
Development and validation of a nomogram to predict pathological complete response in patients with locally advanced gastric adenocarcinoma treated with neoadjuvant chemotherapy in combination with PD-1 antibodies
Highlight box
Key findings
• Neoadjuvant chemotherapy combined with programmed death 1 (PD-1) antibodies may be the preferred option for patients with advanced gastric adenocarcinoma with a small tumor diameter, no or few lymph node metastases, and high combined positive score.
What is known and what is new?
• The factors that impact the efficacy of neoadjuvant chemotherapy and PD-1 antibody combination therapy in gastric adenocarcinoma remain unclear.
• We conducted a comprehensive evaluation and analysis of the factors impacting pathological complete response (pCR) in patients with advanced gastric adenocarcinoma treated at our institution using a two-drug regimen combined with camrelizumab. Subsequently, we developed and validated a nomogram for predicting pCR in these patients.
What is the implication, and what should change now?
• The results of our analysis may improve outcomes of preoperative treatment for gastric cancer patients and serve as a foundation for future related studies. To further validate the accuracy of these findings, large-scale, multicenter, prospective studies should be conducted.
Introduction
Background and objectives
Gastric cancer (GC) remains the most prevalent malignancy worldwide, with over one million new cases and 760,000 reported deaths in 2020 (equivalent to 1 in 13 global fatalities) (1). It ranks fifth globally in terms of incidence and fourth in mortality, with adenocarcinoma accounting for more than 95% of all GCs (1,2). Total neoadjuvant therapy (TNT) and neoadjuvant therapy hold great promise in reducing tumor staging, improving surgical outcomes, and providing novel approaches for long-term disease control (3). This treatment paradigm has resulted in improved surgical as well as downstream survival outcomes for select patients, however, median overall survival remains less than 5 years and more biologically-informed therapeutic strategies are needed (4).
A related study has shown that tumor cells promote tumor progression and metastasis by overexpressing cell-surface programmed death ligand 1 (PD-L1) or by inducing PD-L1 expression on immune cells, using the programmed death 1 (PD-1)/PD-L1 pathway to inhibit the body’s immune response and further promote immune escape (5). This mechanism is currently believed to be a major factor in promoting tumor immune escape (6). Neoadjuvant chemoimmunotherapy involves the use of both chemotherapy and immunotherapy agents. Chemotherapy drugs are administered to target and kill cancer cells, while immunotherapy drugs enhance the body’s immune response to fight against cancer. The immunotherapy approach involves the use of PD-1 monoclonal antibodies to obstruct the PD-1/PD-L1 pathway, thereby stimulating T cells and reinstating the body’s antitumor immunity, thus preventing tumor cells from evading immune surveillance (7). The administration of PD-1 antibodies has been shown to be associated with an increase in the rate of pathological remission in patients with locally advanced gastric adenocarcinoma, facilitating the conversion of unresectable tumors into resectable ones. Moreover, its combination with chemotherapy and targeted therapy further augments the anti-tumor efficacy. PD-1/PD-L1 blockade represents a significant advancement for cancer therapy in recent years (8). The efficacy of neoadjuvant chemotherapy combined with PD-1 antibodies for advanced gastric adenocarcinoma remains a subject of debate.
Previous study has indicated that the survival benefit of neoadjuvant therapy is dependent on the pathological complete response (pCR) to both chemotherapeutic agents and PD-1 antibodies (9). pCR was chosen as the marker of high sensitivity to neoadjuvant therapy because it is a universal, objective and reproducible indicator that reflects the complete eradication of the tumor cells in the resected specimen after neoadjuvant therapy. It provides a reliable and accurate assessment of the effectiveness of neoadjuvant chemotherapy and is widely recognized as a valuable endpoint in clinical trials evaluating neoadjuvant treatment for gastric adenocarcinoma. Several clinical trials have demonstrated that neoadjuvant chemotherapy and PD-1 antibodies is advantageous in reducing tumor stage and achieving pCR as compared to neoadjuvant chemotherapy alone, leading to improved radical resection rates (R0) (10). For resectable GC, neoadjuvant chemoimmunotherapy confers superior survival advantages compared to neoadjuvant chemotherapy (11). Furthermore, there is no increase in postoperative complications, and patients’ quality of life is enhanced (12-15). Superior overall survival and progression-free survival are more likely to occur in patients who achieve pCR after neoadjuvant therapy. For nonresponders, PD-1 antibody and chemotherapy combination therapy offers no survival benefit, only toxicity and the risk of tumor progression during chemotherapy, which may prevent surgical resection. Therefore, pre-screening of patients with a propensity for pCR to neoadjuvant therapy is imperative in order to provide personalized treatment.
At present, the predictive factors determining the effectiveness of neoadjuvant chemotherapy combined with PD-1 antibody therapy in gastric adenocarcinoma remain unclear. Research indicates that the efficacy of immunotherapy may be influenced by factors such as tumor stage, tumor mutation burden (TMB), PD-1 expression, microsatellite instability (MSI), and immune cell infiltration (16-18). However, it should be noted that these factors are not deterministic and may vary depending on the tumor type and treatment approach. Therefore, the ability to predict pCR following neoadjuvant chemotherapy in combination with PD-1 antibody therapy has significant clinical implications for the personalized treatment of patients with advanced gastric adenocarcinoma.
Nomograms are widely regarded as a viable model type for predicting the likelihood of positive outcomes in individuals based on regression analysis (19), through which clinicians can conveniently predict endpoint events. Currently, there is no application of this predictive model in forecasting the efficacy of chemoimmunotherapy for gastric adenocarcinoma. In this study, we conducted an analysis of clinical and pathological data from patients with advanced gastric adenocarcinoma who underwent neoadjuvant chemotherapy and PD-1 antibody combination therapy. We also investigated the factors that influence pCR in gastric adenocarcinoma. The nomogram prediction model, in which multiple relevant factors were integrated, was developed and validated internally. The prediction model provided a more accurate evaluation and reference for predicting the clinical efficacy of neoadjuvant chemotherapy plus PD-1 antibody therapy for patients with gastric adenocarcinoma. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-751/rc).
Methods
Source of data
A retrospective study design was employed to collect clinical data from 52 patients with gastric adenocarcinoma who underwent neoadjuvant chemotherapy and PD-1 antibody (camrelizumab) combination therapy, as well as radical gastrectomy at the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital between January 2019 and December 2020.
Participants
The inclusion criteria were as follows: (I) T3/4N0/+ gastric and gastroesophageal junction adenocarcinoma assessed with computed tomography; (II) preoperative assessment revealing the absence of any unresectable factors, with the patient undergoing a minimum of 3–4 cycles of camrelizumab in combination with 2-drug chemotherapy (fluorouracil plus oxaliplatin); (III) absence of distant metastasis; (IV) undergoing radical gastrectomy with D2 lymph node dissection; (V) complete pathological and clinical data available; and (VI) relevant informed consent obtained prior to surgery. Meanwhile, the exclusion criteria were the following: patients who had undergone palliative resection or were unable to complete radical gastrectomy, as well as those with comorbidities such as mental and nervous system disorders that significantly impact cognitive function. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Review Committee of the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital (No. 2021-499-002) and individual consent for this retrospective analysis was waived.
The clinical data collected from patients in this study included gender; age; cT stage; cN stage; tumor location; degree of differentiation; combined positive score (CPS) (PD-L1 IHC 22C3 pharmDx; Agilent, Santa Clara, CA, USA); levels of carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), CA199, CA125, and CA724 biomarker; and tumor diameter (Table 1).
Table 1
| Characteristics | Total (n=52) | Non-pCR (n=42) | pCR (n=10) | P value |
|---|---|---|---|---|
| Sex | 0.137 | |||
| Male | 35 | 26 | 9 | |
| Female | 17 | 16 | 1 | |
| Age (years) | 57 [16] | 58 [16] | 57 [16] | 0.836 |
| CPS | 0.007 | |||
| <1 | 19 | 19 | 0 | |
| 1–10 | 26 | 19 | 7 | |
| >10 | 7 | 4 | 3 | |
| Clinical T stage | 0.734 | |||
| T3 | 29 | 24 | 5 | |
| T4 | 23 | 18 | 5 | |
| Clinical N stage | 0.015 | |||
| N0 | 15 | 10 | 5 | |
| N1 | 15 | 10 | 5 | |
| N2 | 15 | 15 | 0 | |
| N3 | 7 | 7 | 0 | |
| Tumor differentiation | 0.179 | |||
| Moderately to well differentiated | 1 | 1 | 0 | |
| Moderately differentiated | 4 | 4 | 0 | |
| Poorly to moderately differentiated | 22 | 20 | 2 | |
| Poor differentiation | 25 | 17 | 8 | |
| Tumor location | 0.254 | |||
| Cardia | 31 | 25 | 6 | |
| Fundus of stomach | 4 | 2 | 2 | |
| Stomach body | 10 | 8 | 2 | |
| Gastric antral | 7 | 7 | 0 | |
| Tumor diameter | 0.014 | |||
| <2.25 cm | 18 | 11 | 7 | |
| ≥2.25 cm | 34 | 31 | 3 | |
| CEA | 0.608 | |||
| <4.7 ng/mL | 45 | 37 | 8 | |
| ≥4.7 ng/mL | 7 | 5 | 2 | |
| CA199 | 0.12 | |||
| <34 U/mL | 45 | 38 | 7 | |
| ≥34 U/mL | 7 | 4 | 3 | |
| CA724 | 0.723 | |||
| <6.9 U/mL | 34 | 28 | 6 | |
| ≥6.9 U/mL | 18 | 14 | 4 | |
| CA125 | 0.999 | |||
| <35 U/mL | 47 | 38 | 9 | |
| ≥35 U/mL | 5 | 4 | 1 | |
| AFP | 0.576 | |||
| <25 ng/mL | 48 | 38 | 10 | |
| ≥25 ng/mL | 4 | 4 | 0 |
Age is represented as the median [interquartile range]. The remaining variables are categorical and are represented by counts. pCR, pathological complete response; CPS, combined positive score; CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein.
The treatment strategies for all patients were devised by a multidisciplinary team comprising physicians and surgeons. The regimen included at least 3 to 4 cycles of neoadjuvant chemo-immunotherapy. Neoadjuvant immunotherapy was camrelizumab (20), and neoadjuvant chemotherapy was a 2-drug regimen of a fluorouracil plus oxaliplatin. The regimen was as follows: (I) camrelizumab plus FOLFOX, including camrelizumab (200 mg, ivgtt, D1), oxaliplatin (85 mg/m2), and continuous intravenous injection of fluorouracil (2,800 mg/m2) over 48 h, every 2 weeks; (II) camrelizumab plus SOX, including camrelizumab (200 mg, ivgtt, d1), intravenous injection of oxaliplatin (130 mg/m2), and tegafur gimeracil oteracil potassium capsule (40–60 mg bid D1–D14) every 3 weeks; and (III) camrelizumab plus XELOX, including camrelizumab (200 mg, ivgtt, d1), oxaliplatin (130 mg/m2), and capecitabine (1,000 mg/m2 bid D1–D14) every 3 weeks.
All enrolled patients underwent curative tumor resection, either total or subtotal gastrectomy, accompanied by D2 lymphadenectomy.
Outcome
All resected specimens were submitted to the pathology department and evaluated by the attending pathologist based on Ryan grading criteria (21). The absence of residual cancer cells in both the primary tumor and dissected regional lymph nodes (TRG0) was defined as pCR, while the presence of residual cancer cells in postoperative tissue [tumor regression grade (TRG) 1–3] was defined as non-pCR.
Predictors
The continuous variables examined in this study encompassed age, CPS, tumor diameter, and tumor markers. Patients were categorized into three groups (<1, 1–10, >10) based on the CPS cutoff value established in previous large-scale study of immunotherapy (22). Tumor markers were quantified in the peripheral venous blood of patients using the original reagent from the Roche E601 detection instrument. According to the instrument’s reference values, cutoff values for CEA, CA125, CA199, CA724, and AFP were set to 4.7 ng/mL, 35 U/mL, 34 U/mL, 6.9 U/mL, and 25 ng/mL, respectively. In this study, the receiver operating characteristic (ROC) curve was generated using SPSS software (IBM Corp., Armonk, NY, USA), and the cutoff value corresponding to the maximum Youden index was identified as the optimal threshold for the tumor diameter (23). The final analysis revealed that the optimal cutoff value for tumor diameter was 2.25 cm.
Statistical analysis methods
The normality of continuous data was assessed through the Kolmogorov-Smirnov test. Parameters with a nonnormal distribution are expressed as the median with interquartile range (IQR) and were analyzed using appropriate nonparametric tests including the Mann-Whitney test or Kruskal-Wallis test. The chi-squared test was employed to analyze categorical variables. Variables that showed a significant difference (P<0.05) in univariate analysis were included for further multivariate analysis. Binary logistic regression using the forward method was employed to conduct multivariate analysis of risk factors associated with pCR, while the Hosmer-Lemeshow test was used to assess model fit. A 2-tailed hypothesis test with a significance level of 0.05 was used (Table 2).
Table 2
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| CPS | 6.364 | 1.247–32.485 | 0.026 |
| Clinical N stage | 0.215 | 0.054–0.858 | 0.03 |
| Tumor diameter | 0.112 | 0.016–0.774 | 0.026 |
CPS, combined positive score; OR, odds ratio; CI, confidence interval.
Nomogram construction
Based on the prognostic factors, a nomogram was formulated using the “survival” package in R studio version 4.2.3 software. Each predictor was displayed on a separate row in a plot, with varying numbers of points assigned to different levels of the predictor variable. The cumulative point axis was depicted at the end of the nomogram, with higher total points indicating a lower probability of achieving pCR. The predictive ability of the model was assessed using the Harrell concordance index (C-index). The C-index and 95% CIs were used to assess the predictive discrimination, and the range of values was from 0 to 1, with values closer to 1 indicating superior model discrimination. Internal verification was completed via the bootstrapping method, and the calibration curve of the nomogram was constructed to illustrate the correlation between predicted and observed outcomes.
Results
Participants
Based on the inclusion and exclusion criteria, a total of 52 patients diagnosed with gastric adenocarcinoma were enrolled in this study. There were 35 male and 17 female participants, with a median age of 57 years. The 52 patients were categorized into two groups based on TRG classification (10 pCR and 42 non-pCR). More than half of patients had tumor sites in the cardia (n=31, 60%). The T3 stage (n=29, 56%) was the most prevalent, followed by the T4 stage (n=23, 44%). Poor differentiation (n=25, 48%) was the most common type of differentiation, followed by poorly to moderately differentiated (n=22, 42%), moderately differentiated (n=4, 8.0%), and moderately to well differentiated (n=1, 2.0%). Univariable associations between the clinical parameters and pCR are shown in Table 1. Clinical N stage, CPS, and tumor diameter exhibited statistically significant associations.
Multivariate logistic regression analysis
Multivariate analysis revealed that cN [odds ratio (OR): 0.215; 95% CI: 0.054–0.858], CPS (OR: 6.364; 95% CI: 1.247–32.485), and tumor diameter (OR: 0.112; 95% CI: 0.016–0.774) were independent predictors of pCR following neoadjuvant chemotherapy in combination with PD-1 antibody therapy (Table 2).
Construction of the nomogram
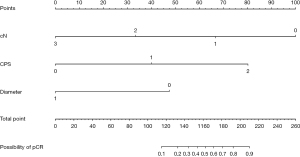
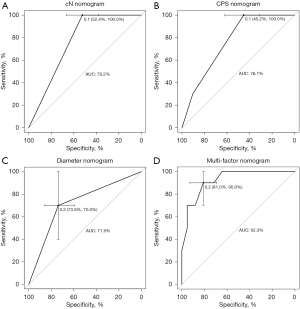
Nomogram prediction model for pCR after neoadjuvant chemotherapy and PD-1 antibody combination therapy was based on multifactorial analysis using R software (Figure 1). The corresponding scores were obtained by projecting each variable onto the “Points” axis and then aggregated to derive a total score that corresponded to the predicted outcome. The results of the ROC curve analysis and the C-index of the univariate nomogram prediction model were the following: cN =0.762, CPS =0.761, and diameter =0.719 (Figure 2A-2C). The multifactor nomogram model exhibited a high predictive accuracy, with a C-index value of 0.923 (Figure 2D). The analysis of the calibration curve demonstrated that the nomogram model evaluated in this study exhibited superior predictive ability for pCR following treatment with PD-1 antibody combined with chemotherapy (Figure 3).
Discussion
Neoadjuvant chemotherapy combined with PD-1 antibody immunotherapy has demonstrated a more complete and durable response compared to chemotherapy alone. As a result, it has the potential to become a standard treatment approach for resectable gastric adenocarcinoma (24). This approach aims to achieve tumor downstaging, volume reduction, and eradication of positive lymph nodes, ultimately improving patient prognosis (25,26). Clinical trials have shown that neoadjuvant PD-1 blockade plus chemotherapy neither delays the timing of surgery nor increases the rate of postoperative complications (16). Such therapy may also be better tolerated than triplet cytotoxic chemotherapy, which has higher risk of cytopenias and neuropathy.
The limitations in the efficacy of current immunotherapies, mainly involving immune checkpoint inhibitors, have emphasized the need to enhance our comprehension of combination therapeutic strategies for improving outcomes among patients who exhibit poor response to single-agent immunotherapy (27). To further enhance the efficacy of neoadjuvant therapy in patients with advanced gastric adenocarcinoma, multimodal treatment regimens incorporating neoadjuvant chemotherapy and PD-1 antibody immunotherapy prior to surgical resection are being increasingly employed (28). However, the choice of population for neoadjuvant chemotherapy in combination with PD-1 antibody therapy remains controversial.
pCR status reflects the sensitivity of tumor cells to preoperative treatment and represents a crucial indicator of the efficacy of combined treatment with PD-1 blockade. Achieving a pCR following neoadjuvant chemotherapy is strongly associated with prolonged survival and the possibility of undergoing radical surgery (29). Therefore, the ability to predict pCR can provide personalized prognostication as well as surrogate for clinical efficacy in drug development of immune checkpoint inhibitors in the neoadjuvant setting. In this study, pCR was observed in 10 out of 52 patients (19.2%) who received neoadjuvant chemotherapy combined with PD-1 immunotherapy for advanced gastric adenocarcinoma. This pCR rate is higher than the reported range of 10.4% to 16.0% for neoadjuvant chemotherapy alone (30-32). The findings of this study demonstrate that the combination of PD-1 antibody and a 2-drug regimen chemotherapy can yield a comparable or possibly higher pCR rate for advanced gastric adenocarcinoma compared to neoadjuvant chemotherapy alone, providing a valuable opportunity for surgical resection treatment (33).
We evaluated and analyzed the factors that impact the efficacy of neoadjuvant chemotherapy with a 2-drug regimen combined with camrelizumab treatment at our institution and developed a nomogram prediction model. The multifactorial analysis of 52 clinical cases demonstrated significant associations between pCR rates and CPS, cN stage, and tumor diameter following neoadjuvant chemotherapy combined with PD-1 antibody treatment for gastric adenocarcinoma. CPS is being increasingly used as a reliable indicator of immunotherapy response in advanced gastric adenocarcinoma, owing to its high predictive value (34). CPS is calculated by percent of PD-L1 positive tumor and immune cells out of total viable tumor cells (35). In this study, CPS was positively correlated with pCR rate, and the high-CPS (CPS >10) group was more sensitive to neoadjuvant chemotherapy combined with PD-1 antibody compared to the low-CPS group. Therefore, preoperative evaluation of CPS is a predictive value for patients with progressive GC (17). The results of the multifactorial regression analysis indicated that cN stage is significantly associated with pCR, likely it correlates with tumor burden and aggressiveness (36). Lymph node micrometastases may decrease the rate of pCR following neoadjuvant PD-1 blockade plus chemotherapy, thereby impacting the prognosis of patients with advanced GC. Therefore, precise evaluation of cN staging and identification of patients with low or no lymph node metastases for this regimen has an important prognostic role in evaluating patients for neoadjuvant chemo-immunotherapy (37).
Tumor size and tumor cell numbers have been considered important determinants of tumor response to treatment. Larger tumors may develop resistance through vascularization and cellular heterogeneity (36). The findings of this investigation revealed a negative correlation between tumor diameter and the rate of achieving pCR. The PD-1 antibody-based combination chemotherapy regimen may be the preferred treatment option for patients with advanced gastric adenocarcinoma with a small tumor diameter.
The PD-1/PD-L1 pathway plays a pivotal role in the pathogenesis of advanced gastric adenocarcinoma, as evidenced by relevant meta-analyses and multiple clinical trials highlighting its significance (6). PD-L1/PD-1 antibodies can be used safely in patients with GC and provide patients with sustained anti-cancer activity (15,24,38). Immunotherapy has the potential to stimulate a systemic immune response, which may help prevent the occurrence of tumor micrometastasis. The positive correlation between CPS and treatment outcome observed in this study is consistent with previous findings, such as those reported in the Keynote-059 phase II trial (39-41).
Our nomogram was constructed based on three easily assessable variables, namely cN stage, CPS and tumor diameter, making it more applicable in clinical settings. By comparing the C-index of the unifactorial model with that of the multifactorial model, it was observed that the predictive performance of the former was inferior to that of the latter. Therefore, all three variables in the nomogram model synergistically contribute to enhancing its discriminative ability. These three variables could also be used to enrich patient population for future studies testing neoadjuvant chemo-immunotherapy regimens. Combination therapies have become an important cornerstone in the treatment of gastric adenocarcinoma, and there are many ongoing clinical trials aimed at identifying new effective combinations of drugs. In the future, we can expect to see more personalized combination therapies that are tailored to the specific needs of each patient.
There are some limitations to this study. To begin, only a small group of individuals in Henan Province were examined, and the applicability of the findings to other neoadjuvant treatments is unknown. Moreover, the sample size was too small to allow for a division into a training and validation set. Additionally, a retrospective design and not a randomized controlled design was employed. Thus, these limitations may have a negative impact on the accuracy of the results obtained in this study. However, our findings may nonetheless serve as a foundation for future related studies. To further validate the accuracy of these findings, large-scale, multicenter, prospective studies should be conducted.
Conclusions
Neoadjuvant chemotherapy combined with PD-1 antibodies may be the preferred option for patients with advanced gastric adenocarcinoma who have a small tumor diameter, no or few lymph node metastases, and high CPS. The presented nomogram model demonstrates the potential to predict pCR in advanced gastric adenocarcinoma patients, showcasing satisfactory predictive performance and potentially facilitating the implementation of personalized treatment strategies.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-751/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-751/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-751/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-751/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Review Committee of the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital (No. 2021-499-002) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167-92. [Crossref] [PubMed]
- Yang J, Greally M, Strong VE, et al. Perioperative versus total neoadjuvant chemotherapy in gastric cancer. J Gastrointest Oncol 2023;14:1193-203. [Crossref] [PubMed]
- Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023;26:1-25.
- Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol 2012;13:1129-32. [Crossref] [PubMed]
- Zhao Y, Bai Y, Shen M, et al. Therapeutic strategies for gastric cancer targeting immune cells: Future directions. Front Immunol 2022;13:992762. [Crossref] [PubMed]
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [Crossref] [PubMed]
- Xie F, Xu M, Lu J, et al. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer 2019;18:146. [Crossref] [PubMed]
- Karagkounis G, Thai L, Mace AG, et al. Prognostic Implications of Pathological Response to Neoadjuvant Chemoradiation in Pathologic Stage III Rectal Cancer. Ann Surg 2019;269:1117-23. [Crossref] [PubMed]
- Li S, Yu W, Xie F, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun 2023;14:8. [Crossref] [PubMed]
- Jeung HC, Moon YW, Rha SY, et al. Phase III trial of adjuvant 5-fluorouracil and adriamycin versus 5-fluorouracil, adriamycin, and polyadenylic-polyuridylic acid (poly A:U) for locally advanced gastric cancer after curative surgery: final results of 15-year follow-up. Ann Oncol 2008;19:520-6. [Crossref] [PubMed]
- Wan T, Zhang XF, Liang C, et al. The Prognostic Value of a Pathologic Complete Response After Neoadjuvant Therapy for Digestive Cancer: Systematic Review and Meta-Analysis of 21 Studies. Ann Surg Oncol 2019;26:1412-20. [Crossref] [PubMed]
- Fields RC, Strong VE, Gönen M, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer 2011;104:1840-7. [Crossref] [PubMed]
- Kawazoe A, Shitara K, Boku N, et al. Current status of immunotherapy for advanced gastric cancer. Jpn J Clin Oncol 2021;51:20-7. [Crossref] [PubMed]
- Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One 2017;12:e0182692. [Crossref] [PubMed]
- Tang X, Li M, Wu X, et al. Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer. Oncoimmunology 2022;11:2135819. [Crossref] [PubMed]
- Zurlo IV, Schino M, Strippoli A, et al. Predictive value of NLR, TILs (CD4+/CD8+) and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Cancer Immunol Immunother 2022;71:45-55. [Crossref] [PubMed]
- Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 Expression and Other Variables With Benefit From Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer: Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. JAMA Oncol 2022;8:1456-65. [Crossref] [PubMed]
- Park SY. Nomogram: An analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg 2018;155:1793. [Crossref] [PubMed]
- Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs 2019;79:1355-61. [Crossref] [PubMed]
- Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141-6. [Crossref] [PubMed]
- Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol 2022;29:1559-74. [Crossref] [PubMed]
- Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med 2013;4:627-35. [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Salas-Benito D, Pérez-Gracia JL, Ponz-Sarvisé M, et al. Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov 2021;11:1353-67. [Crossref] [PubMed]
- Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. [Crossref] [PubMed]
- Yap TA, Parkes EE, Peng W, et al. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov 2021;11:1368-97. [Crossref] [PubMed]
- Yuan Z, Cui H, Wang S, et al. Combining neoadjuvant chemotherapy with PD-1/PD-L1 inhibitors for locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: A systematic review and meta-analysis. Front Oncol 2023;13:1103320. [Crossref] [PubMed]
- Wang T, Wang N, Zhou H, et al. Long-term survival results of patients with locally advanced gastric cancer and pathological complete response after neoadjuvant chemotherapy and resection. Transl Cancer Res 2020;9:529-35. [Crossref] [PubMed]
- Koh YW, Park YS, Ryu MH, et al. Postoperative nodal status and diffuse-type histology are independent prognostic factors in resectable advanced gastric carcinomas after preoperative chemotherapy. Am J Surg Pathol 2013;37:1022-9. [Crossref] [PubMed]
- Anderson E, LeVee A, Kim S, et al. A Comparison of Clinicopathologic Outcomes Across Neoadjuvant and Adjuvant Treatment Modalities in Resectable Gastric Cancer. JAMA Netw Open 2021;4:e2138432. [Crossref] [PubMed]
- Wang X, Li S, Sun Y, et al. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer 2021;21:20. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol 2017;3:1237-44. [Crossref] [PubMed]
- Yamashita K, Iwatsuki M, Harada K, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer 2020;23:95-104. [Crossref] [PubMed]
- Panda A, Mehnert JM, Hirshfield KM, et al. Immune Activation and Benefit From Avelumab in EBV-Positive Gastric Cancer. J Natl Cancer Inst 2018;110:316-20. [Crossref] [PubMed]
- MacGuill M, Mulligan E, Ravi N, et al. Clinicopathologic factors predicting complete pathological response to neoadjuvant chemoradiotherapy in esophageal cancer. Dis Esophagus 2006;19:273-6. [Crossref] [PubMed]
- Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934-9. [Crossref] [PubMed]
- Wu X, Gu Z, Chen Y, et al. Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J 2019;17:661-74. [Crossref] [PubMed]
- Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019;22:828-37. [Crossref] [PubMed]
- Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med 2019;143:330-7. [Crossref] [PubMed]
- Moehler M, Högner A, Wagner AD, et al. Recent progress and current challenges of immunotherapy in advanced/metastatic esophagogastric adenocarcinoma. Eur J Cancer 2022;176:13-29. [Crossref] [PubMed]



