
Effect of ramucirumab plus paclitaxel in advanced gastric cancer according to the status of programmed cell death-ligand 1 (PD-L1) expression
Highlight box
Key findings
• When conducting a comparative analysis among gastric cancer patients receiving ramucirumab plus paclitaxel, it was observed that individuals with a programmed cell death-ligand 1 (PD-L1) combined positive score (CPS) score of 10 or higher exhibited superior survival outcomes compared to those with lower scores.
What is known and what is new?
• No biomarker is available to predict the tumor response to ramucirumab plus paclitaxel in advanced gastric cancer (AGC) patients.
• Vascular endothelial growth factor receptor inhibition might be more effective in tumors with high PD-L1 expression.
What is the implication, and what should change now?
• PD-L1 CPS cutoff of 10 might be a novel biomarker to predict survival following ramucirumab plus paclitaxel in AGC patients.
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer-related death (1). The incidence of GC is highest in East Asian countries, including Korea, Japan, and China. In Korea, GC is the third most common cancer and the fourth leading cause of cancer-related death (2). Currently, the platinum plus fluoropyrimidine doublet combination is used as the first-line treatment (3), and ramucirumab plus paclitaxel is used as the second-line treatment in metastatic advanced GC (AGC) (4-6). Ramucirumab is a recombinant human IgG1-neutralizing monoclonal antibody (mAb) specific for the ectodomain of vascular endothelial growth factor receptor (VEGFR)2. Ramucirumab has been approved for use as monotherapy or in combination with paclitaxel for the treatment of patients with previously treated AGC (7). However, in clinical trials, ramucirumab in combination with paclitaxel demonstrated efficacy in only 28% of metastatic GC patients (5). No biomarker is available to predict the tumor response to these treatments.
Immunotherapy, which has emerged as a novel anticancer therapy, has become a strategy for treating various types of solid cancers, and many such therapies have already been approved and used, including for AGC. In the CheckMate 649 trial, combining nivolumab with chemotherapy offered overall survival (OS) and progression-free survival (PFS) superior to chemotherapy alone (8). Immunotherapy mainly targets programmed cell death-ligand 1 (PD-L1) or programmed cell death protein 1 (PD-1) because they regulate the immune activity of tumors. PD-L1 expression is regulated by various pathways, and tumors with low PD-L1 expression generally have less T cell infiltration than tumors with high PD-L1 expression (9,10). Ramucirumab, an anti-VEGFR mAb, inhibits angiogenesis and reduces tumor activity. It also causes hypoxia to increase the activity of effector T cells (11-13). PD-L1 might act upon VEGFR2 to induce cancer cell angiogenesis and metastasis (14).
In this work, we investigated the efficacy of combining ramucirumab with paclitaxel according to the status of PD-L1 expression in patients with AGC. We present this article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-418/rc).
Methods
Patients
We conducted an analysis on 117 out of 543 patients who received the combination of ramucirumab plus paclitaxel without undergoing clinical trials and had undergone PD-L1 testing (Figure 1). We analyzed retrospectively these 117 AGC patients who received second-line ramucirumab plus paclitaxel and were also tested for PD-L1 expression at Samsung Medical Center, Korea, between December 1, 2018, and February 28, 2022. The following clinicopathologic characteristics were collected for all 117 patients: age, sex, tumor site, initial disease status, pathology, chemotherapy, and survival. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki (as revised in 2013) and the Korea Good Clinical Practice guidelines. This study was approved by the Institutional Review Board at Samsung Medical Center (IRB No. 2022-12-078), and individual consent for this analysis was waived. Patients in the database were identified by patient number only, with personally identifiable information kept confidential according to the IRB protocol.
Tumor samples
Samples for analysis were collected from solid tumors and used to make formalin-fixed paraffin-embedded materials. They were collected as biopsies at diagnosis, surgical specimens, and repeat biopsies at the time of disease progression. The obtained tumor samples were not all collected at the same times. However, they were all collected prior to the start of second-line ramucirumab plus paclitaxel therapy.
Immunohistochemistry (IHC) of PD-L1
Tissue sections were freshly sliced into 4-µm sections, then affixed onto Fisherbrand Superfrost Plus Microscope Slides (Thermo Fisher, Waltham, Massachusetts, USA) and subjected to drying at 60 ℃ for one hour. IHC staining was conducted using a Dako Autostainer Link 48 system (Agilent Technologies, Santa Clara, California, USA) employing the Dako PD-L1 22C3 PharmDx kit (Agilent Technologies) in conjunction with the EnVision FLEX visualization system. Subsequently, the specimens were counterstained with hematoxylin following the manufacturer’s instructions. The quantification of PD-L1 protein expression was performed utilizing the combined positive score (CPS), which was calculated as the ratio of PD-L1-stained cells (including tumor cells, lymphocytes, and macrophages) to the total count of viable tumor cells, multiplied by 100.
Outcomes and statistical analysis
Descriptive statistics are reported as proportions and medians. Data are presented as the number (%) for categorical variables. Response categories were assessed according to response evaluation criteria in solid tumors (RECIST) 1.1 by computed tomography. The primary outcome was PFS, defined as the time from the start of ramucirumab plus paclitaxel until the date of disease progression or death from disease not unexpected events. The secondary outcomes were OS; defined as the time from the start of ramucirumab plus paclitaxel until death from any cause, objective response rate; defined as the proportion of patients who had a best response of complete response or partial response (PR). Survival analyses between pairs of subgroups were performed using the Kaplan-Meier method, and hazard ratios between pairs of subgroups were analyzed using Cox-proportional hazard models. All P values were two-sided, and statistical significance was set at P<0.05. Statistical analysis was performed using IBM SPSS Statistics 25 (Armonk, NY, USA).
Results
Patient characteristics
We conducted an analysis on 117 out of 543 patients who received the combination of ramucirumab plus paclitaxel without undergoing clinical trials and had undergone PD-L1 testing (Figure 1). Table 1 presents patients’ clinical characteristics, including the expression of PD-L1. We analyzed data for 117 patients, whose median age was 55 years. Among the patients, 68 (58%) were male, and 49 (42%) were female. As the primary tumor location, stomach body was the most common, found in 65 (56%) patients, and poorly differentiated stomach cancer was the most frequent histologic grade, found in 89 (76%) patients. Ninety-two patients (79%) received capecitabine plus oxaliplatin as first-line chemotherapy. Eighty patients (68%) had a PD-L1 CPS of one or more, 37 patients (32%) had a PD-L1 CPS of five or more, and 19 patients (16%) had a PD-L1 CPS of ten or more.
Table 1
| Variables | PD-L1 status† (N=117) | P value | ||
|---|---|---|---|---|
| 0 (N=37) | 1–9 (N=61) | ≥10 (N=19) | ||
| Median age [range] | 50 [28–76] | 57 [28–78] | 61 [31–79] | |
| Male | 15 [41] | 36 [59] | 17 [89] | |
| Primary tumor location | 0.57 | |||
| Cardia | 3 [8] | 6 [10] | 3 [16] | |
| Body | 23 [62] | 35 [57] | 7 [37] | |
| Antrum | 6 [16] | 13 [21] | 7 [37] | |
| Site unspecified | 5 [14] | 7 [11] | 2 [10] | |
| Histologic grade | 0.34 | |||
| Well-differentiated | 0 | 0 | 0 | |
| Moderately differentiated | 4 [11] | 14 [23] | 6 [32] | |
| Poorly differentiated | 31 [84] | 45 [74] | 13 [68] | |
| Unknown | 2 [5] | 2 [3] | 0 | |
| ECOG performance status | 0.76 | |||
| 0–1 | 37 [100] | 59 [97] | 19 [100] | |
| ≥2 | 0 | 2 [3] | 0 | |
| Gastrectomy | 0.90 | |||
| Previous gastrectomy | 13 [35] | 15 [25] | 7 [37] | |
| No surgery (de novo stage 4) | 24 [65] | 46 [75] | 12 [63] | |
| Metastasis site | ||||
| Liver | 6 [16] | 15 [25] | 6 [32] | 0.79 |
| Peritoneal seeding | 29 [78] | 49 [80] | 12 [63] | 0.66 |
| Malignant ascites | 21 [57] | 28 [46] | 4 [21] | 0.61 |
| HER2 status | 0.69 | |||
| Positive | 2 [5] | 7 [11] | 3 [16] | |
| Negative | 34 [92] | 52 [85] | 16 [84] | |
| Unknown | 1 [3] | 2 [3] | 0 | |
| MSI status | >0.99 | |||
| MSI-high | 0 | 0 | 0 | |
| MSS | 37 [100] | 61 [100] | 19 [100] | |
| TMB status | 0.14 | |||
| TMB-High | 2 [5] | 8 [13] | 0 | |
| TMB-Low | 35[95] | 53 [87] | 19 [100] | |
| Previous chemotherapy | 0.95 | |||
| XELOX | 26 [70] | 41 [67] | 15 [79] | |
| FOLFOX | 3 [8] | 5 [8] | 1 [5] | |
| SP | 3 [8] | 2 [3] | 1 [5] | |
| XPH | 2 [5] | 5 [8] | 2 [11] | |
| XELOX + nivolumab | 0 | 1 [2] | 0 | |
| XELOX + pembrolizumab | 2 [5] | 4 [7] | 0 | |
| XELOX + pembrolizumab + trastuzumab | 1 [3] | 1 [2] | 0 | |
| UK | 0 | 2 [3] | 0 | |
Data are presented as n [%] unless otherwise specified. †, PD-L1 expression was evaluated using various IHC platforms and the 22C3 antibody and calculated as a CPS. PD-L1, programmed death ligand-1; ECOG, Eastern Cooperative Oncology Group; MSI, microsatellite instability; MSS, microsatellite stable; TMB, tumor mutation burden; XELOX, capecitabine plus oxaliplatin; FOLFOX, 5-fluorouracil plus leucovorin plus oxaliplatin; SP, TS-1 plus cisplatin; XPH, trastuzumab plus capecitabine plus cisplatin; IHC, immunohistochemistry; CPS, combined positive score.
Tumor response to ramucirumab and paclitaxel based on PD-L1 expression status
The tumor response to ramucirumab plus paclitaxel was as follows: PR in 17 patients (15%), stable disease (SD) in 56 patients (48%), and progressive disease (PD) in 28 patients (24%) (Table 2). We re-analyzed the tumor response to ramucirumab and paclitaxel using PD-L1 CPS cutoff values of one, five, and ten. Among the 80 patients with a PD-L1 CPS value of one or more, 12 (15%) had a PR, 39 (49%) had SD, and 19 (24%) had PD. Among the 32 patients with a PD-L1 CPS of five or more, 7 (19%) had a PR, 16 (43%) had SD, and 9 (24%) had PD (P=0.40). Among the 18 patients with a PD-L1 CPS of ten or more, 6 (32%) had a PR, 9 (47%) had SD, and 3 (16%) had PD (Table 2).
Table 2
| Parameters | Patients responded, n (%) | Patients didn’t respond, n (%) | P value‡ |
|---|---|---|---|
| PD-L1 CPS <1† | 5 (16.1) | 26 (83.9) | >0.99 |
| PD-L1 CPS ≥1 | 12 (17.1) | 58 (82.9) | |
| PD-L1 CPS <5 | 10 (14.5) | 59 (85.5) | 0.40 |
| PD-L1 CPS ≥5 | 7 (21.9) | 25 (78.1) | |
| PD-L1 CPS <10 | 11 (13.3) | 72 (86.7) | 0.08 |
| PD-L1 CPS ≥10 | 6 (33.3) | 12 (66.7) |
Patients responded means a person who has received more than a partial response. Patients didn’t respond means a person who achieved a response for stable disease or progressive disease. †, PD-L1 expression was evaluated using various IHC platforms and the 22C3 antibody and calculated as a CPS. ‡, Fisher’s exact test was used to calculate P values. PD-L1, programmed cell death-ligand 1; CPS, combined positive score; IHC, immunohistochemistry.
Survival outcomes based on PD-L1 expression status
We analyzed the survival times following ramucirumab plus paclitaxel treatment based on the PD-L1 CPS. Using a cutoff value of 1, the median OS of patients with a PD-L1 CPS of 0 and one or more was 7.0 and 8.1 months, respectively (P=0.32), and the median PFS was 3.6 and 4.1 months, respectively (P=0.93) (Table 3, Figure 2A,2B).
Table 3
| Parameters | Patients, n [%] |
PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Median months [95% CI] |
P value† | Hazard ratio [95% CI] |
Median months [95% CI] |
P value† | Hazard ratio [95% CI] |
|||
| PD-L1 CPS <1 | 37 [32] | 3.60 [2.38–4.82] | 0.93 | 0.98 [0.63–1.52] | 7.00 [5.44–8.56] | 0.32 | 1.3 [0.78–2.08] | |
| PD-L1 CPS ≥1 | 80 [68] | 4.10 [3.49–4.71] | 8.10 [6.37–9.83] | |||||
| PD-L1 CPS <5 | 80 [68] | 3.90 [3.30–4.50] | 0.57 | 1.14 [0.63–1.52] | 7.40 [6.5–8.3] | 0.07 | 1.61 [0.10–2.78] | |
| PD-L1 CPS ≥5 | 37 [32] | 4.40 [2.97–5.83] | 10.00 [1.1–18.9] | |||||
| PD-L1 CPS <10 | 98 [84] | 3.80 [3.31–4.29] | 0.05 | 1.72 [0.98–3.03] | 7.20 [6.50–7.90] | 0.04 | 2.00 [1.02–4.00] | |
| PD-L1 CPS ≥10 | 19 [16] | 5.70 [4.13–7.27] | 18.90 [6.46–31.34] | |||||
†, Kaplan-Meier methods and Cox-proportional hazards models were used to calculate P values and hazard ratios, respectively. PD-L1, programmed death ligand-1; PFS, progression-free survival; OS, overall survival; CI, confidence interval; CPS, combined positive score.

At a cutoff value of 5, OS and PFS did not differ significantly between patients with a PD-L1 CPS of less than five and five or more (P=0.07 and P=0.57, respectively) (Table 3, Figure 3A,3B).

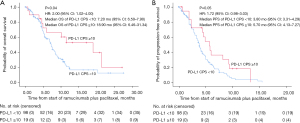
However, at a cutoff value of 10, the median OS of patients with a PD-L1 CPS of less than 10 and ten or more was 7.2 and 18.9 months, respectively (P=0.04), and the median PFS was 3.8 and 5.7 months, respectively (P=0.05). Thus, a significant difference in survival outcomes following treatment with ramucirumab plus paclitaxel was observed at a PD-L1 CPS cutoff value of 10 (Table 3, Figure 4A,4B).
Discussion
In this study, we analyzed the tumor response and survival outcomes following the use of ramucirumab plus paclitaxel as a second-line therapy in AGC using various PD-L1 CPS cutoff values. We analyzed the response rate by PD-L1 CPS cutoff values using cross-tabulation. When the cutoff value was a PD-L1 CPS of 1, the P value was 0.32; when the cutoff value was a PD-L1 CPS of 5, the P value was 0.07, and when the cutoff value was a PD-L1 CPS of 10, the P value was 0.04 in OS analysis. The survival outcomes (OS and PFS) differed significantly between patients with a PD-L1 CPS of less than ten and ten or more. Although the survival outcomes did not differ statistically at cutoff values of 1 and 5, the survival outcomes showed a trend of increasing as the cutoff values increased. Moreover, when assessing the survival outcomes of patients who underwent immune-checkpoint inhibitor (ICI) therapy (Figure S1) or cytotoxic chemotherapy (Figure S2) subsequent to second-line administration of ramucirumab plus paclitaxel, no significant disparity was observed between the two cohorts when categorized by a PD-L1 cutoff of 10. This implies that the PD-L1 CPS cutoff value of 10 might delineate a more distinctive demarcation among patients subjected to the ramucirumab plus paclitaxel. These findings suggest that a PD-L1 CPS cutoff of 10 might be a novel biomarker to predict the survival of patients who receive ramucirumab plus paclitaxel to treat AGC.
What makes these findings special is the absence of biomarkers for anti-angiogenic agents. Vascular endothelial growth factor A (15) and tumor microvessel density (16) were expected to predict the response to anti-angiogenic agents, but they did not provide significant results. Ramucirumab, an anti-VEGFR mAb, inhibits angiogenesis and causes hypoxia to increase the activity of effector T cells (12). Therefore, if a tumor cell has high PD-L1 expression, a large effect from effector T cells is expected (13). Furthermore, PD-L1 might act upon VEGFR2 to induce cancer cell angiogenesis and metastasis (14). Therefore, VEGFR2 inhibition might be more effective in tumors with high PD-L1 expression. Also, VEGF drives immunosuppression in the tumor microenvironment (TME) by inducing vascular abnormalities, suppressing antigen presentation and immune effector cells, or augmenting the immune suppressive activity of regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages (11). Thus, VEGFR inhibitors improve immunosuppression in the TME (14,17) and this theoretical concept allowed us to predict that a combination of an anti-angiogenic agent and an ICI would be effective.
In our study, 4 patients with a PD-L1 CPS of 10 or more received an ICI after treatment with ramucirumab plus paclitaxel, and 3 of those 4 patients discontinued the drug after a few months due to disease progression. However, one patient maintained a PR for more than 2 years of continuous ICI treatment (Table 4, Figure S1A). And, it was apparent that patients who demonstrated a response lasting beyond a span of 2 years showed a notably elevated PD-L1 CPS score of 80.
Table 4
| Variables | PD-L1 status (N=117), n [%] | P value | ||
|---|---|---|---|---|
| 0 (N=37) | 1–9 (N=61) | ≥10 (N=19) | ||
| Post-treatment after ramucirumab plus paclitaxel | 0.17 | |||
| Include immune-checkpoint inhibitor† | 7 [19] | 18 [30] | 4 [21] | |
| Only cytotoxic chemotherapy‡ | 6 [16] | 18 [30] | 5 [26] | |
| Other treatments¶ | 1 [3] | 2 [3] | 3 [16] | |
| No more chemotherapy | 22 [59] | 22 [36] | 7 [37] | |
| Unknown | 1 [3] | 1 [2] | 0 | |
†, immune-checkpoint inhibitor includes pembrolizumab and nivolumab. ‡, cytotoxic chemotherapy includes FOLFIRI, EP and irinotecan monotherapy. ¶, other treatments include study chemo, monoclonal antibody, ADC and other targeted agents. PD-L1, programmed death ligand-1; FOLFIRI, 5-FU, leucovorin, irinotecan; EP, etoposide, cisplatin; ADC, antibody-drug conjugate.
Our study has several limitations. First, it was small and retrospective, so our results should be confirmed in a prospective study. Second, only Asian patients with AGC were analyzed in this study, limiting its generalizability because of differences in molecular profiles and clinical features between Western and Eastern patients with AGC. Third, PD-L1 was scored using IHC samples taken before the start of first-line therapy. The status of PD-L1 expression might have been changed by the effect of the first-line therapy. Our finding of a link between the PD-L1 CPS and the efficacy of ramucirumab plus paclitaxel should thus be interpreted with caution. Lastly, it is important to note that the PD-L1 CPS diagnostic tool is 22C3. Since the PD-L1 CPS score has not been validated for each IHC method, there is a possibility that the approval criteria for nivolumab plus XELOX may differ when relying on the PD-L1 IHC 28-8 pharmDx assay (Dako, Santa Clara, CA, USA) (8), which is currently in use. Specifically, the 28-8 assay is known to have a PD-L1 cutoff value higher than that of 22C3 (1), thus necessitating additional studies to address this discrepancy.
Conclusions
In conclusion, no biomarker has been established to predict survival following ramucirumab plus paclitaxel treatment for AGC. This analysis suggests that a PD-L1 CPS cutoff of 10 might be a novel biomarker to predict survival following ramucirumab and paclitaxel treatment for AGC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-418/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-418/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-418/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-418/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki (as revised in 2013) and the Korea Good Clinical Practice guidelines. This study was approved by the Institutional Review Board at Samsung Medical Center (IRB No. 2022-12-078), and individual consent for this analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDeri
References
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Katoh H, Ishikawa S. Lifestyles, genetics, and future perspectives on gastric cancer in east Asian populations. J Hum Genet 2021;66:887-99. [Crossref] [PubMed]
- Jatoi A, Murphy BR, Foster NR, et al. Oxaliplatin and capecitabine in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction and gastric cardia: a phase II study from the North Central Cancer Treatment Group. Ann Oncol 2006;17:29-34. [Crossref] [PubMed]
- Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7. [Crossref] [PubMed]
- Oholendt AL, Zadlo JL. Ramucirumab: A New Therapy for Advanced Gastric Cancer. J Adv Pract Oncol 2015;6:71-5. [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Schoemig-Markiefka B, Eschbach J, Scheel AH, et al. Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 2021;24:1115-22. [Crossref] [PubMed]
- Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol 2020;11:598877. [Crossref] [PubMed]
- de Almeida PE, Mak J, Hernandez G, et al. Anti-VEGF Treatment Enhances CD8(+) T-cell Antitumor Activity by Amplifying Hypoxia. Cancer Immunol Res 2020;8:806-18. [Crossref] [PubMed]
- Yi M, Niu M, Xu L, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol 2021;14:10. [Crossref] [PubMed]
- Yang Y, Xia L, Wu Y, et al. Programmed death ligand-1 regulates angiogenesis and metastasis by participating in the c-JUN/VEGFR2 signaling axis in ovarian cancer. Cancer Commun (Lond) 2021;41:511-27. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med 1991;324:1-8. [Crossref] [PubMed]
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475-85. [Crossref] [PubMed]



