
Prospective observational study of zinc deficiency symptoms during first-line chemotherapy for gastric and colorectal cancer
Highlight box
Key findings
• Before first-line chemotherapy, 38% of the patients had zinc deficiency.
• The least-square means of serum zinc levels significantly decreased at 3 and 6 months of chemotherapy in 30 patients without zinc deficiency at the beginning of chemotherapy (both P<0.01).
• Changes in serum zinc levels during chemotherapy were negatively correlated with taste changes, rash, and itching (all P<0.04) in patients without zinc deficiency before treatment initiation.
What is known and what is new?
• Zinc deficiency has been implicated as a cause of taste and skin disorders.
• The serum level of zinc decreased during chemotherapy in patients who were zinc-non-deficient before chemotherapy, and the decrease in zinc was related to taste changes, rash, and itching.
What is the implication, and what should change now?
• Investigating whether zinc supplementation ameliorates these symptoms is necessary.
Introduction
Zinc is an essential trace element required for the activities of more than 300 enzymes (1). Zinc deficiency has been reported to cause various symptoms including dermatitis, hair loss, anemia, taste disorders, stunting, hypogonadism, diarrhea, osteoporosis, and delayed wound healing (1-5). Zinc is absorbed in the distal duodenum and proximal jejunum and distributed throughout the human body (2). A storage system for zinc does not exist in the body, and plasma zinc constitutes only 0.1% of the total body zinc (1). Homeostatic mechanisms for zinc are fragile; therefore, a modest shortage of zinc intake causes its deficiency in the blood (6).
A few reports on the relationship between serum zinc levels and its symptoms (taste disorders and oral mucositis) during cancer chemotherapy exist (7,8). However, the clinical state of zinc deficiency and its symptoms during long-term courses of cancer chemotherapy have not been sufficiently investigated.
This study aimed to determine the prevalence of zinc deficiency before chemotherapy and changes in zinc levels during chemotherapy. In addition, we elucidated the symptoms that may be related to zinc deficiency during first-line chemotherapy for unresectable gastric and colorectal cancers. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-517/rc).
Methods
Study design and patients
This study was an exploratory and prospective observational study of zinc deficiency in patients with gastric and colorectal cancers who underwent standard first-line chemotherapy at the National Hospital Organization Shikoku Cancer Center from August 2020 to September 2021. The cut-off date for data collection was August 20, 2023.
The eligibility criteria were age ≥20 years; unresectable advanced or recurrent gastric or colorectal cancer; previously untreated disease except for neoadjuvant and adjuvant chemotherapy; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, 1, or 2; and adequate organ function for standard chemotherapy. Patients were excluded if they took zinc acetate hydrate supplements at registration, could not tolerate oral intake or enteral nutrition, or were appeared to be unable to answer the questionnaire. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the National Hospital Organization Shikoku Cancer Center (No. 2020-3) and complied with the Japanese ethical guidelines (9). Written informed consent was obtained from all patients before enrollment in the study.
Data collection and assessments
The following clinical parameters were assessed: sex, age, ECOG PS, primary tumor site, organ metastasis, peritoneal dissemination, ascites, gastrojejunostomy or duodenal resection, body mass index, and serum levels of albumin. Duodenal resection included distal/total gastrectomy and pancreaticoduodenectomy.
Tumor response, progression-free survival (PFS), and overall survival (OS) were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 to investigate whether zinc deficiency affected the efficacy of chemotherapy and prognosis. PFS was defined as the time from the start of chemotherapy to disease progression or death from any cause. OS was defined as the time from the start of chemotherapy until death from any cause.
Serum zinc levels were measured before and 1, 3, and 6 months after chemotherapy, and eight symptoms were assessed using the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®), version 1.0 (10). PRO-CTCAE is a measurement system for 78 symptom items drawn from the CTCAE by self-reports from patients in cancer clinical trials (11). The response score of the PRO-CTCAE was 0–4 (frequency, severity, interference, and amount) or 0–1 (presence/absence). We selected eight symptoms of interest (mouth/throat sores, taste changes, rash, skin dryness, acne, hair loss, itching, and nail ridging) that were presumed to be associated with zinc deficiency (1-8).
Blood samples for measurement of serum zinc levels were obtained a few hours after breakfast. Serum zinc levels were measured using Nitro-PAPS (FALCO Biosystems Ltd., Kyoto, Japan) (12). According to the Practice Guideline for Zinc Deficiency, zinc deficiency was defined as a serum level of <60 µg/dL, and borderline zinc deficiency was defined as a serum level of ≥60 to <80 µg/dL (13).
The physician in charge of each patient administered zinc acetate hydrate when necessary. Data after the administration of zinc acetate hydrate or discontinuation of chemotherapy were excluded from the analyses.
Statistical analyses
Data were analyzed using JMP Pro Version 16.0. (SAS Institute Inc., Cary, NC, USA), EZR version 1.5 and R version 4.0.2 (14). Statistical significance was set at P<0.05. Chi-square and Wilcoxon rank-sum tests were used to evaluate proportional differences between the two groups. Repeated measures were analyzed using linear and generalized linear mixed models. Five factors believed to affect PRO-CTCAE and zinc deficiency were set as fixed effects in the generalized linear mixed model: age, chemotherapy regimen (SOX, tegafur/gimeracil/oteracil and oxaliplatin; FOLFOX, 5-fluorouracil/leucovorin and oxaliplatin; XELOX, capecitabine and oxaliplatin), anti-epidermal growth factor receptor (EGFR) antibody, gastrojejunostomy or duodenal resection, and primary tumor (gastric and colorectal cancer).
Results
Patients
Sixty-one patients were enrolled in the study. All patients were Japanese ethnicity. Fifty-nine patients were treated with various regimens (Figure 1) after excluding two ineligible patients according to the protocol (ECOG PS 3 and administration of zinc acetate hydrate). We analyzed 48 patients who underwent standard first-line chemotherapy with fluoropyrimidine plus oxaliplatin (FPOX) to reduce the bias of the different regimens used. The median patient age was 71 years (Table 1). Most patients had an ECOG PS of 0 or 1. Thirty patients had gastric cancer, and 18 had colorectal cancer. Six patients underwent adjuvant chemotherapy and had more than a year gap between the adjuvant chemotherapy and the start of first-line chemotherapy. Several patients were treated with anti-human EGFR 2 (HER2), anti-vascular endothelial growth factor, and anti-EGFR antibodies, in addition to FPOX. Median follow-up for all patients was 20.6 [interquartile range (IQR), 10.5–27.5] months.

Table 1
| Factors | All patients (n=48) | Zinc deficiency status | P value† | |
|---|---|---|---|---|
| ND group (n=30) | D group (n=18) | |||
| Sex | 0.18 | |||
| Male | 26 [54] | 14 [47] | 12 [67] | |
| Female | 22 [46] | 16 [53] | 6 [33] | |
| Age (years) | 71 [34–89] | 69 [34–85] | 74 [64–89] | 0.04 |
| ECOG PS | 0.26 | |||
| 0 | 22 [46] | 16 [53] | 6 [33] | |
| 1 | 22 [46] | 11 [37] | 11 [61] | |
| 2 | 4 [8] | 3 [10] | 1 [6] | |
| Primary site | 0.28 | |||
| Stomach | 30 [63] | 17 [57] | 13 [72] | |
| Colorectum | 18 [38] | 13 [43] | 5 [28] | |
| No. of metastatic sites | 0.19 | |||
| 0, 1 | 20 [42] | 13 [43] | 7 [39] | |
| 2 | 12 [25] | 5 [17] | 7 [39] | |
| ≥3 | 16 [33] | 12 [40] | 4 [22] | |
| Peritoneal dissemination | 0.71 | |||
| Yes | 23 [48] | 15 [50] | 8 [44] | |
| No | 25 [52] | 15 [50] | 10 [56] | |
| Ascites | 0.60 | |||
| Yes | 7 [15] | 5 [17] | 2 [11] | |
| No | 41 [85] | 25 [83] | 16 [89] | |
| Gastrojejunostomy or resection of duodenum‡ | 0.77 | |||
| Yes | 9 [19] | 6 [20] | 3 [17] | |
| No | 39 [81] | 24 [80] | 15 [83] | |
| Body mass index (kg/m2) | 22.3 [15.3–31.2] | 22.3 [15.3–31.2] | 22.3 [15.7–27.9] | 0.47 |
| Serum albumin levels (g/dL) | 3.5 [1.9–4.6] | 3.6 [2.4–4.6] | 3.3 [1.9–4.2] | 0.08 |
| Serum zinc levels (μg/dL) | 64.0 [31–85] | 69.5[60–85] | 55.0 [31–59] | <0.0001 |
| Received adjuvant therapy | 0.66 | |||
| Yes | 6 [13] | 3 [10] | 3 [17] | |
| No | 42 [88] | 27 [90] | 15 [83] | |
| Regimen | 0.77 | |||
| FPOX | 34 [71] | 20 [67] | 14 [78] | |
| FPOX + trastuzumab | 2 [4] | 1 [3] | 1 [6] | |
| FPOX + bevacizumab | 8 [17] | 6 [20] | 2 [11] | |
| FPOX + panitumumab | 4 [8] | 3 [10] | 1 [6] | |
Data are presented as n [%] or median [range]. †, chi-square test and Wilcoxon rank-sum test; ‡, resection included distal gastrectomy, total gastrectomy, and pancreaticoduodenectomy. FPOX, fluoropyrimidine plus oxaliplatin; ND, zinc-non-deficiency; D, zinc-deficiency; ECOG PS, Eastern Cooperative Oncology Group performance status.
Zinc deficiency
Before first-line chemotherapy, 38% of the patients had zinc deficiency. The incidence of abnormal PRO-CTCAE symptoms in patients with or without zinc deficiency before chemotherapy is shown in Figure 2. Although mouth/throat sores, taste, and rash changes tended to be frequent in patients with zinc deficiency before chemotherapy, the incidence of these symptoms did not differ significantly between patients with and without zinc deficiency. The severity of all symptoms graded using the PRO-CTCAE was almost mild.

Analyses in zinc-non-deficient (ND) and zinc-deficient (D) patients
Considering that a substantial number of patients had zinc deficiency before chemotherapy, we analyzed the data by dividing the patients into two groups: ND group (n=30) and D group (n=18). Table 1 shows the patient characteristics in the two groups before chemotherapy. The median age was significantly lower in the ND group than in the D group (P=0.04). However, other parameters, including the anti-EGFR antibody combination, did not differ between the two groups.
The response (complete plus partial response) rates were 24% (4/17) and 38% (5/13) in the ND and D groups, respectively, in patients with gastric cancer (P=0.38). The response rates were 38% (5/13) and 40% (2/5) in the ND and D groups, respectively, in patients with colorectal cancer (P=0.95). The PFS and OS did not differ between the ND and D groups in patients with gastric or colorectal cancer (Figure 3).

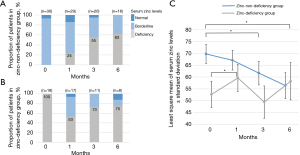
The proportion of zinc deficiency in the ND group increased as treatment progressed (Figure 4A), whereas that in the D group improved to approximately half of that in patients 1 month after chemotherapy (Figure 4B). Figure 4C shows the changes in serum zinc levels in the ND and D groups using a linear mixed model. The least-squares mean (LSM) of serum zinc levels at 3 and 6 months of chemotherapy were significantly lower than those before chemotherapy in the ND group (P=0.007 and P<0.001, respectively). In contrast, LSM at 1 month of chemotherapy was significantly higher than that before chemotherapy in the D group (P=0.037). The zinc levels of eight patients in the D group increased to over 60 µg/dL after 1 month of chemotherapy. The initial responses included a partial response (n=2) and stable disease (n=6).
Correlations of PRO-CTCAE symptoms and serum zinc levels in the ND and D groups
The incidence of the eight PRO-CTCAE symptoms that emerged during first-line chemotherapy is shown in Table 2. Although the incidence of ≥ grade 2 and ≥ grade 3 taste changes, skin dryness, hair loss, and itching tended to be higher in the ND group than in the D group, no statistically significant differences were observed between the two groups at the various cutoff grades.
Table 2
| Symptoms (attribute, grade range) | ND group (n=30) | D group (n=18) | |||||
|---|---|---|---|---|---|---|---|
| Grade 1 or more |
Grade 2 or more |
Grade 3 or more |
Grade 1 or more |
Grade 2 or more |
Grade 3 or more |
||
| Mouth/throat sores (severity, 0–4) | 11 [37] | 2 [7] | 0 [0] | 5 [28] | 1 [6] | 0 [0] | |
| Taste changes (severity, 0–4) | 15 [50] | 6 [20] | 3 [10] | 10 [56] | 2 [11] | 0 [0] | |
| Rash (presence/absence, 0–1) | 9 [30] | – | – | 5 [28] | – | – | |
| Skin dryness (severity, 0–4) | 18 [60] | 6 [20] | 1 [3] | 7 [39] | 0 [0] | 0 [0] | |
| Acne (severity, 0–4) | 9 [30] | 2 [7] | 1 [3] | 2 [11] | 1 [6] | 1 [6] | |
| Hair loss (amount, 0–4) | 9 [30] | 6 [20] | 3 [10] | 2 [11] | 1 [6] | 0 [0] | |
| Itching (severity, 0–4) | 15 [50] | 5 [17] | 2 [7] | 4 [22] | 0 [0] | 0 [0] | |
| Nail ridging (presence/absence, 0–1) | 7 [23] | – | – | 5 [28] | – | – | |
Data are presented as n [%]. PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; ND, zinc-non-deficiency; D, zinc-deficiency.
The correlations between symptoms (present or absent during chemotherapy) and serum zinc levels were analyzed using a generalized linear mixed model (Table 3). Taste changes, skin rashes, and itching were significantly negatively correlated with serum zinc levels in the ND group (P=0.038, 0.031, and 0.037, respectively). No symptoms correlated with serum zinc levels in the D group.
Table 3
| Factors | Odds ratio | Estimate | Standard error | P value† |
|---|---|---|---|---|
| ND group (n=30) | ||||
| Mouth/throat sores | 0.988 | −0.013 | 0.025 | 0.611 |
| Taste changes | 0.943 | −0.059 | 0.029 | 0.038 |
| Rash | 0.887 | −0.120 | 0.056 | 0.031 |
| Skin dryness | 0.952 | −0.049 | 0.025 | 0.053 |
| Acne | 0.952 | −0.049 | 0.033 | 0.140 |
| Hair loss | 0.973 | −0.028 | 0.033 | 0.407 |
| Itching | 0.951 | −0.051 | 0.024 | 0.037 |
| Nail ridging | 0.960 | −0.041 | 0.035 | 0.247 |
| D group (n=18) | ||||
| Mouth/throat sores | 1.049 | 0.048 | 0.032 | 0.134 |
| Taste changes | 1.070 | 0.067 | 0.035 | 0.052 |
| Rash | 1.017 | 0.017 | 0.034 | 0.613 |
| Skin dryness | 0.974 | −0.027 | 0.035 | 0.442 |
| Acne | – | – | – | – |
| Hair loss | – | – | – | – |
| Itching | 1.067 | 0.065 | 0.035 | 0.067 |
| Nail ridging | 1.043 | 0.043 | 0.034 | 0.205 |
†, all analyses were performed by categorizing symptoms as absent (grade 0) or present (grade 1 or higher). The models were adjusted for age, chemotherapy regimen (SOX, FOLFOX, and XELOX), anti-EGFR antibody, gastrojejunostomy or resection of the duodenum, and primary tumors (gastric and colorectal cancer). ND, zinc-non-deficiency; D, zinc-deficiency; SOX, tegafur/gimeracil/oteracil and oxaliplatin; FOLFOX, 5-fluorouracil/leucovorin and oxaliplatin; XELOX, capecitabine and oxaliplatin; EGFR, epidermal growth factor receptor.
Discussion
In this study, the serum zinc levels in the ND group significantly decreased during chemotherapy, whereas those in the D group showed no further decrease. Serum zinc behavior differed between ND and D patients. Furthermore, serum zinc levels were negatively correlated with the incidence of PRO-CTCAE symptoms, including taste changes, skin rashes, and itching, in the ND group.
The proportion of zinc deficiency before first-line chemotherapy with FPOX in patients with gastric and colorectal cancer is high at approximately 40%, relative to that reported in the Japanese literature on voluntary health checkups at 0.4–0.6% (15). This indicated that these patients were highly likely to be in a D state before chemotherapy. This may be due to decreased zinc intake and absorption, inflammation, and downregulation of ZRT and IRT-like proteins (ZIP; a zinc transporter) in cancers (16-18). Low zinc intake is also reportedly related to the risk of gastrointestinal cancer, and cancer biology appears to be strongly associated with zinc intake (19).
A significant difference in age was observed between the ND and D groups. This reflects the natural course of aging, as previously reported epidemiologically (15,20). No significant differences were noted between the two groups in terms of other baseline characteristics. Serum albumin levels were slightly lower in the D group, suggesting impaired nutrition.
The incidences of symptoms before chemotherapy between the ND and D groups were not significantly different. However, there were higher tendencies of incidence in many symptoms (1.4–3.6 times in mouth/throat sores, taste changes, rash, and itching). We considered this was due to a small number of patients detecting the statistical difference.
As the treatment progressed, the number of patients with zinc deficiency increased in the ND group. Serum zinc levels in the ND group decreased, whereas those in the D group did not. One reason for the decrease in zinc in the ND group was the direct effect of the zinc-chelating ability of fluorouracil (21). Another reason may be the decreased food intake and absorption due to chemotherapy-induced gastrointestinal mucosal injury. However, the maintained zinc levels in group D were inconsistent, suggesting the presence of some mechanisms for maintaining blood zinc levels in a D state. Further studies are required to elucidate the underlying molecular mechanisms.
Interestingly, serum zinc levels significantly increased in the D group at 1 month of chemotherapy, and the proportion of normal zinc levels over 80 µg/dL appeared to increase in the ND and D groups (Figure 4). These findings suggest an improvement in oral intake and nutritional status owing to effective chemotherapy in some patients. The first tumor responses of patients in the D group, whose serum zinc levels changed from deficient to non-deficient after 1 month of chemotherapy, were all partial responses and stable disease, and no patient progressed. Serum zinc levels are likely to be affected by cancer control for at least 1 month. The ND group initially had high zinc levels, so even if the nutritional status improved, the effect on the zinc level might be limited.
We considered various cutoff points for the PRO-CTCAE grades but did not find any significant differences in the incidence and grade of symptoms between the ND and D groups. However, generalized mixed model analyses showed that serum zinc levels were negatively correlated with the incidence of taste changes, rashes, and itching among the symptoms of interest in the ND group over time. Recently, an interventional study showed that zinc acetate hydrate did not improve chemotherapy-induced dysgeusia in patients with gastrointestinal cancer, suggesting no correlation between zinc deficiency and dysgeusia (7). This result was inconsistent with our findings. However, comparing our results with theirs is difficult because the patient selection differed between the studies, and a correlation between taste changes and serum zinc levels was observed only in the ND group in our study. The previous study showed polaprezinc improved dysgeusia and suggested some components other than zinc may contribute to the symptom improvement. This is worth to study further, especially in ND group patients. The skin is one of the systems most susceptible to zinc deficiency, resulting in rash and itching (1). We also reported that a zinc homeostasis disorder might cause skin xerosis in patients with colorectal cancer treated with FPOX plus an anti-EGFR antibody (22). Skin conditions and zinc levels appear to be closely correlated.
This study has some limitations. Although this was an exploratory study, its sample size was small. Given low patient numbers, it may be difficult to draw statistical conclusions from an analysis of the D group. However, we identified possible symptoms related to zinc deficiency, and further investigations with larger sample sizes are warranted. Furthermore, ensuring that the patients’ backgrounds are as uniform as possible is necessary. Since this study was observational, the selection of therapeutic regimens was based on the judgment of attending physicians and patients’ agreement. We should plan a next interventional study with zinc administration in an appropriate cancer and regimen fixed. Symptoms were assessed using the PRO-CTCAE. However, the feasibility of this tool has not yet been adequately assessed. Therefore, concurrently using other measurement tools is necessary.
Conclusions
In conclusion, serum zinc levels decreased during the course of chemotherapy in patients who were ND before chemotherapy, and this decrease was related to the frequency of taste changes, rash, and itching. Therefore, we planned to conduct an interventional study using zinc to improve these symptoms.
Acknowledgments
We would like to thank the Editage (https://www.editage.com/) for the English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-517/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-517/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-517/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-517/coif). T.N. reports honoraria from Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol Myers Squibb, Eli Lilly Japan K.K., Takeda Pharmaceutical Co., Ltd., Merck Serono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd and Yakult Honsha Co., Ltd. T.K. reports honoraria from Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd., Bristol Myers Squibb K.K., Taiho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. I.H. received consulting fees from Taiho Pharmaceutical Co., Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Asahi Kasei Co., Ltd., and Daiichi Sankyo Co., Ltd., and reports honoraria from Chugai Pharmaceutical Co., Takeda Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the National Hospital Organization Shikoku Cancer Center (No. 2020-3), and informed consent was obtained from all participants before enrollment in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc 2000;59:541-52. [Crossref] [PubMed]
- Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr 2000;130:1374S-7S. [Crossref] [PubMed]
- Saper RB, Rash R. Zinc: an essential micronutrient. Am Fam Physician 2009;79:768-72. [PubMed]
- Yagi T, Asakawa A, Ueda H, et al. The role of zinc in the treatment of taste disorders. Recent Pat Food Nutr Agric 2013;5:44-51. [Crossref] [PubMed]
- Lin PH, Sermersheim M, Li H, et al. Zinc in Wound Healing Modulation. Nutrients 2017;10:16. [Crossref] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Zinc. In: Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington: National Academies Press; 2001:442-501.
- Ito K, Yuki S, Nakatsumi H, et al. Multicenter, prospective, observational study of chemotherapy-induced dysgeusia in gastrointestinal cancer. Support Care Cancer 2022;30:5351-9. [Crossref] [PubMed]
- Mehrzad V, Mahmood-Zadeh M, Feizi A, et al. Determination Relation of the Zinc Serum Level in Acute Leukemia Adult Patients with Mucositis and Neutropenic Prevalence before and after Treatment in Isfahan' Seyed-Al-Shohada Hospital, 2012-2013. Adv Biomed Res 2018;7:31. [Crossref] [PubMed]
- Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol 2022;52:539-44. [Crossref] [PubMed]
- National Cancer Institute. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®). [Cited 2023 Feb 20]. Available online: https://healthcaredelivery.cancer.gov/pro-ctcae/
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. 2017. [Cited 2023 Feb 20]. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- Makino T, Saito M, Horiguchi D, et al. A highly sensitive colorimetric determination of serum zinc using water-soluble pyridylazo dye. Clin Chim Acta 1982;120:127-35. [Crossref] [PubMed]
- The Japanese Society of Clinical Nutrition. Practice Guideline for zinc Deficiency. [Cited 2023 Feb 20]. Available online: http://www.jscn.gr.jp/pdf/aen20190423.pdf
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Yokokawa H, Fukuda H, Saita M, et al. Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. J Gen Fam Med 2020;21:248-55. [Crossref] [PubMed]
- Lindsey AM, Piper BF. Anorexia, serum zinc, and immunologic response in small cell lung cancer patients receiving chemotherapy and prophylactic cranial radiotherapy. Nutr Cancer 1986;8:231-8. [Crossref] [PubMed]
- Federico A, Iodice P, Federico P, et al. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur J Clin Nutr 2001;55:293-7. [Crossref] [PubMed]
- Costello LC, Franklin RB. Decreased zinc in the development and progression of malignancy: an important common relationship and potential for prevention and treatment of carcinomas. Expert Opin Ther Targets 2017;21:51-66. [Crossref] [PubMed]
- Li P, Xu J, Shi Y, et al. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin Nutr 2014;33:415-20. [Crossref] [PubMed]
- Kurosawa R, Kubori S. Zinc deficiency and its clinical features in the cases found in Kitamimaki, a rural area in Japan. Biomed Res Trace Elements 2006;17:91-3.
- Fukasawa T, Orii T, Tanaka M, et al. Statistical approach to drug-induced taste disorders based on zinc chelating ability. Yakugaku Zasshi 2005;125:377-87. [Crossref] [PubMed]
- Tohyama M, Sakaguchi C, Nishina T, et al. Possible involvement of zinc deficiency in epidermal growth factor receptor inhibitor-induced xerotic dermatitis. J Dermatol 2021;48:1579-83. [Crossref] [PubMed]


