days), mean [range] | 1.7 [1–8] | 2 [1–26] | <0.001 |
Mann-Whitney U test for continuous variables with not normal distribution and Student’s t-test for continuous variables with normal distribution, Fisher’s exact for nominal variables (n<120). DEB-TACE, drug-eluting beads transarterial chemoembolization; TAE, transarterial bland embolization.
Patients in the DEB-TACE-group had a shorter hospital stay than patients in the TAE-group (mean: 1.7 vs. 3.8 days; P<0.001, Mann-Whitney U test, Table 3).
Radiological response
The radiological analyses showed no significant difference in the radiological response between the DEB-TACE and the TAE group (P=0.59, Chi-square test; Table 4). At the first follow-up imaging 1–3 months after the first intervention, 33 (45.8%) out of 72 DEB-TACE-lesions and 24 (48.0%) out of 50 TAE-lesions showed a CR. PR rate and stable disease and PD rate were similar between the two groups. After up to a maximum of 3 embolizations (range, 1–3), a total of 42 (58.3%) DEB-TACE lesions and 25 (50.0%) TAE lesions showed a CR. Twenty-five (34.7%) of DEB-TACE lesions and 19 (38.0%) of TAE lesions showed a PR, 2 (2.8%) of DEB-TACE and 4 (8.0%) of TAE lesions showed stable disease. In the DEB-TACE group, 3 (4.2%) lesions presented with a PD compared to 2 (4.0%) lesions in the TAE group (P=0.53; Chi-square test), results are not shown in Table 4. Of the lesions showing a CR after 1–3 treatments, 17 (40.5%) of all 42 DEB-TACE lesions and 8 (33.3%) of all 24 TAE lesions re-occurred after a median time of 7.5 months (range, 1.8–62.0 months) and 19.4 months (range, 4.4–61.2 months), respectively (P=0.8, Chi-square test; P=0.20, respectively, Mann-Whitney U test, Table 4).
Table 4
| Variables | DEB-TACE (n=72) | TAE (n=50) | P value |
|---|---|---|---|
| Radiological response†, n (%) | 0.59 | ||
| Complete response | 33 (45.8) | 24 (48.0) | |
| Partial response | 37 (50.0) | 19 (38.0) | |
| Stable disease | 2 (2.8) | 5 (10.0) | |
| Progressive disease | 1 (1.4) | 2 (4.0) | |
| Local recurrence after complete response, n (%) | 17 (40.5) | 8 (33.3) | 0.70 |
| Time to local re-occurrence (months), median [range] | 7.5 [1.8–62.0] | 19.4 [4.4–61.2] | 0.8 |
| Local progression, n (%) | 9 (24.3) | 6 (31.6) | 0.66 |
| Time to local progression (months), median [range] | 9.1 [0.83–48.9] | 6.7 [1.0–11.0] | 0.74 |
| Subsequent treatment, n (%) | 28 (38.9) | 28 (56.0) | 0.06 |
Mann-Whitney U test for continuous variables with not normal distribution. Chi-square test and Fisher’s exact test for nominal variables. †, response after the first embolization. DEB-TACE, drug-eluting beads transarterial chemoembolization; TAE, transarterial bland embolization.
Fifty-four (44.3%) lesions with residual activity received either stereotactic microwave ablation, systemic therapy (sorafenib) or were operated including atypical resection, hemihepatectomy or LT. In the long-time follow-up, local PD occurred in 9 (24.3%) of 37 DEB-TACE lesions with PR and 6 (31.6%) of 19 TAE lesions with PR (P=0.74; Chi-square test; Table 4).
Progression free survival (PFS)
Overall disease progression occurred in 12 (23.1%) of 52 DEB-TACE patients and 6 (14.3%) of 42 TAE patients (P=0.31; Fisher’s exact test). Time to overall disease progression was not significantly different [mean 6.8 (range, 1.1–27.5) vs. mean 6.9 (range, 0.9–11.4) months; P=0.78; Mann-Whitney U test]. Further, PFS was not significantly different (P=0.41; Kaplan-Meier curve; Figure 2A). Patients with alpha-fetoprotein (AFP) >20 ng/mL (=16.5 kU/L) showed significant more overall disease progression (P=0.001; Kaplan-Meier curve, Figure S1). No other association was found (Table S3).
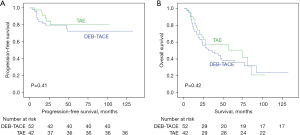
OS
Mean OS was 42.4 months (range, 3–132 months) for DEB-TACE patients and 34.7 months (range, 1–102 months) for TAE patients until death or end of the study period (P=0.42; Kaplan-Meier curve, Figure 2B). Additionally, mean survival considering transplantation or death as endpoint was not different (see Figure S2). The overall 1-, 2- and 3-year survival rate was 75.0%, 55.8% and 48.1% in the DEB-TACE group 81.0%, 52.4% and 35.7% in the TAE group, respectively (P=0.30; Fisher’s exact test). Again, patients with an AFP above 20 ng/mL (=16.5 kU/L) showed a significantly worse survival irrespective of whether they received DEB-TACE or TAE (P=0.005; Kaplan-Meier curve, Figure S3). No other association was found (Table S3).
During the study period, 29 (55.8%) DEB-TACE patients and 19 (45.2%) TAE patients were listed for LT (P=0.41, Fisher’s exact test). Seven (24.1%) of these 29 DEB-TACE patients and 4 (21.1%) of the 19 listed TAE patients dropped out of the waiting list due to disease progression (P=0.53, Fisher’s exact test, Table 4).
Post-transplant histological analysis
In total, 30 (81.1%) explanted livers of 37 transplanted patients with 32 (71.1%) of 45 embolized hepatic lesions were available for histological analysis (DEB-TACE =19, TAE =13). Six (31.6%) and 5 (38.5%) completely necrotic lesions were detected in DEB-TACE and TAE treated patients, respectively (P=0.50; Fisher’s exact test). Remaining vital HCC nodules were identified in 13 (68.4%) and 6 (46.2%) of DEB-TACE and TAE treated lesions, respectively. Two (15.3%) lesions with local progression were treated by microwave ablation after TAE so the effect of embolization could not be elicited.
Discussion
Our retrospective single-center analysis showed no significant differences in terms of local tumor control and OS in patients treated with DEB-TACE or TAE for very early and early HCC.
In 2009, Malagari et al. compared DEB-TACE with TAE finding better local response, fewer recurrences and a longer time to progression in favor of DEB-TACE without a survival benefit within 1 year (10). In contrast, the more recently published randomized controlled trial by Brown et al. found no difference in terms of response rates and survival comparing bland embolization with DEB-TACE (11). Our data supports the latter results for patients with early and very early HCC.
According to the BCLC guidelines, embolization is commonly recommended for patients with BCLC stage B in a non-curative approach (12,18). Furthermore, embolization is a therapeutic option for patients who do not qualify for resection or ablation or as a bridging or downstaging therapy for patients awaiting LT (19-21). In our cohort, 51.1% of all patients were listed for transplantation, of which 77.1% could be transplanted. Until 2014, patients with HCC in a very early or early stage, unsuitable for surgery or ablation, were routinely treated with DEB-TACE. In 2014 stereotactic microwave ablation was introduced offering an additional curative therapy for inoperable patients with HCC (20).
Despite the retrospective design of our study, the clinical features of the two cohorts were very similar, except for the degree of thrombocytopenia, which was significantly more severe in the TAE group. In the literature, most studies excluded patients with thrombocytopenia below 50×109/L or the thrombocytes were not considered in the patients’ characteristics at all (22,23). However, it’s known, that thrombocytopenia is correlated with the severity of portal hypertension in cirrhosis pointing to the fact that patients in the TAE group had a more advanced underlying liver disease compared to those in the DEB-TACE group (24).
In the literature, an upper size limit between 7 and 10 cm is discussed, in our patients, only 2 patients (4.8%) presented with a tumor above 7 cm, both treated by TAE (25-27).
We detected an overall complication rate of 11.8%, including 3.4% post-embolization syndromes in the DEB-TACE-group and 6.8% in the TAE-group, respectively, with no difference between the two treatment groups. The retrospective character of available studies may underestimate mild forms of post-embolization syndromes.
The length of hospital stay was also significantly different between the two groups, which can be explained in part by changing reimbursement policies in Switzerland during the study period requiring either an ambulant procedure or a 2-day hospital stay. Further reasons may include the fact that TAE is a painful procedure, even this study found no differences in the adverse event (28). Principally, however, embolization is an intervention that can be performed in an out-patient setting (18). However, a large range of hospitalization time up to 41 days has been described (29).
Due to the retrospective character of the study, embolization was not standardized. For DEB-TACE, DC beads were used and the particle size was chosen depending on the supplying artery of the hepatic lesion and the preferences of the interventionalist in charge. For TAE, overall size of the particles was smaller and more adapted to the individual patient as the most necrotic effect was expected if the embolization material reaches the microvasculature of the tumor. However, the subanalysis according to the size and type of the embolization material showed no significant difference.
To analyze the radiological effect, we used mRECIST criteria which are recommended to evaluate the response rate after embolization (30). The overall CR rate was 45.9% without significant difference between the 2 groups. Confirming this radiological finding, no difference of histologically proven necrosis was observed in the liver explants among the two groups. The much longer time to progression in the TAE group might be, at least partly, explained by the more selective and therefore maybe longer-lasting embolization. Due to the small sample size this would have to be validated in a separate and ideally prospective study.
For a more detailed analysis, we further stratified in OS and PFS. Differences were solely found in AFP >20 ng/mL (=16.5 kU/L) (Table S3 and Figure S1). Finally, OS, PFS and recurrence after CR was equal in both groups.
Even though combination of different therapies is known to potentiate the effect (23), this study is lacking a comparison with percutaneous or systemic treatments. In the recent years, the introduction of stereotactic image-guided microwave ablation led to a change in our treatment algorithm offering a curative approach even to invisible lesions or those being deemed as not amenable for ablation due to their complicated anatomic location in the liver (31,32).
Clearly, the retrospective character of the study, the potential bias due to the change in our treatment algorithm and the change of clinical practice over time as well as the small patient collective with no possible matching, limits the interpretation of our results. In particular, the further development of minimal invasive procedures, imaging modalities and also the compounds and devices used for embolotherapy in the treatment landscape of HCC most likely limit the interpretation of the current study results. On top of that, no comparison to conventional TACE is available. Nevertheless, our data demonstrate the feasibility and safety of TAE compared with DEB-TACE in early and very early-stage HCC, in particular considering the fact that patients in the TAE group had more advanced and larger tumors.
Conclusions
In conclusion, our monocentric retrospective study showed no difference in local tumor control and OS between DEB-TACE and TAE. These findings indicate that TAE might be a valid treatment alternative for patients with very early and early HCC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-261/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-261/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-261/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-261/coif). IB is receiving consulting fees/honoraria from educational grant Terumo und Boston Scientific. AB is receiving consulting fees from Boehringer Ingelheim and General Electrics. MHM has been receiving consulting fees/honoraria from Johnson&Johnson, Bayer Healthcare and CAScination. AL is receiving consulting fees/honoraria from Johnson&Johnson, Histoconics, CAScination and the Swiss Association of the Study of the Liver. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Regional Ethics Review Board of Bern, Switzerland (No. KEK-Nr.2018-00416). All patients signed a general informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth GS, Benhamou M, Teyssier Y, et al. Comparison of Trans-Arterial Chemoembolization and Bland Embolization for the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers (Basel) 2021;13:812. [Crossref] [PubMed]
- Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293-313. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817. [Crossref]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Malagari K, Alexopoulou E, Chatzimichail K, et al. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging 2008;33:512-9. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Kang YJ, Lee BC, Kim JK, et al. Conventional Versus Small Doxorubicin-eluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2020;43:55-64. [Crossref] [PubMed]
- Razi M, Safiullah S, Gu J, et al. Comparison of tumor response following conventional versus drug-eluting bead transarterial chemoembolization in early- and very early-stage hepatocellular carcinoma. J Interv Med 2021;5:10-4. [Crossref] [PubMed]
- Hong S, Choi WS, Purushothaman B, et al. Drug delivery in transarterial chemoembolization of hepatocellular carcinoma: Ex vivo evaluation using transparent tissue imaging. Acta Biomater 2022;154:523-35. [Crossref] [PubMed]
- Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. [Crossref] [PubMed]
- Brown KT, Do RK, Gonen M, et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol 2016;34:2046-53. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Elsayes KM, Hooker JC, Agrons MM, et al. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics 2017;37:1994-2017. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Vincenzi B, Di Maio M, Silletta M, et al. Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis. PLoS One 2015;10:e0133488. [Crossref] [PubMed]
- Leung DA, Goin JE, Sickles C, et al. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001;12:321-6. [Crossref] [PubMed]
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2017;28:1432-1437.e3. Erratum in: J Vasc Interv Radiol 2018;29:146. [Crossref] [PubMed]
- Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers (Basel) 2020;12:791. [Crossref] [PubMed]
- Makary MS, Khandpur U, Cloyd JM, et al. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel) 2020;12:1914. [Crossref] [PubMed]
- Lachenmayer A, Tinguely P, Maurer MH, et al. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int 2019;39:1975-85. [Crossref] [PubMed]
- Samant H, Amiri HS, Zibari GB. Addressing the worldwide hepatocellular carcinoma: epidemiology, prevention and management. J Gastrointest Oncol 2021;12:S361-73. [Crossref] [PubMed]
- Duran R, Chapiro J, Schernthaner RE, et al. Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: state of the art and future directions. Br J Radiol 2015;88:20140564. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. [Crossref] [PubMed]
- Mitchell O, Feldman DM, Diakow M, et al. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med 2016;8:39-50. [PubMed]
- Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212-20. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol 2017;67:173-83. [Crossref] [PubMed]
- Kucukay F, Badem S, Karan A, et al. A Single-Center Retrospective Comparison of Doxorubicin-Loaded HepaSphere Transarterial Chemoembolization with Conventional Transarterial Chemoembolization for Patients with Unresectable Hepatocellular Carcinoma. J Vasc Interv Radiol 2015;26:1622-9. [Crossref] [PubMed]
- Meyer T, Kirkwood A, Roughton M, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer 2013;108:1252-9. [Crossref] [PubMed]
- Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306. [Crossref] [PubMed]
- Tinguely P, Frehner L, Lachenmayer A, et al. Stereotactic Image-Guided Microwave Ablation for Malignant Liver Tumors-A Multivariable Accuracy and Efficacy Analysis. Front Oncol 2020;10:842. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]


