
Patterns and kinetics of hepatocellular carcinoma relapse post-liver transplantation: oligorecurrence and role of local therapies
Highlight box
Key findings
• Patients with recurrent hepatocellular carcinoma (HCC) following liver transplantation with ≤3 foci of recurrence [i.e., oligorecurrence (oligoM1)] had favorable overall survival (OS) outcomes compared to patients with ≥4 metastases (polyrecurrence). Moreover, amongst oligoM1 patients, receipt of local metastasis-directed therapy to all sites of disease (MDT-All) was associated with improved OS.
What is known and what is new?
• Randomized trial data supports the benefit of MDT in patients with limited metastatic cancer (i.e., oligometastasis/oligoM1), but benefit may depend upon patient- and disease-specific characteristics. Patients with HCC recurrence post-transplant have limited systemic therapy options owing to the need for immunosuppression.
• This study is among the first to characterize the potential benefit of MDT in HCC patients with oligoM1 specifically following liver transplantation.
What is the implication, and what should change now?
• MDT-All may prolong survival of patients with HCC recurrence post-transplant, providing impetus for prospective study.
Introduction
Liver transplant is a potentially curative procedure for patients with hepatocellular carcinoma (HCC). Despite use of bridging and downstaging therapies and strict selection criteria, HCC relapse can occur following transplant. Systemic therapy options for patients with relapsed HCC following liver transplantation are limited by functional status and treatment-related toxicity. Immune checkpoint inhibition is infrequently administered to the immunosuppressed, transplanted patient. The benefits of local therapy, also called metastasis-directed therapy (MDT), in the setting of oligometastatic cancer have been studied in prospective, randomized controlled trials in a number of settings, with results from trials conducted in prostate (1,2), lung (3), and mixed disease sites (4) demonstrating that MDT may improve progression-free survival (PFS) or overall survival (OS). In aggregate, these studies of MDT provide significant evidence of the benefit of MDT across multiple disease sites, but the benefit of MDT may still depend in part upon both the disease site being treated and how the oligometastatic state is defined (5). Thus, there is value in assessing the benefit of local therapies in metastatic disease based upon specific disease sites and specific biological scenarios, such as the post-transplant immunosuppressed state.
No single definition of oligometastatic cancer has been applied across trials or disease sites, although studies commonly restrict the definition to a limited number of grossly evident metastases, (e.g., fewer than 3 to 5 lesions), and often with a requirement that all sites of disease may be treated with local therapies (6). Within the realm of HCC, a number of retrospective studies have attempted to identify cohorts of patients with metastatic HCC who may benefit from local therapies (7-10), which commonly take the form of surgery, radiation therapy, transarterial chemoembolization (TACE), or thermal ablative therapies. To our knowledge, however, no report has attempted to define the oligorecurrent (oligoM1)/oligometastatic state specifically in patients with post-transplant recurrence and to quantify the impact of MDT in this context.
Hence, we aimed to (I) characterize the patterns of HCC recurrence and progression following liver transplantation at a high-volume transplant center, (II) identify pre-transplant characteristics associated with oligoM1 vs. polyrecurrence (polyM1); (III) identify clinical characteristics associated with differential outcomes; and (IV) evaluate the impact of MDT on outcomes in patients with limited disease at the time of initial recurrence. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-541/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Johns Hopkins University institutional review board (No. IRB00344985) and individual consent for this retrospective analysis was waived. A single-institution retrospective review was conducted to identify patients with HCC who received liver transplantation and subsequently experienced disease recurrence. Eligibility for liver transplant was determined using Milan criteria. Following transplant, surveillance involved imaging scans every 2 months for 2 years, then every 6 months thereafter. Imaging scans typically consisted of magnetic resonance imaging (MRI) or computed tomography (CT) of the abdomen and pelvis, and CT chest every 6 months. Baseline demographic and clinical characteristics were recorded. CT, positron emission tomography, and MRI were reviewed to determine radiographic recurrence and subsequent disease progression, which was characterized using RECIST criteria. Any site of recurrence was considered a metastatic lesion. Patients with three or fewer metastases were classified as having oligoM1 disease at the time of recurrence, while patients with more than three metastases were considered to have polyM1 disease. Progression was defined as progression of a previously detected metastasis, including ones previously treated with local or systemic therapy, or the appearance of new metastases. In patients with initial oligoM1 disease, polyPFS was defined as the time from initial recurrence to the polyM1 state or death. OS was the time from initial recurrence to death. Patients who did not experience an event of interest were censored at the time of last clinical follow-up.
Sites of recurrence were categorized into the following anatomical regions: liver, abdominal nodes, lungs, mediastinal nodes, adrenal glands, bone, brain, and peritoneal cavity. Any other recurrences that did not correspond to one of the pre-defined regions were classified as “other”.
MDT was defined as any locally directed therapy administered with the intention of eradication of the site of metastatic disease. For example, surgical resection, stereotactic ablative radiation therapy, radioembolization of metastatic disease were considered MDT, while palliative-intent radiotherapy alone was not considered MDT. In some cases, however, MDT could have both a therapeutic and palliative rationale.
Statistical analysis
Pre- and post-transplant categorical data are presented as the number and percentage, and continuous data as the median and range. Patients with missing data were excluded from the analysis related to the data in question. The relationship between clinical variables among the two groups was evaluated using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables, where appropriate. Univariable and multivariable logistic regressions were used to assess the predictive performance of various clinical variables on oligoM1 vs. polyM1 recurrence following liver transplantation. Predictive performance was evaluated using the Hosmer-Lemeshow goodness-of-fit test and receiver operating characteristic (ROC) analysis.
Time-to-event outcomes including polyPFS and OS were compared between patients with initial oligoM1 vs. polyM1 disease at the time of recurrence using the Kaplan-Meier method. Univariable Cox regression was performed to identify clinical prognostic factors. A multivariable model was constructed based upon statistically significant and clinically relevant covariates in univariable analysis. Model fitness was assessed via chi-square goodness-of-fit test. Further subgroup analysis of patients with oligoM1 disease stratified by receipt of MDT to all sites of disease (MDT-All) was also conducted using the Kaplan-Meier method. In all multivariable regressions collinearity and dependency were examined and variables showing a high level of correlation and dependency were removed from the final model. Statistical analyses were performed using IBM SPSS Statistics (version 28.0) and Stata Statistical Software: Release 17 (StataCorp LLC, College Station, TX, USA). A P value <0.05 was set as the threshold for statistical significance in all analyses.
Results
Three hundred and ninety-seven patients with HCC who underwent liver transplantation at our institution were reviewed, of whom 43 (11%) patients experienced HCC recurrence between 2005 and 2022. The median follow-up time was 8.4 (range, 1.6–173.5) months. At the time of recurrence, 16 (37%) patients presented with >3 metastases (polyM1 disease), while 27 (63%) presented with ≤3 metastases (oligoM1 disease). Table 1 shows baseline demographic and clinical characteristics of the two groups. There was no significant difference in the time to recurrence between patients with oligoM1 and polyM1 disease (median 10.6 vs. 11.3 months, P=0.9). The regions of first recurrence were liver (n=14), abdominal nodes (n=14), lungs (n=11), mediastinal nodes (n=4), adrenals (n=3), brain (n=1), and peritoneal cavity (n=1). Eighteen (42%) patients received systemic therapy after recurrence, consisting of sorafenib alone (n=11), lenvatinib or cabozantinib (n=6), and gemcitabine/cisplatin (n=2) as either first or second-line therapy.
Table 1
| Characteristics | PolyM1 (n=16) | OligoM1 (n=27) | Overall (n=43) | P value |
|---|---|---|---|---|
| Age (years) | 58.9 [44.3, 70.7] | 63.2 [46.6, 72.4] | 61.7 [44.3, 72.4] | 0.34 |
| Sex | 0.688 | |||
| Male | 14 (87.5) | 21 (77.8) | 35 (81.4) | |
| Female | 2 (12.5) | 6 (22.2) | 8 (18.6) | |
| Race | 0.648 | |||
| White | 12 (75.0) | 17 (63.0) | 29 (67.4) | |
| Black | 4 (25.0) | 8 (29.6) | 12 (27.9) | |
| Asian | 0 (0.0) | 2 (7.4) | 2 (4.7) | |
| ECOG PS at recurrence | 0.739 | |||
| 0 to 1 | 10 (62.5) | 19 (70.4) | 29 (67.4) | |
| 2 and above | 6 (37.5) | 8 (29.6) | 14 (32.6) | |
| Number of metastatic lesions at recurrence | 8 [4, 47] | 1 [1, 3] | 2 [1, 47] | <0.001 |
| Number of metastatic sites at recurrence | 0.002 | |||
| 1 | 5 (31.3) | 22 (81.5) | 27 (62.8) | |
| 2 | 8 (50.0) | 5 (18.5) | 13 (30.2) | |
| 3 | 2 (12.5) | 0 (0.0) | 2 (4.7) | |
| 4 | 1 (6.3) | 0 (0.0) | 1 (2.3) | |
| AFP at recurrence (ng/mL) | 166.85 | 26.4 [1.8, 3,864] | 34 [1.2, 365,210] | 0.332 |
| AFP ≥400 ng/mL | 5/14 (35.7) | 6/26 (23.1) | 11/40 (27.5) | 0.469 |
| MDT modality | 0.019 | |||
| Cryoablation | 0 (0.0) | 2 (7.4) | 2 (4.7) | |
| IMRT | 0 (0.0) | 2 (7.4) | 2 (4.7) | |
| MWA | 0 (0.0) | 2 (7.4) | 2 (4.7) | |
| MWA, SBRT | 0 (0.0) | 1 (3.7) | 1 (2.3) | |
| SBRT | 0 (0.0) | 6 (22.2) | 6 (14.0) | |
| SRS | 0 (0.0) | 1 (3.7) | 1 (2.3) | |
| Surgery | 0 (0.0) | 2 (7.4) | 2 (4.7) | |
| Surgery + palliative PORT | 0 (0.0) | 1 (3.7) | 1 (2.3) | |
| TACE | 1 (6.3) | 0 (0.0) | 1 (2.3) | |
| Y90 | 1 (6.3) | 0 (0.0) | 1 (2.3) | |
| Sites of first failure† | ||||
| Liver | 9 (56.3) | 5 (18.5) | 14 (32.6) | 0.018 |
| Lungs | 8 (50.0) | 3 (11.1) | 11 (25.6) | 0.01 |
| Abdominal nodes | 5 (31.3) | 9 (33.3) | 14 (32.6) | >0.99 |
| Bone | 4 (25.0) | 6 (22.2) | 10 (23.3) | 0.835 |
| Mediastinal nodes | 2 (12.5) | 2 (7.4) | 4 (9.3) | 0.621 |
| Adrenals | 1 (6.3) | 2 (7.4) | 3 (7.0) | >0.99 |
| Peritoneal cavity | 1 (6.3) | 0 (0.0) | 1 (2.3) | 0.372 |
| Brain | 0 (0.0) | 1 (3.7) | 1 (2.3) | >0.99 |
| Other‡ | 1 (6.3) | 2 (7.4) | 3 (7.0) | >0.99 |
Data are presented as median [range] or n (%). †, patients could have multiple sites of first recurrence; ‡, other sites include perirectal nodes, broad ligament of the uterus, kidney, and abdominal wall. PolyM1, polyrecurrent; oligoM1, oligorecurrent; ECOG, Eastern Cooperative Oncology Group; PS, performance status; AFP, alpha-fetoprotein; MDT, metastasis-directed therapy; IMRT, intensity-modulated radiation therapy; MWA, microwave ablation; SBRT, stereotactic body radiation therapy; SRS, stereotactic radiosurgery; PORT, post-operative radiation therapy; TACE, transarterial chemoembolization; Y90, Yttrium-90 radioembolization.
Nineteen (44%) patients received MDT, including seven patients who received stereotactic ablative radiotherapy, and two who received conventionally-fractionated radiotherapy to a definitive dose. The majority of patients receiving MDT had oligoM1 disease at recurrence (18/20; 90%).
Association of pre-transplant characteristics with extent of recurrence
Among the pre-transplant clinical variables, age, sex, and baseline HCC status (i.e., within or not within Milan criteria) before transplant were similar between the oligoM1 and polyM1 groups (Table 2). The number of loco-regional treatments of HCC prior to transplant appeared to have no statistically significant impact on oligoM1 or polyM1 recurrence. Transplant waitlist duration for the polyM1 group (266 days) was longer than that of the oligoM1 group (199 days) but this difference was not statistically significant. Those with oligoM1 recurrence more frequently had low pre-transplant alpha-fetoprotein (AFP; ≤100 ng/mL) compared to those with polyM1 (P=0.043). Explant pathology showed that a greater proportion of patients with polyM1 than those with oligoM1 had microvascular invasion (94% vs. 41%, P=0.001) and poor cellular differentiation of their transplant specimen (50% vs. 12%, P=0.0081). There was no difference between the two groups when comparing necrosis status of the largest lesion, the number of lesions, or the area of the largest lesion (Table 2).
Table 2
| Characteristics | OligoM1 (n=27) | PolyM1 (n=16) | P value |
|---|---|---|---|
| Age (years) | 61 [45–72] | 58 [44–69] | 0.5374 |
| Baseline HCC within Milan criteria† | >0.99 | ||
| No | 6 [22] | 3 [19] | |
| Yes | 21 [78] | 13 [81] | |
| AFP before transplant (ng/mL) | 0.043 | ||
| >100 | 5 [19] | 8 [50] | |
| ≤100 | 22 [81] | 8 [50] | |
| Listing to transplant (days) | 199 [1–936] | 266 [6–1,929] | 0.3124 |
| Number of TACE before transplant | 0.719 | ||
| >2 | 7 [26] | 3 [19] | |
| ≤2 | 20 [74] | 13 [81] | |
| Explant pathological examination | |||
| Cellular differentiation | n=25‡ | 0.0081 | |
| Poor | 3 [12] | 8 [50] | |
| Moderate/well | 22 [88] | 8 [50] | |
| Microvascular invasion | 0.001 | ||
| Yes | 11 [41] | 15 [94] | |
| No | 16 [59] | 1 [6] | |
| Necrosis status of largest lesion | >0.99 | ||
| Partial | 21 [78] | 13 [81] | |
| Complete | 6 [22] | 3 [19] | |
| Number of lesions | >0.99 | ||
| >3 | 10 [37] | 6 [38] | |
| ≤3 | 17 [63] | 10 [63] | |
| Area of largest tumor (cm2) | 0.186 | ||
| >5 | 16 [59] | 13 [81] | |
| ≤5 | 11 [41] | 3 [19] |
Data are presented as median [range] or n [%]. †, patients outside of Milan criteria were downstaged prior to transplant; ‡, two patients had no residual viable tumor in the liver specimen. OligoM1, oligorecurrent; polyM1, polyrecurrent; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization.
Regression analyses (Table 3) showed the following pre-treatment clinical variables to be independent predictors of polyM1 recurrence: AFP level before transplantation >100 ng/mL (OR: 4.40; 95% CI: 1.11–17.48), poor cellular differentiation (OR: 7.33; 95% CI: 1.549–34.695) and presence of microvascular invasion in the explanted liver (OR: 21.82; 95% CI: 2.50–190.12). However, in multivariable analysis, only microvascular invasion predicted for polyM1 recurrence.
Table 3
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Cellular differentiation | |||||
| Poor | 5.25 (1.23–22.39) | 0.025 | 3.60 (0.64–20.14) | 0.145 | |
| Moderate/well | 1 (reference) | ||||
| Tumor necrosis status | |||||
| Partial | 1.24 (0.26–5.83) | 0.787 | |||
| Complete | 1 (reference) | ||||
| Microvascular invasion | |||||
| Yes | 21.82 (2.50–190.12) | 0.005 | 14.64 (1.48–144.77) | 0.022 | |
| No | 1 (reference) | ||||
| Number of tumors | |||||
| >3 | 1.02 (0.28–3.66) | 0.976 | |||
| ≤3 | 1 (reference) | ||||
| Area of largest tumor (cm2) | |||||
| >5 | 2.98 (0.68–12.98) | 0.146 | |||
| ≤5 | 1 (reference) | ||||
| Number of TACE before transplantation | |||||
| >2 | 0.66 (0.144–3.02) | 0.592 | |||
| ≤2 | 1 (reference) | ||||
| AFP level before transplantation (ng/mL) | |||||
| >100 | 4.40 (1.11–17.48) | 0.035 | 1.25 (0.23–6.82) | 0.797 | |
| ≤100 | 1 (reference) | ||||
| Baseline HCC within Milan criteria† | |||||
| No | 0.81 (0.17–3.80) | 0.787 | |||
| Yes | 1 (reference) | ||||
†, patients outside of Milan criteria were downstaged prior to transplant. PolyM1, polyrecurrent; OR, odds ratio; CI, confidence interval; TACE, transarterial chemoembolization; AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma.
OligoM1 vs. polyM1 disease at initial recurrence
Patients with oligoM1 disease had superior OS compared to patients with polyM1 disease at the time of recurrence with a median survival of 16.2 (95% CI: 11.3, 21.1) vs. 4.0 (95% CI: 1.4, 6.6) months (log-rank P=0.001; Figure 1), and 3-year OS rates of 28.4% vs. 6.3%. Amongst patients with oligoM1 disease, an AFP level ≥400 ng/mL at recurrence was associated with a median OS of 9.5 (95% CI: 4.5, 14.6) compared to 17.7 (95% CI: 16.1, 19.4) months for patients with AFP <400 ng/mL (log-rank P=0.015).

Association of clinical characteristics with survival
Among all patients with HCC recurrence (Table 4), AFP ≥400 ng/mL at recurrence was associated with inferior OS [hazard ratio (HR): 2.63; 95% CI: 1.17, 5.91; P=0.02] while oligoM1 disease (HR: 0.34; 95% CI: 0.17, 0.68; P=0.002) and receipt of MDT-All (HR: 0.37; 95% CI: 0.17, 0.81; P=0.012) were associated with improved OS. Age, sex, race, and Eastern Cooperative Oncology Group (ECOG) performance status at the time of recurrence were not associated with OS. Given the expected dependency between the receipt of MDT-All and oligoM1 disease, MDT-All was not included in the multivariable regression model, while elevated AFP and oligoM1 disease were included. Both elevated AFP ≥400 ng/mL (HR: 2.44; 95% CI: 1.08, 5.52; P=0.033) and oligoM1 disease (HR: 0.42; 95% CI: 0.21, 0.87; P=0.018) remained independently associated with OS in multivariable analysis.
Table 4
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 0.99 (0.94, 1.03) | 0.512 | |||
| Sex | |||||
| Male | 1 (reference) | – | |||
| Female | 0.49 (0.17, 1.39) | 0.179 | |||
| Race | |||||
| White | 1 (reference) | 0.432 | |||
| Black | 1.62 (0.75, 3.52) | 0.22 | |||
| Asian | 0.83 (0.19, 3.56) | 0.803 | |||
| ECOG PS at recurrence | |||||
| 0 to 1 | 1 (reference) | – | |||
| 2 and above | 1.43 (0.72, 2.85) | 0.306 | |||
| AFP ≥400 ng/mL | |||||
| No | 1 (reference) | – | 1 (reference) | – | |
| Yes | 2.63 (1.17, 5.91) | 0.02 | 2.44 (1.08, 5.52) | 0.033 | |
| OligoM1 | |||||
| No | 1 (reference) | – | 1 (reference) | – | |
| Yes | 0.34 (0.17, 0.68) | 0.002 | 0.42 (0.21, 0.87) | 0.018 | |
| MDT to all sites | |||||
| No | 1 (reference) | – | |||
| Yes | 0.37 (0.17, 0.81) | 0.012 | |||
| Systemic therapy received | |||||
| No | 1 (reference) | – | |||
| Yes | 1.07 (0.54, 2.1) | 0.847 | |||
| Time to recurrence (years) | 0.98 (0.94, 1.01) | 0.22 | |||
| Liver disease at recurrence | |||||
| No | 1 (reference) | – | |||
| Yes | 0.91 (0.42, 1.98) | 0.819 | |||
OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; AFP, alpha-fetoprotein; oligoM1, oligorecurrent; MDT, metastasis-directed therapy.
Effect of MDT on outcomes in patients with initial oligoM1 disease
Amongst the 27 patients with oligoM1 disease at recurrence, 15 (55.6%) received MDT-All, 3 (11.1%) received MDT to at least one metastasis, and 9 (33.3%) received no MDT. There was no significant difference in the receipt of systemic therapy, ECOG performance status or AFP at recurrence between MDT-All and MDT to limited or no sites (Table 5). Patients who received MDT-All more frequently had liver involvement (5/15, 33.3%) than those who did not receive MDT-All (0/12, 0.0%; P=0.047), but otherwise there was no significant difference in the site of metastases between the two groups. Two patients underwent surgery as part of MDT-All in our cohort. One patient underwent a Whipple resection in the setting of a mass causing extrinsic compression of the common bile duct, with negative margins; this patient subsequently developed polymetastatic recurrence however maintained local control at the resection site. The second patient had a humeral metastasis causing pathological fracture and underwent radical resection with placement of a humeral head prosthesis, followed by post-operative palliative radiation therapy at an outside facility. The patient maintained local control but subsequently was found to have bone metastases of the skull which were treated with stereotactic radiation therapy. Only 27% of patients who received MDT-All initiated systemic therapy prior to polyprogression or death compared to 58% amongst patients who did not receive MDT-All, though this difference did not reach statistical significance.
Table 5
| Characteristics | Received MDT-All | Overall (n=27) | P value | |
|---|---|---|---|---|
| No (n=12) | Yes (n=15) | |||
| Age (years) | 58.3 (44.3, 70.7) | 67.3 (44.3, 70.7) | 58.9 (44.3, 70.7) | 0.548 |
| Sex | 0.182 | |||
| Male | 11 (91.7) | 10 (66.7) | 21 (77.8) | |
| Female | 1 (8.3) | 5 (33.3) | 6 (22.2) | |
| Race | 0.107 | |||
| White | 5 (41.7) | 12 (80.0) | 17 (63.0) | |
| Black | 6 (50.0) | 2 (13.3) | 8 (29.6) | |
| Asian | 1 (8.3) | 1 (6.7) | 2 (7.4) | |
| ECOG PS at recurrence | 0.398 | |||
| 0 to 1 | 7 (58.3) | 12 (80.0) | 19 (70.4) | |
| 2 and above | 5 (41.7) | 3 (20.0) | 8 (29.6) | |
| Number of lesions at recurrence | 2.0 (1.0, 3.0) | 1.0 (1.0, 3.0) | 1.0 (1.0, 3.0) | 0.083 |
| Number of metastatic sites at first failure | 0.612 | |||
| 1 | 10 (83.3) | 12 (80.0) | 22 (81.5) | |
| 2 | 2 (16.7) | 3 (20.0) | 5 (18.5) | |
| AFP at recurrence (ng/mL) | 166.85 | 26.4 (1.8, 3,864) | 34 (1.2, 365,210) | 0.332 |
| AFP ≥400 ng/mL‡ | 3 (25.0) | 3 (21.4) | 6 (23.1) | >0.99 |
| Site of first failure | ||||
| Liver | 0 (0.0) | 5 (33.3) | 5 (18.5) | 0.047 |
| Abdominal nodes | 5 (41.7) | 4 (26.7) | 9 (33.3) | 0.448 |
| Lungs | 2 (16.7) | 1 (6.7) | 3 (11.1) | 0.569 |
| Mediastinal nodes | 1 (8.3) | 1 (6.7) | 2 (7.4) | >0.99 |
| Adrenals | 1 (8.3) | 1 (6.7) | 2 (7.4) | >0.99 |
| Bone | 3 (25.0) | 3 (20.0) | 6 (22.2) | >0.99 |
| Brain | 0 (0.0) | 1 (6.7) | 1 (3.7) | >0.99 |
| Other† | 2 (16.7) | 0 (0.0) | 2 (7.4) | >0.99 |
| Peritoneal cavity | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Time to recurrence (months) | 10.2 (2.7, 32.8) | 13.8 (0.1, 41.3) | 10.6 (0.1, 41.3) | 0.719 |
| Year of recurrence | ||||
| 2005–2016 | 7 (58.3) | 7 (46.7) | 14 (51.9) | 0.704 |
| 2017–2022 | 5 (41.7) | 8 (53.3) | 13 (48.1) | |
| Initiated systemic therapy prior to polyprogression‡ | 7 (58.3) | 4 (28.6) | 11 (42.3) | 0.233 |
Data are presented as n (%) or median (range). †, other sites include kidney and abdominal wall; ‡, n=26 for this analysis due to 1 patient in the MDT-All arm with missing data for this characteristic. OligoM1, oligorecurrent; MDT, metastasis-directed therapy; MDT-All, MDT to all sites of disease; ECOG, Eastern Cooperative Oncology Group; PS, performance status; AFP, alpha-fetoprotein; N/A, not available.
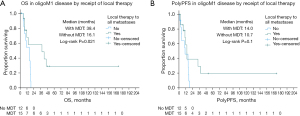
OS was improved amongst oligoM1 patients who received MDT-All compared to those who did not (median 38.4 vs. 16.1 months, log-rank P=0.021; Figure 2A). Progression of disease was common in patients with initial oligoM1 disease, with 21 (77.8%) patients experiencing progression following initial recurrence. PolyPFS was numerically improved with MDT-All but this difference was non-significant (median 14.0 vs. 10.7 months, log-rank P=0.1; Figure 2B). Among OligoM1 patients who did not receive MDT-All, 14 patients (93%) experienced progression to polyM1 state and all died within 19 months of initial recurrence. Among those who did receive MDT-All, 3 (11%) patients survived to 4 or more years of follow-up, with survival durations of 48 and 52 months in two patients, while a third patient remained alive without evidence of disease at greater than 14 years of follow-up.
Discussion
In this study we comprehensively characterized patterns of HCC recurrence following liver transplantation. We demonstrated that a significant subset of patients initially presented with limited sites of grossly evident recurrence. Most of these patients progressed to the polyM1 state and OS was poor. However, long-term survival was achieved in a subset of these patients who received MDT at all recurrence sites, suggesting that MDT may play an important role in achieving prolonged survival.
We defined and studied the oligoM1 state amongst patients with recurrent HCC following liver transplantation, and evaluated the benefit of MDT in this specific clinical context. HCC recurrence, at least at first detection, was often limited, as 63% of patients met criteria for oligoM1 recurrence. Patients who undergo liver transplantation for HCC require continued, lifelong immunosuppression, complicating their eligibility for systemic therapies including immune checkpoint inhibitors (11). Immune suppression has been demonstrated in other disease sites to be associated with poorer outcomes (12). Given possible variability in the benefit of MDT for oligometastatic disease for specific cancer disease sites (5), it is therefore valuable to attempt to identify a cohort of patients with recurrent HCC following liver transplantation who may benefit from aggressive application of local therapies. We demonstrated that treatment of all sites of recurrence with local therapies for oligoM1 recurrence was associated with improved OS outcomes relative to oligoM1 patients who did not undergo this treatment. While the numerical benefit of MDT-All on polyPFS outcomes amongst oligoM1 patients in this study did not reach statistical significance, possibly owing to the small study size, there again appeared to be a subset of patients at the tail of the survival curve for whom receipt of MDT-All may have delayed polyprogression or polydeath, consistent with the results for OS.
The potential benefit of curative-intent surgery or ablation in patients with recurrent HCC following liver transplantation has been reported previously in a cohort of patients treated from 2000–2012, in which ineligibility for curative-intent therapy at the time of recurrence, in addition to early recurrence and elevated AFP levels, were independently associated with poor survival outcomes (10). A variety of metastasis-directed local therapies are available, including high-dose/ablative radiation therapy. The choice of which particular MDT is used should depend upon the clinical context, in particular factoring in tumor location and size and expected efficacy and morbidity of the treatment. Many trials evaluating the role of MDT for oligometastatic disease have used radiation as the MDT.
Taken together, our study suggests aggressive MDT at initial recurrence may delay disease progression and improve survival in patients presenting with limited recurrence. Moreover, the ongoing long-term survival (>4 years) of multiple patients with oligoM1 recurrence who received MDT-All suggests that MDT may be curative in select patients.
Additionally, our findings suggest that pre-transplant characteristics, specifically the presence of microvascular invasion and poorly differentiated HCC, are associated with polymetastatic recurrence. A number of clinical factors have previously been identified as a risk factor for overall recurrence, including pre-treatment AFP (13) and microvascular invasion (14). Our study further underscores a potential negative impact of microvascular invasion on survival outcomes in patients with HCC following liver transplantation who recur. Consideration of pre-transplant clinical factors associated with microvascular invasion, including a prior history of microvascular invasion on prior resection (15) may aid in identifying patients who are at higher risk of microvascular invasion and subsequent polyM1, however further investigation of the impact on outcomes of such an approach would be required prior to clinical implementation.
Limitations of this study include the heterogeneity in modalities that constituted MDT in this study, which may reflect the need to consider multiple MDT modalities depending on the anatomical location of recurrence as well as patient performance status and co-morbidities. The retrospective nature of the study also makes selection bias a possibility. For example, patients with oligometastatic disease who received MDT may have been selected based upon favorable prognostic factors compared to those who did not. However, in our study there did not appear to be differences in the performance status or frequency of receipt of systemic therapy amongst patient with oligometastatic disease who did or did not receive MDT-All, reducing concerns for selection bias. Furthermore, lack of blinding when assessing imaging progression is a limitation of this study, owing to its retrospective nature. Notably, OS was significantly improved with MDT-All, suggesting that this limitation did not impact our results. In the absence of a multi-institutional prospective trial, this study provides valuable information to clinicians both in terms of prognostic guidance for patients as well as the potential value of local MDT in patients with post-transplant recurrence of HCC.
Conclusions
The presence of microvascular invasion on explant pathology appears to be a risk factor for aggressive polyM1 recurrence. Patients with recurrent HCC following liver transplantation with oligoM1 disease (≤3 metastases) exhibit favorable survival outcomes compared to those with polymetastatic disease at recurrence. Furthermore, in selected patients with oligoM1, the receipt of local MDT-All may improve survival outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-541/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-541/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-541/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-541/coif). A.K. previously received consulting fees from AstraZeneca. J.J.M. receives royalties from UpToDate and Springer and previously received research support from Boston Scientific (funds paid to institution). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Johns Hopkins Medicine (No. IRB00344985) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:650-9. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. [Crossref] [PubMed]
- Tsai CJ, Yang JT, Guttmann DM, et al. Final analysis of Consolidative Use of Radiotherapy to Block (CURB) oligoprogression trial-a randomized study of stereotactic body radiotherapy for oligoprogressive metastatic lung and breast cancers. Int J Radiat Oncol Biol Phys 2022;114:1061. [Crossref]
- Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol 2020;148:157-66. [Crossref] [PubMed]
- Kim K, Kim TH, Kim TH, et al. Efficacy of Local Therapy for Oligometastatic Hepatocellular Carcinoma: A Propensity Score Matched Analysis. J Hepatocell Carcinoma 2021;8:35-44. [Crossref] [PubMed]
- Kornberg A, Küpper B, Tannapfel A, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol 2010;36:275-80. [Crossref] [PubMed]
- Regalia E, Fassati LR, Valente U, et al. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg 1998;5:29-34. [Crossref] [PubMed]
- Sapisochin G, Goldaracena N, Astete S, et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol 2015;22:2286-94. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Tam S, Yao CMKL, Amit M, et al. Association of Immunosuppression With Outcomes of Patients With Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol Head Neck Surg 2020;146:128-35. [Crossref] [PubMed]
- Sapena V, Enea M, Torres F, et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut 2022;71:593-604. [Crossref] [PubMed]
- Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108-13. [Crossref] [PubMed]
- Heinrich S, Mittler J, Theurer J, et al. Microvascular invasion of hepatocellular carcinoma predicts microvascular invasion of its recurrence: potential implications for salvage liver transplantation? Hepatobiliary Surg Nutr 2023;12:183-91. [Crossref] [PubMed]


