
Comparison of the effects of sufentanil-dominant anaesthesia/analgesia and epidural anaesthesia/analgesia on postoperative immunological alterations, stress responses and prognosis in open hepatectomy: a randomized trial
Highlight box
Key findings
• Intravenous opioid use appears not to be inferior to epidural anaesthesia and can be used safely in hepatocellular carcinoma (HCC) patients without worsening patients’ prognosis.
What is known and what is new?
• Many preclinical studies and retrospective studies suggest that perioperative opioid use may be linked to cancer-promoting effects and lead to inferior oncologic outcomes, while clinical results have been contradictory and elusive for perioperative opioid use.
• We designed a randomized controlled trial (RCT) in HCC patients. Our study was designed to find out whether sufentanil-dominant anaesthesia/analgesia was comparable to epidural anaesthesia/analgesia in affecting immunological alterations, perioperative stress response and also prognosis.
What is the implication, and what should change now?
• Our study results provide evidence for supporting perioperative opioid use in cancer patients without worsening short- and long-term prognosis. More large and well-designed RCTs are needed to ascertain the perioperative opioid use in other types of tumours and surgeries.
Introduction
Globally, cancer is the second major contributor to morbidity and mortality (1,2), and its burden is rapidly increasing. Hepatocellular carcinoma (HCC) is the most frequent primary type of liver cancer and the third leading cause of cancer-related death worldwide (3). Surgical resection is potentially curative with proven survival benefits (2,3), but the 5-year recurrence rate is surprisingly high (~60%) in patients who undergo hepatic resection (4).
Among various perioperative factors linked to cancer recurrence, anaesthesia-related factors, including anaesthetic agents, analgesia techniques and analgesia, remain elusive (5-9). Epidural anaesthesia is used to enhance recovery after surgery because it provides high-quality analgesia (10), thus attenuating the surgery-mediated stress response and preserving the patient’s innate immune system (8). A concern regarding thoracic epidural anaesthesia in hepatectomy is possible postoperative prolongation of the prothrombin time, which may delay epidural catheter removal and increase the administration of corrective blood products (11). In addition, a relative contraindication of epidural anaesthesia is thromboprophylaxis, which is common in patients with atherosclerotic cardiovascular disease, the leading cause of morbidity and mortality globally (12,13). Thromboprophylaxis is also widely used in the perioperative period for deep vein thrombosis prevention, which raises the same concern as that for hepatectomy based on the potential for epidural hematomas.
Unlike epidural anaesthesia, intravenous opioids, as an essential element of general anaesthesia and the standard potent pain-relieving agents for pain in the perioperative period (2,14,15), could be safely used in patients with coagulation disorders. However, in cancer patients, opioids often exert immunosuppressive effects, including modulating cellular and humoral responses, inhibiting natural killer (NK) cell cytotoxicity (16), directly acting on tumour cells, stimulating angiogenesis and interacting with the μ-receptor (17,18), in the regulation of tumour growth and metastasis. Many preclinical studies and retrospective studies suggest that perioperative opioid use may be linked to cancer-promoting effects and lead to inferior oncologic outcomes (19). However, the majority of research focusing on the immune system has involved in vitro or animal studies and clinical retrospective studies, which have low translational value, with most evidence deriving from laboratory and retrospective clinical studies (19). Well-designed randomized controlled trials (RCTs) are needed to clarify the relationship between cancer recurrence and anaesthesia-related factors.
In the development of HCC, immunoregulation plays a central role and the T helper (Th) cell subgroups are important parts in orchestrating host immune patterns in perioperative states (20). Under HCC scenarios, Th1 cell subsets mediate anti-tumour effects and result in tumour regression by producing interferon (IFN)-γ. In the meantime, Th2 and regulatory T (Treg) may in contrast inhibit the anti-tumour efficacy (21). Various anaesthesia managements may result in different polarizations of Th subsets, which determine the directions of anti-tumour responses.
In this study, we compared the effects of sufentanil-dominant anaesthesia/analgesia and epidural anaesthesia/analgesia in HCC patients undergoing open liver resection, including immunological alterations, perioperative stress response and also prognosis by assessing cytokines, immune cells (B cells, T cells, Th cell subsets, NK cells), hormonal changes, short and long-term follow-up (survival, metastasis and recurrence). This entire study was conducted to determine whether the effects of general anaesthesia combined with sufentanil target-controlled infusion (SGA) and patient-controlled intravenous analgesia (PCIA) on the immune system and tumour prognosis are comparable to those of general anaesthesia combined with epidural anaesthesia (EGA) and patient-controlled epidural analgesia (PCEA). We present this article in accordance with the CONSORT reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-711/rc).
Methods
Study design and patients
This study was a randomized, non-inferiority study conducted at a single site (Zhongshan Hospital Affiliated with Fudan University, Shanghai, China). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study procedures were approved by the Ethics Committee of Zhongshan Hospital affiliated with Fudan University (No. B2020-080R2), and informed consent was taken from all the patients. Patients aged 18–68 years with American Society of Anaesthesiologists (ASA) classification I–II who were diagnosed with liver neoplasms by magnetic resonance imaging (MRI) or ultrasound examination and scheduled to undergo elective open hepatectomy at Zhongshan Hospital were enrolled. The exclusion criteria were: (I) emergency operation; (II) contradictions of epidural (including abnormal coagulation function, platelet count under 100×109/L, and skeletal deformity); (III) allergies to drugs used in the study; (IV) pregnant or lactating; (V) severe heart, lung, liver, kidney, or endocrine disease; and (VI) use of immunosuppressive drugs or corticosteroids.
A total of 170 patients were ultimately enrolled in this study and all participants enrolled were randomly assigned (1:1) into one of two groups: the EGA group and the SGA group. The randomization sequence was generated by a study researcher who was blinded to the enrolment, using the RAND function in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) before patient recruitment. The allocation sequence was concealed from the researcher enrolling and assessing participants in sequentially numbered, opaque, sealed envelopes. The corresponding envelopes were opened only on the morning of the surgery.
Anaesthesia technique and grouping method
All patients received a preoperative intramuscular injection of midazolam (0.05 mg/kg; Jiangsu Enhua Pharmaceutical Co., Xuzhou, China). Subjects in the EGA group underwent epidural puncture at T8 and T9 for instillation of 2% lidocaine (3 mL; Shandong Hualu Pharmaceutical Co., Liaocheng, China) by catheter to confirm epidural analgesia, thus indicating that the epidural block was successful. Patients in both the EGA and SGA groups underwent right internal jugular vein catheterization, and the first blood sample was obtained. Routine monitoring parameters were established, including electrocardiogram (ECG), heart rate, peripheral capillary oxygen saturation (SpO2), capnography, central temperature, and invasive blood pressure.
As for our anaesthesia protocol, patients were induced with 4 µg/mL propofol (B. Braun Melsungen AG, Berlin, Germany, using the Marsh model) and 4 ng/mL remifentanil (Renfu Pharmaceutical Co., Beijing, China) by target-controlled infusion (TCI) (using the Braun Space) to the target plasma concentrations. Intravenous rocuronium (Zhejiang Xianju Pharmaceutical Co., Taizhou, China) was administered at a dose of 0.6 mg/kg. Patients then underwent intubation after about 120 s and were connected to a Drager Fabius anaesthesia machine (Dragerwerk AG, Lübeck, Germany) for the onset of surgery. Anaesthesia was maintained with sevoflurane [Drager AbbVie; minimal alveolar concentration (MAC), 0.8–1.2]. Patients in the EGA group were given a loading dose of 0.375% ropivacaine (10 mL; NAROPIN, AstraZeneca Pharmaceutical Co., Wuxi, China), 2 mg of morphine administered epidurally before the incision, and then 0.375% ropivacaine (4–5 mL) administered to maintain the epidural block every hour depending on the height and weight of the patient. Patients in the SGA group were given intravenous sufentanil (Renfu Pharmaceutical Co.) by TCI at a plasma concentration of 0.2–0.8 ng/mL, and the plasma concentration was set based on the haemodynamics and surgical procedure. The arterial blood pressure and heart rate fluctuations in both groups are controlled under 20%. Sufentanil TCI was stopped 30 min before the surgery ended. Before extubation, ramosetron was used for preventing postoperative nausea and vomiting (PONV). After extubation, all patients were monitored in the post-anaesthesia care unit for at least 50 min.
Postoperative analgesia
For patients in the EGA group, PCEA was administered using an electronic analgesia pump (Apon ZZB-300, Nantong, China). The analgesic drugs included ropivacaine (0.12%) and morphine (0.02 mg/mL), which were diluted in 0.9% normal saline to a final volume of 250 mL. The analgesia pump settings were as follows: background dose of 3 mL/h, bolus volume of 4 mL for each PCA aliquot and a lockout interval of 8 min. For patients in the SGA group, the PCIA pump contained sufentanil (1 µg/mL) and ramosetron (0.6 mg in total), which were administered via the jugular vein catheter. The pump had a total volume of 250 mL, a background dose of 0.03 mL/kg, a bolus volume of 5 mL, and a lockout interval of 8 min. The PCA pumps were removed 48 h after surgery. All patients were followed up on postoperative day (POD)0, POD1, and POD2 using a visual analogue scale (VAS) to evaluate their pain levels. Side effects, including PONV, urinary retention, pruritus, and respiratory depression, were also recorded after surgery.
Baseline and operative characteristics
The following baseline demographic and clinical characteristics were collected: age, sex, Child-Pugh score, and presence/absence of hepatitis. The following intraoperative characteristics were recorded: operation time, blood loss, and use of norepinephrine. The following postoperative characteristics were collected: postoperative pathological diagnosis; size, number, and stage of tumours; and length of hospital stay.
Study outcomes
IFN-γ, interleukin (IL)-4, and transforming growth factor (TGF)-β1 are considered to be the typical cytokines produced by Th1, Th2, and Treg cells, respectively. The primary study outcome was the plasma concentrations of IL-4/IFN-γ [baseline/before surgery (T0) and 24 h after surgery (T3)] because IFN-γ and IL-4 are most upregulated at T3 (20).
The secondary outcomes were the plasma concentrations of cortisol, tumour necrosis factor (TNF)-α, IL-6 [T0 and 1 h after surgery (T2)], glucose [T0, 5 min after portal block release (T1), and T2], IL-4, IFN-γ, and TGF-β1 (T0 and T3); the proportions of circulating CD3+, CD4+, CD8+, CD4+/CD8+ T cells, NK cells, and B cells [T0, T3, and on a POD5 (T4)]; the VAS scores, assessed at POD0, POD1, and POD2, both at rest and during active coughing; and length of hospital stay; the determination of 3-month and 1-year recurrence and mortality rates.
Blood samples and follow-up
Plasma was sampled at five-time points (T0, T1, T2, T3, and T4; Figure 1). At each time point, peripheral blood (30 mL in total) was drawn into ethylenediamine tetraacetic acid (EDTA)-spiked tubes and immediately transferred to the hospital laboratory for analysis of cortisol, IL-6, TNF-α and circulating immune cell levels. The plasma cortisol level was measured by radioimmunoassay, and circulating immune cell counts were measured by flow cytometry. For IFN-γ, IL-4, and TGF-β1 analysis, the samples were centrifuged and stored at −80 ℃ for use in the enzyme-linked immunosorbent assay. Patients were required to take routine test for surveillance, respecting ultrasound, tumour markers or computed tomography (CT)/MRI in 1 month, 3 months and 1 year after surgery. Telephone follow-up was used to determine patients’ survival.
Sample size calculation
The study was a non-inferiority RCT, designed to investigate whether the effect of Th cell subsets (IFN-γ/IL-4) in the SGA group at T3 was comparable to the EGA group. In a previous study, IFN-γ/IL-4 was {mean [standard deviation (SD)], 1.39 (1.00)} at T3 for patients undergoing general anaesthesia, and [mean (SD), 1.75 (2.27)] at T3 for epidural anaesthesia combined with general anaesthesia (22). We chose the non-inferiority margin (δ) as 0.35. NCSS-PASS 11 software (NCSS, Kaysville, UT, USA) was used to select the method of non-inferiority test for the mean of two independent samples, and α=0.05 and 1 − β=0.8 were set. The ratio of sample size in the two groups was 1:1, and the calculated sample size was 77 in each group, giving a total of 154. Considering missing samples, we added a 10% loss of follow-up rate, giving a final sample size of each group of 85, for a total of 170.
Statistical analysis
Statistical analyses were performed with SPSS 26.0 software (SPSS, Chicago, IL, USA). Continuous variables with normal distributions are presented as mean (SD) and compared by independent samples t-tests. Continuous variables with abnormally distributed data are presented as median [interquartile range (IQR)] and compared by the nonparametric Mann-Whitney U-test. Categorical variables are presented as numbers with percentages (%) and compared by the χ2 test. P<0.05 (two-side test) indicated statistical significance.
Results
Patients characteristics
Between October 2020 and February 2022, 180 patients were screened and 170 were ultimately included in the study. The CONSORT flow diagram (Figure 2) shows the details of patient assessment and exclusion. Tables 1,2 give an overview of the patients’ baseline characteristics and operative information, including age, sex, body mass index (BMI), tumour size, Barcelona Clinic Liver Cancer (BCLC) stage, preoperative liver cirrhosis, herpesvirus B infection, surgery-related factors, intensive care unit (ICU) stay, and hospital stay. The final diagnoses were confirmed by postoperative histological analyses. Among the 162 patients who underwent open hepatectomy and were analysed, 139 (85.8%) were diagnosed with HCC, 4 (2.5%) were diagnosed with intrahepatic cholangiocellular carcinoma (ICC), 5 (3.1%) were diagnosed with focal nodular hyperplasia (FNH), and 14 (8.6%) were diagnosed with other hepatic diseases (based on inflammatory changes). We compared the postoperative treatments for HCC patients, including transarterial chemoembolization, targeted therapy or none. No differences were found between the two groups (Table 2).

Table 1
| Characteristics | SGA (n=81) | EGA (n=81) | P value |
|---|---|---|---|
| Age (years) | 52.48 (1.10) | 53.20 (1.00) | – |
| Sex (male/female) | 71 (87.7) | 70 (86.4) | – |
| BMI (kg/m2) | 24.05 (2.91) | 23.46 (2.97) | – |
| Tumour size (≤5/>5 cm) | 48 (67.6) | 54 (66.7) | – |
| BCLC stage | |||
| BCLC 0 | 0 (0.0) | 0 (0.0) | – |
| BCLC A | 28 (34.6) | 26 (32.1) | 0.739† |
| BCLC B | 51 (63.0) | 53 (65.5) | 0.743† |
| BCLC C | 2 (2.4) | 2 (2.4) | >0.99† |
| Liver cirrhosis (yes/no) | 51 (63.0) | 44 (54.3) | – |
| HBeAg (+/−) | 60 (74.1) | 67 (82.7) | – |
| Blood loss (<200/≥200 mL) | 53 (65.4) | 49 (60.4) | – |
| Surgery time (≤3/>3 h) | 69 (85.2) | 64 (79.0) | – |
| Hepatic portal occlusion times (<2/≥2) | 45 (55.6) | 46 (56.8) | – |
| Postoperative ICU stay (yes/no) | 17 (21.0) | 22 (27.2) | – |
| Length of hospital stay (days) | 7.40 (1.80) | 7.80 (1.80) | – |
| Postoperative treatment for HCC | n=66 | n=73 | |
| TACE | 23 | 25 | 0.941† |
| Targeted therapy | 8 | 15 | 0.182† |
| None | 35 | 34 | 0.447† |
Data are expressed as mean (SD), n (%), or n. †, χ2 test. SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; BMI, body mass index; BCLC, Barcelona Clinic Liver Cancer; HBeAg, hepatitis B e antigen; ICU, intensive care unit; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; SD, standard deviation.
Table 2
| Parameters | SGA (n=81) | EGA (n=81) | P value |
|---|---|---|---|
| HCC | 66 (81.5) | 73 (90.1) | 0.115 |
| ICC | 3 (3.7) | 1 (1.2) | – |
| FNH | 4 (4.9) | 1 (1.2) | – |
| Other | 8 (9.9) | 6 (7.4) | – |
| HCC tumour stage (I–II/III–IV) | 54 (66.7) | 58 (71.6) | 0.725 |
Data are expressed as n (%). Analysis by χ2 test. SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocellular carcinoma; FNH, focal nodular hyperplasia.
Plasma concentrations of IFN-γ, IL-4, and TGF-β1
As shown in Figure 3 and Table 3, no significant differences in the plasma concentrations of IFN-γ, IL-4, IFN-γ/IL-4, and TGF-β1 were observed between the groups. In the two groups, the plasma concentrations of IL-4 and IFN-γ were increased slightly at T3 compared with the baseline values (T0). The IFN-γ/IL-4 ratio remained comparable between the two groups at T3 [median (IQR), 20.78 (12.73–29.18) vs. 19.52 (13.98–29.29), P=0.607]. The plasma concentration of TGF-β1 in the SGA group was increased and comparable to that of T3 in the EGA groups [median (IQR), 228.01 (61.33–372.48) vs. 238.01 (65.18–303.67) pg/mL, P=0.748].
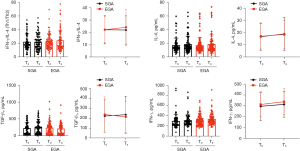
Table 3
| Parameters | SGA (n=81) | EGA (n=81) | P value |
|---|---|---|---|
| IFN-γ/IL-4 | |||
| T0 | 18.14 (14.58–30.54) | 20.35 (14.68–26.12) | 0.612 |
| T3 | 20.78 (12.73–29.18) | 19.52 (13.98–29.29) | 0.607 |
| IFN-γ (pg/mL) | |||
| T0 | 278.01 (217.38–334.82) | 291.89 (225.48–365.97) | 0.209 |
| T3 | 290.29 (242.80–354.80) | 310.02 (258.99–385.68) | 0.085 |
| IL-4 (pg/mL) | |||
| T0 | 13.48 (10.13–18.82) | 13.48 (11.23–19.26) | 0.594 |
| T3 | 15.11 (10.94–22.13) | 14.63 (11.33–20.58) | 0.905 |
| TGF-β1 (pg/mL) | |||
| T0 | 218.07 (62.09–344.06) | 239.00 (94.13–321.23) | 0.636 |
| T3 | 228.01 (61.33–372.48) | 238.01 (65.18–303.67) | 0.748 |
Data are expressed as median (IQR) and were compared by the Mann-Whitney nonparametric U-test. T0: baseline/before surgery; T3: 24 h after surgery. IFN, interferon; IL, interleukin; TGF, transforming growth factor; SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; IQR, interquartile range.
Circulating immune cells
As shown in Figure 4 and Table 4, the circulating levels of CD3+, CD4+, CD8+, and CD4+/CD8+ T cells were all decreased on POD1, but those of CD3+ and CD4+ T cells nearly recovered to baseline values at T4 in both groups; the ranges at T4 were comparable between the two groups. The CD4+/CD8+ ratio was also comparable between groups at T4 [median (IQR), 1.80 (1.40–2.30) vs. 1.70 (1.30–2.40)]. In both groups, the proportion of CD8+ T cells continued to decrease until T4. Moreover, as shown in Figure 4, the B cell proportion was obviously increased in both groups at T3 and remained nearly unchanged at T4; the proportions of NK cells were increased on T3 in both groups and decreased significantly on T4. The circulating immune cell levels did not significantly differ between the SGA and EGA groups at T0, T3, or T4.
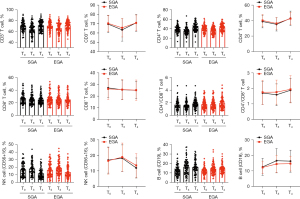
Table 4
| Parameters | SGA (n=81) | EGA (n=81) | P value |
|---|---|---|---|
| CD3+ T cells (%) | |||
| T0 | 69.50 (9.14) | 70.25 (8.80) | 0.876† |
| T3 | 64.10 (57.70–70.60) | 65.10 (58.55–73.70) | 0.252‡ |
| T4 | 70.89 (8.96) | 70.80 (8.51) | 0.374† |
| CD4+ T cells (%) | |||
| T0 | 38.63 (8.18) | 39.97 (7.75) | 0.763† |
| T3 | 34.98 (8.60) | 36.15 (7.58) | 0.944† |
| T4 | 42.80 (9.27) | 42.41 (8.04) | 0.357† |
| CD8+ T cells (%) | |||
| T0 | 26.00 (20.35–32.65) | 24.80 (20.15–29.05) | 0.382‡ |
| T3 | 24.30 (18.20–28.80) | 23.80 (19.40–29.60) | 0.888‡ |
| T4 | 23.50 (19.40–28.35) | 23.80 (19.55–28.65) | 0.932‡ |
| CD4+/CD8+ T cells | |||
| T0 | 1.60 (1.30–2.10) | 1.60 (1.20–2.15) | 0.206‡ |
| T3 | 1.50 (1.15–1.95) | 1.60 (1.20–2.15) | 0.585‡ |
| T4 | 1.80 (1.40–2.30) | 1.70 (1.30–2.40) | 0.988‡ |
| B cells (%) | |||
| T0 | 12.00 (8.60–15.75) | 12.40 (10.00–14.50) | 0.949‡ |
| T3 | 17.00 (0.15–21.15) | 13.50 (10.70–17.40) | 0.071‡ |
| T4 | 15.30 (12.55–20.25) | 14.40 (11.45–16.50) | 0.162‡ |
| NK cells (%) | |||
| T0 | 16.20 (9.35–24.35) | 15.40 (10.05–22.90) | 0.813‡ |
| T3 | 17.00 (12.00–24.00) | 17.50 (12.15–24.80) | 0.615‡ |
| T4 | 10.60 (6.90–15.90) | 11.60 (8.30–18.65) | 0.164‡ |
Data are expressed as mean (SD) or median (IQR). †, independent sample t-test; ‡, Mann-Whitney nonparametric U-test. T0: baseline/before surgery; T3: 24 h after surgery; T4: POD5. SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; NK, natural killer; SD, standard deviation; IQR, interquartile range; POD, postoperative day.
Plasma concentrations of cortisol, glucose, IL-6, and TNF-α
As shown in Figure 5 and Table 5, the plasma levels of TNF-α at T2 were significantly higher in the SGA group than in the EGA group [median (IQR), 7.45 (6.20–9.80) vs. 5.95 (4.95–7.45) pg/mL, P<0.001]. Similarly, the plasma IL-6 and cortisol levels were notably increased in both groups at T2 but showed comparable ranges. The blood glucose concentrations were remarkably increased in both groups at T1 compared with those at T0 and recovered toward the baseline at T2.

Table 5
| Parameters | SGA (n=81) | EGA (n=81) | P value |
|---|---|---|---|
| IL-6 (pg/mL) | |||
| T0 | 3.75 (2.50–6.00) | 3.70 (2.70–6.20) | 0.997‡ |
| T2 | 76.30 (40.00–110.00) | 49.20 (33.60–109.50) | 0.083‡ |
| TNF-α (pg/mL) | |||
| T0 | 6.75 (5.55–9.70) | 6.10 (5.20–8.85) | 0.147‡ |
| T2 | 7.45 (6.20–9.80) | 5.95 (4.95–7.45) | <0.001**‡ |
| Cortisol (nmol/mL) | |||
| T0 | 303.50 (233.00–376.50) | 283.00 (223.00–375.50) | 0.766‡ |
| T2 | 557.13 (163.53) | 530.36 (170.93) | 0.799† |
| Glucose (mmol/mL) | |||
| T0 | 5.65 (5.10–6.45) | 5.60 (5.20–6.20) | 0.737‡ |
| T1 | 10.38 (2.67) | 10.69 (2.65) | 0.646† |
| T2 | 8.15 (7.05–9.55) | 8.30 (6.95–9.75) | 0.783‡ |
Data are expressed as median (IQR) or mean (SD). †, independent sample t-test; ‡, Mann-Whitney nonparametric U-test; **, compared between the two groups, P<0.01. T0: baseline/before surgery; T1: 5 min after portal block release; T2: 1 h after surgery. IL, interleukin; TNF, tumour necrosis factor; SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; IQR, interquartile range; SD, standard deviation.
VAS scores
As shown in Table 6, the VAS scores (at rest, and while coughing) of the SGA and EGA groups were not different on POD0. Notably, on POD1 and POD2, significant differences were observed, because more patients suffered moderate pain both at rest and while coughing in the SGA group than in the EGA group.
Table 6
| Parameters | SGA (n=81) | EGA (n=81) | P value | |||
|---|---|---|---|---|---|---|
| 0–3 | 4–6 | 0–3 | 4–6 | |||
| POD0at rest | 74 | 7 | 79 | 2 | 0.086 | |
| POD1at rest | 72 | 9 | 79 | 2 | 0.029* | |
| POD1while coughing | 42 | 39 | 71 | 10 | 0.000** | |
| POD2at rest | 74 | 7 | 81 | 0 | 0.007** | |
| POD2while coughing | 58 | 23 | 77 | 4 | 0.000** | |
Data are expressed as n. VAS scores: 0–3 represents mild pain, 4–6 represents moderate pain. Data were compared by χ2 test. *, compared between the two groups, P<0.05; **, compared between the two groups, P<0.01. VAS, visual analogue scale; SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; POD, postoperative day.
Recurrence, metastasis and survival
In this perspective cohort, 139 of 162 patients were diagnosed as HCC and included in the 3-month and 1-year follow-up. As shown in Table 7, there were 3 (4.5%) HCC recurrence in the SGA group and 4 (5.5%) in the EGA group; one patient in the SGA group died during the 3-month follow-up period, but none in the EGA group. In the 1-year follow-up, there were 10 cases of recurrence in the SGA group and 14 in the EGA group. As for HCC metastasis, there were 2 (3.0%) in SGA, 1 for lung metastasis, 1 for bone metastasis, 1 (1.4%) was found in EGA (lung metastasis). No significant difference was found in either short- or long-term follow-up for HCC recurrence, metastasis and survival in both groups.
Table 7
| Parameters | SGA (n=66) | EGA (n=73) | P value |
|---|---|---|---|
| 3-month | |||
| Recurrence (y/n) | 3 (4.5) | 4 (5.5) | 0.801 |
| Metastasis (y/n) | 0 (0.0) | 0 (0.0) | – |
| Death (y/n) | 1 (1.5) | 0 (0.0) | 0.291 |
| 1-year | |||
| Recurrence (y/n) | 10 (15.2) | 14 (19.2) | 0.531 |
| Metastasis (y/n) | 2 (3.0) | 1 (1.4) | 0.501 |
| Death (y/n) | 1 (1.5) | 0 (0.0) | 0.291 |
Data are expressed as n (%). SGA, general anaesthesia combined with sufentanil target-controlled infusion group; EGA, general anaesthesia combined with epidural anaesthesia group; y/n, yes/no.
Discussion
Surgery is the mainstay treatment for patients with hepatic carcinoma. In addition to the surgical technique and (neo)adjuvant therapies, anaesthesia- and analgesia-related factors have attracted increasing attention due to their potential influence on postoperative outcomes (5,18). Our current study showed that the effect of perioperative intravenous opioids on the postoperative immune response was comparable to that of epidural anaesthesia.
Systemic immune responses to surgical stress include increased tumour-promoting Treg and Th2 cell counts as well as decreases in both the numbers and capacities of NK cells (innate immune system) and cytotoxic CD8+ T cells (adaptive immune system) (23,24). These alterations have been shown to increase the risk of cancer recurrence in both animal models (25-27) and a variety of tumour types in clinical scenarios (28). Furthermore, patients with advanced cancer have a Th2-dominant status (29). IFN-γ and IL-4 are cytokines produced by Th1 and Th2 cells, respectively (30). In the current study, the IFN-γ/IL-4 ratio was used to represent the Th1/Th2 balance (31-34). Although spinal anaesthesia with sevoflurane was shown to preserve IFN-γ/IL-4, whereas sevoflurane alone significantly reduced IFN-γ/IL-4 under laparotomy conditions in mice (30), our results showed that the IL-4 and IFN-γ levels as well as the IFN-γ/IL-4 ratio were comparable between the SGA and EGA groups on POD1, which indicated intravenous opioids did not further worsen the balance of Th1/Th2 cells. Unlike in the animal studies, sufentanil was administered systemically in our study which could partly explain the different results.
Compared with morphine, fentanyl and related agents are more effective at modifying the hormonal responses to surgery. TCI of opioids has been shown to improve intraoperative hemodynamic stability and decrease the intraoperative opioid requirements (35). TCI of sufentanil, even over a long period, has demonstrated predictive pharmacokinetic accuracy (36).
Cumulative evidence from retrospective and preclinical research shows that interventions used to modulate the sympathetic nervous system and hypothalamus-pituitary-adrenal (HPA) axis are helpful to reduce cancer recurrence. Systemic opioids are a vital part of the analgesic component of anaesthesia. Opioids modulate nociception at several levels of neuraxis and centrally mediated neuroendocrine responses to reduce the surgical stress response. Epidural blocks are also an effective sympathetic blockade strategy and attenuate immunosuppression when used in combination with general anaesthesia. In the current study, the levels of blood glucose and plasma cortisol, IL-6 and TNF-α were used to assess the intraoperative stress response. The levels of glucose, cortisol and IL-6 were comparable between the two groups, while the levels of TNF-α were significantly higher in the SGA group than that in the EGA group. The nervous system branches as an extension of the circulatory system, and peripheral nerve-driven neurotransmitters regulate the progression of diverse cancers (37). In addition to stress effects on tumours, the activation of sympathetic nerves promotes the metastasis of solid tumours by other mechanisms, including inflammation (38). Further studies should be performed to assess the effect of increased IL-6 and TNF-α levels on tumour progression.
Poorly controlled pain may drive malignant processes, possibly due to the increased activity of the sympathetic system and HPA axis, and the subsequent increases in catecholamine and glucocorticoid levels suppress the immune system (38). In addition to systemic opioids, postoperative epidural analgesia is recommended for patients undergoing upper abdominal and thoracic surgeries. Our study focused on perioperative immune changes after two anaesthesia strategies and compared the effectiveness of postoperative analgesics. Patients in both groups reported comparable pain control both at rest and while coughing on POD1, but patients in the EGA group reported better pain relief on POD2.
NK cells are key participants in effective tumour immunosurveillance, and the TGF-β1 cytokine is produced by Treg cells and correlates with reduced patient survival and recurrence-free rates (20). In an animal study, TGF-β1 was shown to promote Treg cell differentiation and was identified as an inhibitory mechanism of CD8+ T cells (39), which play opposing roles in antitumour surveillance (20). In a recent single-cell RNA sequencing (scRNA-seq) study, early-relapse tumours exhibited increased CD8+ T cell infiltration (40), and circulating CD4+ T cells are independent predictors of disease-free survival and overall survival after HCC resection (41). In this study, the TGF-β1 levels and numbers of postoperative NK and CD3+, CD4+, and CD8 T cells did not significantly differ between the two groups. The numbers of B lymphocytes (CD19+) were also comparable between the two groups.
Compared with epidural blockade, systemic opioids have more severe effects on immune functions, including attenuating innate immunity by inhibiting NK cytotoxicity and activating the HPA axis, consequentially resulting in immunosuppression (17,42). However, different opioids have different effects on NK cell cytotoxicity (17). In addition, NK cell cytotoxicity-related cancer outcomes have not been assessed in human clinical settings. Opioid receptors are reported to be expressed in cancer cells, thereby enabling the opioid-related stimulation of tumourigenic processes, including migration, angiogenesis, and metastasis (43). In clinical retrospective studies, conflicts exist regarding the influence of systemically used opioids on metastasis (18). Retrospective studies on the effect of neuraxial blockade also report mixed results regarding overall survival (17). Patients receiving abdominal surgery for cancer resection were randomized to receive either epidural analgesia or postoperative systemic morphine analgesia, with no difference in cancer recurrence and mortality rates at 2–3 years in either group (44). A prospective cohort study of 34,188 cancer survivors who had had surgery for early breast cancer showed that opioids were not associated with cancer recurrence (45). In a recent RCT on breast cancer, there was no difference in cancer recurrence rates between patients receiving regional anaesthesia analgesia and systemic opioids (46).
Some limitations of this study should be mentioned. The subjects of the study were mainly male patients, as HCC occurs more in male than female patients (with a 10:1 male-to-female ratio). Therefore, the results may not be representative of the whole population and other types of cancer. Second, the study was a single-site study with a small sample size. Third, IFN-γ/IL-4 (Th1/Th2) was designed as the primary outcome in this prospective study, while studies focused on patients’ survival and prognosis outcomes should also be carried out, therefore, bigger size and multiple-site studies should be designed. Also, we mainly focused on T cell subsets and other immune factors, as the immune system was far more complex, and other factors need to be detected and investigated in the future.
Conclusions
Our results showed that although the proinflammatory factor TNF-α was expressed at higher levels in the SGA group than in the EGA group, the tumour-related immune responses were comparable between the two groups. More importantly, the short- and long-term follow-ups showed no difference between groups in liver cancer recurrence and survival rate. SGA appears not to be inferior to EGA regarding tumour-related immunity and prognosis. Intravenous opioid use appears not to be inferior to epidural anaesthesia, and can be used safely in HCC patients without worsening patients’ prognosis.
Acknowledgments
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-711/rc
Trial Protocol: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-711/tp
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-711/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-711/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-711/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study procedures were approved by the Ethics Committee of Zhongshan Hospital affiliated with Fudan University (No. B2020-080R2), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. Erratum in: JAMA Oncol 2017;3:418. [Crossref] [PubMed]
- Sekandarzad MW, van Zundert AAJ, Lirk PB, et al. Perioperative Anesthesia Care and Tumor Progression. Anesth Analg 2017;124:1697-708. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Hiller JG, Perry NJ, Poulogiannis G, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2018;15:205-18. [Crossref] [PubMed]
- Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 2001;90:191-9. [Crossref] [PubMed]
- Wodehouse T, Demopoulos M, Petty R, et al. A randomized pilot study to investigate the effect of opioids on immunomarkers using gene expression profiling during surgery. Pain 2019;160:2691-8. [Crossref] [PubMed]
- Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015;12:213-26. [Crossref] [PubMed]
- Zhao R, Xu X, Sun L, et al. Long-term effect of anesthesia choice on patients with hepatocellular carcinoma undergoing open liver resection. Front Oncol 2023;12:960299. [Crossref] [PubMed]
- Hasselager RP, Hallas J, Gögenur I. Epidural Analgesia and Recurrence after Colorectal Cancer Surgery: A Danish Retrospective Registry-based Cohort Study. Anesthesiology 2022;136:459-71. [Crossref] [PubMed]
- Sakowska M, Docherty E, Linscott D, et al. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg 2009;33:1802-8. [Crossref] [PubMed]
- Dalen JE, Alpert JS, Goldberg RJ, et al. The epidemic of the 20(th) century: coronary heart disease. Am J Med 2014;127:807-12. [Crossref] [PubMed]
- Bundy JD, He J. Hypertension and Related Cardiovascular Disease Burden in China. Ann Glob Health 2016;82:227-33. [Crossref] [PubMed]
- Nimmo SM, Foo ITH, Paterson HM. Enhanced recovery after surgery: Pain management. J Surg Oncol 2017;116:583-91. [Crossref] [PubMed]
- Jipa M, Isac S, Klimko A, et al. Opioid-Sparing Analgesia Impacts the Perioperative Anesthetic Management in Major Abdominal Surgery. Medicina (Kaunas) 2022;58:487. [Crossref] [PubMed]
- Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol 2018;175:2726-36. [Crossref] [PubMed]
- Wall T, Sherwin A, Ma D, et al. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth 2019;123:135-50. [Crossref] [PubMed]
- Singleton PA, Mirzapoiazova T, Hasina R, et al. Increased μ-opioid receptor expression in metastatic lung cancer. Br J Anaesth 2014;113:i103-8. [Crossref] [PubMed]
- Thomas TE, Bowers K, Gomez D, et al. The association between perioperative opioids and breast cancer recurrence: a narrative review of the literature. Transl Breast Cancer Res 2023;4:12. [Crossref]
- Ringelhan M, Pfister D, O'Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222-32. [Crossref] [PubMed]
- Wang L, Liang S, Chen H, et al. The effects of epidural anaesthesia and analgesia on T lymphocytes differentiation markers and cytokines in patients after gastric cancer resection. BMC Anesthesiol 2019;19:102. [Crossref] [PubMed]
- Yan C. The effects of different liver resection methods, different anesthesia and analgesia methods on postoperative metabolism and immune function. Hangzhou: Zhejiang University; 2011.
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018;32:1267-84. [Crossref] [PubMed]
- Alazawi W, Pirmadjid N, Lahiri R, et al. Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann Surg 2016;264:73-80. [Crossref] [PubMed]
- Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 2010;184:2449-57. [Crossref] [PubMed]
- Benish M, Bartal I, Goldfarb Y, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 2008;15:2042-52. [Crossref] [PubMed]
- Yakar I, Melamed R, Shakhar G, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol 2003;10:469-79. [Crossref] [PubMed]
- Cata JP, Conrad C, Rezvani K. Potential Use of Natural Killer Cell Transfer Therapy in the Perioperative Period to Improve Oncologic Outcomes. Scientifica (Cairo) 2015;2015:732438. [Crossref] [PubMed]
- Tabata T, Hazama S, Yoshino S, et al. Th2 subset dominance among peripheral blood T lymphocytes in patients with digestive cancers. Am J Surg 1999;177:203-8. [Crossref] [PubMed]
- Wada H, Seki S, Takahashi T, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology 2007;106:499-506. [Crossref] [PubMed]
- Morita M, Motoki K, Akimoto K, et al. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem 1995;38:2176-87. [Crossref] [PubMed]
- Motoki K, Maeda K, Ueno H, et al. Antitumor activities of combined treatment with a novel immunomodulator, (2S,3S,4R)-1-O-(alpha-D-Galactopyranosyl)-2-(N-Hexacosanoylamino)-1,3,4 - octadecanetriol (KRN7000), and radiotherapy in tumor-bearing mice. Oncol Res 1996;8:155-62. [PubMed]
- Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997;278:1626-9. [Crossref] [PubMed]
- Burdin N, Brossay L, Koezuka Y, et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol 1998;161:3271-81. [Crossref] [PubMed]
- Richebé P, Pouquet O, Jelacic S, et al. Target-controlled dosing of remifentanil during cardiac surgery reduces postoperative hyperalgesia. J Cardiothorac Vasc Anesth 2011;25:917-25. [Crossref] [PubMed]
- Pandin PC, Cantraine F, Ewalenko P, et al. Predictive accuracy of target-controlled propofol and sufentanil coinfusion in long-lasting surgery. Anesthesiology 2000;93:653-61. [Crossref] [PubMed]
- Monje M, Borniger JC, D'Silva NJ, et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell 2020;181:219-22. [Crossref] [PubMed]
- Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 2015;15:563-72. [Crossref] [PubMed]
- Wang Y, Liu T, Tang W, et al. Hepatocellular Carcinoma Cells Induce Regulatory T Cells and Lead to Poor Prognosis via Production of Transforming Growth Factor-β1. Cell Physiol Biochem 2016;38:306-18. [Crossref] [PubMed]
- Sun Y, Wu L, Zhong Y, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 2021;184:404-421.e16. [Crossref] [PubMed]
- Fu J, Zhang Z, Zhou L, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2013;58:139-49. [Crossref] [PubMed]
- Franchi S, Panerai AE, Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 2007;21:767-74. [Crossref] [PubMed]
- Wigmore T, Farquhar-Smith P. Opioids and cancer: friend or foe? Curr Opin Support Palliat Care 2016;10:109-18. [Crossref] [PubMed]
- Myles PS, Peyton P, Silbert B, et al. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ 2011;342:d1491. [Crossref] [PubMed]
- Cronin-Fenton DP, Heide-Jørgensen U, Ahern TP, et al. Opioids and breast cancer recurrence: A Danish population-based cohort study. Cancer 2015;121:3507-14. [Crossref] [PubMed]
- Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394:1807-15. [Crossref] [PubMed]


