
A narrative review of the evolving landscape of the management of metastatic gastric cancer: the role of targeted therapies
Introduction
Gastric cancer is the fifth most common cancer worldwide and the fourth leading cause of cancer-related death, accounting for over one million new cases and estimated to account for approximately 769,000 deaths in 2020 (1). Notably, in the United States of America (USA), there has been a steady decrease in the incidence and mortality attributable to gastric cancer, with 26,380 estimated new cases and 11,090 estimated deaths in 2022 (2,3). The incidence is two-fold higher in males than in females and is higher in Asians, African Americans, and Hispanics when compared to non-Hispanic whites. African Americans have the highest mortality rate, followed by Asians, Hispanics, and Native Americans (3).
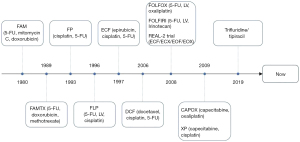
Unfortunately, there continues to be a high incidence of metastatic disease at diagnosis worldwide, which is directly related to the high mortality observed with this disease. Universal or population-based screening is not recommended in the USA, however several countries (South Korea, Japan) with high incidence of gastric cancer have developed screening programs (4). In the USA, approximately 37% of patients diagnosed with gastric cancer are found to have metastatic disease at presentation which is associated with a 5-year relative survival of approximately 6% (3). Thus, it is paramount for the patient’s care team to understand the latest management of metastatic gastric cancer. This review article will focus on the role of targeted therapies in the care of patients with metastatic gastric cancer. This is intended to build upon a backbone of traditional, cytotoxic chemotherapy (Figure 1). We present this article in accordance with the Narrative Review reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-464/rc).
Methods
We performed an extensive review of articles in the PubMed database pertaining to targeted therapies and immunotherapies in the treatment of metastatic gastric cancer. The search strategy used is summarized in the table below. Guidelines from the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) were also reviewed (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | Between 1st January 2020 and 30th April 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “Gastric cancer”, “immunotherapy”, “checkpoint inhibitor”, “targeted therapy”, “HER2”, “VEGF”, “tyrosine kinase inhibitor” |
| Timeframe | 1990–2023 |
| Inclusion criteria | Only papers in English were included. Phase 1, 2, and 3 clinical trials were included |
| Selection process | All authors collected and assembled the data |
HER2, human epidermal growth factor receptor 2; VEGF, vascular endothelial growth factor.
Molecular classification
Traditionally, gastric cancer has been classified in terms of either the anatomic location of the tumor or the histologic subtype. The Lauren classification is the most commonly used in clinical trials (5). However, recent advancements in the cancer genome atlas have reclassified gastric adenocarcinoma into four different molecular subtypes: (I) Epstein-Barr virus-positive (EBV+); (II) microsatellite instability (MSI); (III) genomically stable; and (IV) chromosomal instability (CIN) (6,7).
Targeted therapies
Human epidermal growth factor receptor 2 (HER2 or ERBB2)
The HER2 oncogene encodes a transmembrane receptor (HER2, EGFR2, or ERBB2) which expresses the HER2 protein and has intracellular tyrosine kinase activity. The HER2 receptor is part of the epidermal growth factor receptor (EGFR) superfamily of receptors (8,9). In 2005, Tanner et al. discovered in one study that 12% of patients with gastric adenocarcinoma (n=131) had HER2 amplification, most commonly in the intestinal histology subtype, which was associated with a more aggressive disease course compared with non-HER2 amplified tumors. This finding was similar to the effect of HER2 amplification in breast cancer (10). Molecularly, HER2 (ERBB2) amplification in gastric cancer is associated with the EBV+ subtype and, to a lesser extent, with the MSI subtype (6).
Trastuzumab is a monoclonal antibody that binds to the HER2 receptor. This binding prevents HER2 mediated signaling and triggers antibody dependent cellular cytotoxicity in HER2 expressing cells (11). This drug, used in combination with chemotherapy was previously shown to provide a survival advantage for patients with HER2-amplified breast cancer (9). The ToGA trial, an open-label, randomized, controlled, phase III trial evaluated the safety and efficacy of trastuzumab in combination with chemotherapy [cisplatin plus 5-fluorouracil (5-FU) or capecitabine] compared with chemotherapy alone as a first-line option for patients with HER2-positive advanced gastric/gastroesophageal junction (GEJ) cancer (Figure 2). In this study, gastric cancer was considered to be HER2-positive if it had a score of 3+ per immunohistochemistry (IHC) or if it was positive per fluorescence in-situ hybridization (FISH) (12). The study demonstrated a statistically significant improvement in median overall survival (mOS) for the trastuzumab group (13.8 months) compared to the standard therapy group (11.1 months) with a 26% reduction in the death rate. Median progression-free survival (mPFS) and overall relative risk (RR) were also significantly improved with the addition of trastuzumab. The combination was well tolerated with slightly higher incidence of diarrhea, stomatitis, and myelosuppression (12). Interestingly, a pre-planned exploratory analysis suggested that in patients receiving trastuzumab, high HER2 expression (IHC 3+ or IHC 2+ and FISH positive) was associated with longer OS compared to patients with low expression (IHC 0 and FISH positive or IHC 1+ and FISH positive). Patients with high HER2 expression receiving trastuzumab had a mOS of 16 months compared to 11.8 months for those not receiving trastuzumab with a hazard ratio (HR): 0.65 [95% confidence interval (CI): 0.51–0.83] (12).
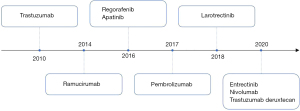
Following the ToGA study, additional studies were completed to evaluate the efficacy of trastuzumab when added to other chemotherapy backbones. The GERCOR investigators retrospectively assessed the efficacy and safety of trastuzumab in combination with oxaliplatin plus 5-FU or capecitabine and found the combination to be safe and effective with an overall RR of 41% (95% CI: 25–56%) (13). The HERXO trial was a phase II, nonrandomized, open-label study that showed that the CAPOX (oxaliplatin and capecitabine)-trastuzumab combination was also effective and a promising first-line treatment option with 8.9%, 37.8%, and 31.1% of patients achieving complete response (CR), partial response and stable disease, respectively (14). The cumulative data from these studies led to the recommendation that trastuzumab should be added to a chemotherapy backbone of a fluoropyrimidine + platinum in the first-line treatment of metastatic gastric cancers with HER2-amplification (15,16).
Pertuzumab is another HER2-directed monoclonal antibody. The JACOB phase III trial evaluated the effect of pertuzumab when added to chemotherapy (cisplatin plus capecitabine or 5-FU) and trastuzumab in the first-line treatment of metastatic gastric or GEJ adenocarcinoma. This study showed a mOS of 17.5 months with pertuzumab which was not significantly different from the mOS of 14.2 months seen in the placebo group (HR: 0.84; 95% CI: 0.71–1.00; P=0.057) (17). The end of study analysis after >44 months of median follow-up was presented at ESMO 2020, and showed an overall RR of 57% for the pertuzumab group compared to 48.6% for the placebo group suggesting a treatment benefit with the addition of pertuzumab. The overall safety profile was considered acceptable (18). These results indicate that there is potential for this drug to improve response rates in patients with advanced HER2-positive gastric cancer, however further work is needed to understand which subset of patients are most likely to benefit. The addition of pertuzumab as part of the first-line management of gastric/GEJ cancer is not currently recommended.
The continuation of trastuzumab beyond tumor progression on first-line therapy has provided clinical benefits in HER2-positive breast cancer, prompting an investigation into whether the same strategy would be effective in gastric cancer. The T-ACT (Trial to Assess the Concept of Using Trastuzumab Beyond Progression) was an open-label, randomized, phase II study that evaluated the PFS and safety of paclitaxel and trastuzumab (PT) compared with paclitaxel alone in HER2-positive advanced gastric/GEJ adenocarcinoma that was refractory to trastuzumab-containing first-line chemotherapy (19). The mPFS for patients receiving paclitaxel alone compared to PT was of 3.2 vs. 3.7 months, respectively (HR: 0.91; 80% CI: 0.67–1.22; P=0.33), indicating that trastuzumab beyond progression did not improve PFS in gastric cancer (19). In a subsequent exploratory analysis of patients who had additional tissue samples collected after progression on first-line therapy found that 69% (11 of 16) had loss of HER2 positivity (19), suggesting a need to reassess HER2 status at the time of progression.
Breakthroughs and new therapies in HER2-positive metastatic breast cancer have led to studies of those new therapies in advanced gastric cancer. Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate formed by trastuzumab, a linker, and a tubulin inhibitor. This drug was investigated as a potential second-line agent for patients with advanced HER2-positive gastric/GEJ adenocarcinoma in the GATSBY study (20). The phase III part of the study compared T-DM1 to a taxane (docetaxel or paclitaxel) as second-line therapy with a primary endpoint of OS. The study did not show a survival advantage with T-DM1, with a mOS of 7.9 vs. 8.6 months (HR: 1.15; 95% CI: 0.87–1.51; one-sided P=0.86) for T-DM1 and taxane, respectively (20). Another anti-HER2 antibody-drug conjugate, trastuzumab deruxtecan (T-DXd), was studied in advanced gastric/GEJ adenocarcinoma in the Destiny-Gastric-01 trial (21). This was a randomized, open-label, phase II trial conducted in Japan and South Korea comparing chemotherapy (irinotecan or paclitaxel) to T-DXd in patients with advanced HER2-positive gastric/GEJ adenocarcinoma who had progression after receiving at least two previous therapies, including trastuzumab (21). Objective response was 51% (95% CI: 42–61%) in the T-DXd group and 14% (95% CI: 6–26%) in the chemotherapy groups (P<0.001) (21). Survival was also longer in the T-DXd group with a mOS 12.5 vs. 8.4 months for the chemotherapy group (HR: 0.59; 95% CI: 0.39–0.88; P=0.01) (21). Pneumonitis and myelosuppression were more common in the T-DXd group (21). More recently the results have been reproduced in a Western population in the Destiny-Gastric-02 trial (22). This was a single-arm, phase II trial in patients with HER2-positive unresectable/metastatic gastric/GEJ cancer who had progressed on trastuzumab-based therapy. mOS was found to be 12.1 months (95% CI, 9.4–15.4) and overall response rate (ORR) was 42% (4 complete and 29 partial responses). The safety profile was found to be tolerable and similar to results from the Destiny-Gastric-01 trial (22). Largely based on findings from Destiny-Gastric-01, T-DXd has been approved for use in locally advanced or metastatic HER2-positive gastric adenocarcinoma in patients who have progressed on at least two prior lines of therapy, including trastuzumab, a fluoropyrimidine- and a platinum-containing chemotherapy (23).
Zanidatamab is an investigational HER2-targeted bispecific antibody. Recently preliminary results of a phase II trial evaluating zanidatamab in combination with chemotherapy in the first-line setting in HER2-positive metastatic gastroesophageal adenocarcinoma was presented at the 2023 ASCO Gastrointestinal Cancers Symposium. Reported survival data was promising with patients receiving zanidatamab in combination with chemotherapy demonstrating an 18-month OS rate of 84% (95% CI: 68–93%). Response data was also promising with a confirmed ORR of 79% (95% CI: 63–90%) and disease control rate of 92% (95% CI: 79–98%) (24). Results from HERIZON-GEA-01, a phase III study evaluating zanidatamab in combination with chemotherapy with or without the PD-1 inhibitor, tislelizumab in HER2-positive metastatic gastric cancer patients are eagerly awaited (25). Trastuzumab with fluoropyrimidine plus platinum-containing chemotherapy remains the backbone of treatment for HER2-positive gastric adenocarcinoma in the first-line setting as established by the ToGA trial. Preliminary data of zanidatamab combined with chemotherapy in the first-line setting has been promising, with data from a phase III trial eagerly awaited. Recent results from the recent KEYNOTE-811 trial have supported the use of pembrolizumab in the first-line setting and will be discussed in detail later in this review. HER2 targeted therapy past the first-line setting has been disappointing with multiple negative trials, but recently T-DXd has shown modest benefit in later lines of treatment.
Vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2)
The VEGF- and VEGFR-2-mediated signaling and angiogenesis contributes to the pathogenesis and progression of gastric cancer (26,27). Furthermore, higher circulating and tumoral concentrations of VEGF are associated with increased tumor aggressiveness and reduced survival (28). The AVAGAST study was a phase III, multinational, randomized, placebo-controlled trial in advanced gastric cancer that evaluated the efficacy of the first-line combination of chemotherapy [capecitabine/cisplatin (XP) or 5-FU/cisplatin (FP)] with bevacizumab, a monoclonal antibody which binds to and neutralizes VEGF (28). Although bevacizumab showed activity, this study did not reach its primary endpoint of OS with a mOS of 12.1 and 10.1 months in the bevacizumab and control groups, respectively (HR: 0.87; 95% CI: 0.73–1.03; P=0.100). The combination did show a significant increase in the mPFS (6.7 vs. 5.3 months; HR: 0.80; 95% CI: 0.68–0.93; P=0.004) and overall RR (46.0% vs. 37.4%; P=0.032) (28). Baseline plasma VEGF-A and tumor neuropilin-1 levels were highlighted as potential biomarkers for predicting clinical outcomes with bevacizumab (29). The AVATAR study was a similarly designed phase III study in Chinese patients which revealed no significant difference in OS between chemotherapy (XP) with bevacizumab compared with chemotherapy alone in the first-line setting (HR: 1.11; 95% CI: 0.79–1.56; P=0.557) (30).
Ramucirumab, a fully human immunoglobulin G1 (IgG1) monoclonal antibody that antagonizes VEGFR-2, provided a survival advantage of 1.4 months over placebo in the REGARD study and was well tolerated (27). This was an international, randomized, placebo-controlled, phase III trial that evaluated the efficacy and safety of ramucirumab compared with placebo in patients with advanced gastric/GEJ adenocarcinoma who had disease progression after a first-line platinum-containing or fluoropyrimidine-containing regimen. The mOS was 5.2 and 3.8 months in the ramucirumab and placebo groups, respectively (HR: 0.776; 95% CI: 0.603–0.998; P=0.047). The results with single-agent ramucirumab were statistically significant, but correlated with only a modest clinical benefit (27). The RAINBOW study was a randomized, double-blind, placebo-controlled, phase III trial that evaluated the efficacy of ramucirumab in combination with paclitaxel compared with paclitaxel alone in the second-line setting, after receiving a combination of a fluoropyrimidine and a platinum with or without an anthracycline (31). The primary endpoint of OS was met and the combination showed a mOS of 9.6 months compared with 7.4 months in the single-agent paclitaxel group (HR: 0.807; 95% CI: 0.678–0.962; P=0.017) with an acceptable safety profile (31). The RAINFALL study was a randomized, double-blind, placebo-controlled, phase III trial that evaluated ramucirumab in combination with chemotherapy (XP or FP) in the first-line setting for patients with advanced HER2-negative gastric/GEJ adenocarcinoma (32). Although the investigator-assessed PFS was 0.3 months longer in the ramucirumab-chemotherapy group (HR: 0.753; 95% CI: 0.607–0.935; P=0.011), a central independent review of the radiological images was non-confirmatory. Moreover, the combination did not improve OS (32). Ramucirumab has also been combined with FOLFIRI (leucovorin, 5-FU, and irinotecan) in the second-line setting for patients unable to tolerate additional platinum agents after a fluoropyrimidine-platinum doublet in the first-line setting. A retrospective study showed a mOS of 13.4 months in patients receiving this combination (33). On the basis of these studies, the combination of ramucirumab-paclitaxel (preferred) and single-agent ramucirumab are category 1 recommendations for patients with advanced gastric/GEJ adenocarcinoma in the second-line setting. The combination of ramucirumab-FOLFIRI received a category 2B recommendation in selected patients (15,16).
Tyrosine kinase inhibitors (TKIs)
Sunitinib, a TKI which inhibits multiple receptors including VEGFR, was evaluated in a phase II, two-arm, open-label, randomized trial in South Korea (34). This trial comparing single agent docetaxel with docetaxel in combination with sunitinib in patients with advanced gastric cancer in the second line failed to show an improvement in its primary endpoint of time to progression (TTP) (34). Apatinib, a TKI that selectively inhibits VEGFR-2, was also studied in a randomized, double-blind, placebo-controlled phase III trial in China in patients who had progressed after two or more lines of chemotherapy (35). This study met its primary endpoints of OS and PFS showing a statistically significant improvement in mOS of 6.5 vs. 4.7 months (HR: 0.709; 95% CI: 0.537–0.937; P=0.016) and a mPFS of 2.6 vs. 1.8 months (HR: 0.444; 95% CI: 0.331–0.595; P<0.001) for patients receiving apatinib and placebo, respectively (35). While the results of the study were statistically significant, the absolute margin of benefit was modest. More recently, the ANGEL trial was a placebo-controlled, multinational, phase III trial which studied apatinib in patients with advanced/metastatic gastric or GEJ adenocarcinoma who had treatment failure with two prior therapies (36). Overall, the results of this trial were disappointing. There was no statistically significant difference in mOS between the treatment and placebo groups (5.78 vs. 5.13 months; HR: 0.93; 95% CI: 0.74–1.15; P=0.4850). Although mOS was not significantly improved, other endpoints did show improvement including mPFS (2.83 vs. 1.77 months; HR: 0.57; 95% CI: 0.46–0.79; P<0.0001) and ORR (6.87% vs. 0%; P=0.0020) (36). Given the negative ANGEL trial results for OS improvement in a Western population, apatinib is not currently approved in the USA for treatment of gastric cancer.
Regorafenib, an oral multikinase inhibitor that targets angiogenic, stromal, and oncogenic pathways was evaluated in the INTEGRATE IIa trial (37). INTEGRATE IIa was a phase III, randomized, placebo-controlled trial which evaluated whether regorafenib improves OS in patients with advanced gastric/GEJ adenocarcinoma who have progressed after two lines of therapy (NCT02773524). Positive results from this trial were presented at the 2023 ASCO Gastrointestinal Cancers Symposium (38). The mOS was found to be 4.5 months in the regorafenib arm compared to 4.0 months in the placebo arm (HR: 0.70; 95% CI: 0.53–0.92; P=0.011). The 12-month OS rate was also superior in the treatment arm at 19% compared to 6% in the placebo arm. Regorafenib was also found to delay progression with mPFS found to be 1.8 months in the treatment arm compared to 1.6 months in the placebo arm (HR: 0.52; 95% CI: 0.40–0.69; P≤0.0001) (38). Though statistically significant, the margins of benefit were small, given patients were receiving third-line or subsequent therapy. INTEGRATE IIb is a randomized, phase 3 trial comparing regorafenib and nivolumab to standard chemotherapy in patients with advanced gastric/GEJ adenocarcinoma who have progressed on two prior lines of treatment. Results of this trial are eagerly awaited.
Lapatinib is another TKI which acts on both the HER2 and EGFR pathways. TRIO-013/LOGiC was a multicenter, double-blind, placebo-controlled, randomized phase III trial designed to evaluate the effectiveness of lapatinib in previously untreated HER2-positive advanced gastroesophageal adenocarcinoma (39). Patients were randomized to receive capecitabine and oxaliplatin with either lapatinib or placebo. The primary end point was mOS which was 12.2 months in the lapatinib arm compared to 10.5 months in the placebo arm, but the difference was not statistically significant (HR: 0.91; 95% CI: 0.73–1.12; P=0.3492) (39). TyTAN was a phase III trial comparing paclitaxel with and without lapatinib in HER2-positive advance gastric cancer. No statistically significant difference was found in mOS, mPFS, or TTP (40). Given these disappointing results, the use of lapatinib is not currently recommended for gastric cancer.
Tumor-agnostic therapies targeting kinase pathways have been approved by the Food and Drug Administration (FDA) and play a role in the treatment of gastric cancer after the first-line. These therapies are approved in patients with unresectable or metastatic solid tumors which have progressed following prior treatment, have no satisfactory alternative treatment options, and have mutations including BRAF V600E, rearranged during transfection (RET) fusion, or neurotrophic receptor tyrosine kinase (NTRK) fusion (41-44). These mutations are known to lead to aberrant kinase activity and oncogenesis (45-47). Support of these tumor-agnostic therapies comes from various basket trials which have demonstrated their benefit (48-51). It is of note that none of these basket trials included patients with gastric cancer. As second-line or subsequent therapy, the NCCN recommends dabrafenib in combination with trametinib for BRAF V600E mutated tumors, selpercatinib for RET gene fusion-positive tumors, and larotrectinib or entrectinib for NTRK gene fusion-positive tumors (15). Although it is encouraging that tumor-agnostic therapies are now approved, these therapies are likely to only benefit a very small minority of gastric cancer patients as they are rarely found in gastric cancer with well under 1% of tumors harboring these targetable mutations (52-54).
Claudin-18 isoform 2 (CLDN18.2)
CLDN18.2 is a tight junction protein that is found exclusively in gastric mucosa cells, both normal and malignant. In malignant cells, the cell polarity is lost and becomes exposed on the surface (55). Given this finding it has been hypothesized that this protein is more accessible to antibodies in malignant cells and a suitable target for therapies. Zolbetuximab is a chimeric IgG1 monocolonal antibody that targets and binds to CLDN18.2 leading to cell death. The efficacy of zolbetuximab has been evaluated in SPOTLIGHT, a global, randomized, placebo-controlled, phase 3 trial (56). Patients with CLDN18.2-positive (defined as ≥75% of tumor cells showing moderate-to-strong membranous CLDN18 staining), HER2-negative, locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma who had previously not received treatment were randomized to receive either zolbetuximab plus mFOLFOX6 (5-FU, leucovorin, and oxaliplatin) (283 patients) or placebo plus mFOLFOX6 (282 patients). mPFS was superior in the investigational group at 10.61 months (95% CI: 8.90–12.48) compared to 8.67 months (95% CI: 8.21–10.28) in the placebo group. A survival benefit was also demonstrated in the investigational group with a significant reduction in the risk of death vs. placebo (HR: 0.75; 95% CI: 0.60–0.94; P=0.0053) (56). Similar results were replicated in the GLOW trial, a global, randomized, placebo-controlled, double-blind, phase 3 trial (57). Here, CAPOX was used instead of mFOLFOX6. Both mOS (14.39 vs. 12.16 months; HR: 0.771; P=0.0118) and mPFS (8.21 vs. 6.80 months; HR: 0.687; P=0.0007) were found to be significantly prolonged with zolbetuximab compared to placebo (57). Of note, CLDN18.2-positivity was fairly common, seen in 40% of screened patients in both trials (56,57). Zolbetuximab remains investigational, but both the GLOW and SPOTLIGHT trials support its use in CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic gastric cancer in the first-line setting.
Fibroblast growth factor receptor 2b (FGFR2b)
Fibroblast growth factors play a role in oncogenesis through their influence on tumor development and progression (58). Bemarituzumab (FPA144) is a humanized, G1 monoclonal antibody directed against FGFR2b which inhibits FR2b signaling and triggers antibody-dependent cell-mediated cytotoxicity. The FIGHT trial was a randomized, double-blind, placebo-controlled phase 2 study evaluating the effectiveness of first-line bemarituzumab in patients with HER2-negative, FGFR2b-selected advanced gastric/GEJ adenocarcinoma (59). Of the 910 patients screened for the study 30% had overexpression of FGFR2b or amplification of FGFR2. Patients were randomized to receive mFOLFOX6 plus bemarituzumab (n=77) or placebo (n=78). The primary outcome, mPFS, was 9.5 months in the bemarituzumab arm vs. 7.4 months in the placebo arm but was not statistically significant. ORR was improved in the bemarituzumab arm (59). Although the primary efficacy endpoint was not met, results suggest a benefit. Bemarituzumab is being further investigated in HER2-negative, previously untreated advanced gastric or GEJ adenocarcinoma with FGFR2b overexpression in the ongoing phase III trials FORTITUDE-101 (mFOLFOX6 and bemarituzumab) and FORTITUDE-102 (mFOLFOX6, bemarituzumab, and nivolumab) (59).
Checkpoint inhibitors
Malignant tumors often develop immune suppressive and tolerance mechanisms which inactivate T-cells, allowing tumors to evade immune destruction. The programmed-death receptor 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways are naturally occurring safety mechanisms to dampen T cell responses, but are commonly used by tumor cells to evade the immune system. Tumor cells can express the programmed death-ligand 1 and 2 (PD-L1 and PD-L2) which bind to PD-1 on T-cells, inhibiting T-cell function. Tumor cells can also express CTLA-4 which binds to B7 on antigen presenting cells and prevents T cell activation (60). Immune checkpoint inhibitors (ICIs) block these tumor inhibitory signals, enabling T-cells to recognize tumor antigens and reactivate (61). ICIs have revolutionized the treatment of many cancer types.
Pembrolizumab
Pembrolizumab is an IgG4 antibody targeting PD-1. The KEYNOTE-158 study was a phase II trial of pembrolizumab in patients with previously treated, advanced metastatic MSI-high (MSI-H) or deficient mismatch repair (dMMR) tumors of 27 different types (62). Tumors with MSI-H are hypermutated compared to cancer cells with proficient mismatch repair. It has been theorized that these mutations create neoantigens which make the cells more immunogenic and susceptible to ICIs. In this study gastric cancer (n=24) was the second most common tumor type. The outcomes of the 24 patients with gastric cancer were impressive with an ORR of 46% (95% CI: 25.6–67.2%) (four with a CR) and mPFS of 11 months [95% CI: 2.1–not reached (NR)] (62). These findings led the FDA to grant accelerated approval of pembrolizumab for the management of patients with unresectable MSI-H or dMMR solid tumors that progressed following prior treatment and who had no satisfactory alternative treatments (63). In gastric cancer, pembrolizumab is currently recommended for the treatment of advanced gastric cancer in the second or subsequent line of therapy for patients with MSI-H or dMMR tumors (15). Approximately 5–10% of gastric tumors are found to be MSI-H or dMMR (64-68). So, while this therapy will impact a small subset of patients with gastric cancer, the response rate and durability of response for these patients can be significant.
KEYNOTE-059 is a phase II, open-label, non-randomized trial evaluating pembrolizumab in patients with advanced gastric/GEJ adenocarcinoma (69). Cohort one received pembrolizumab as monotherapy in patients with disease progression after two or more lines of therapy. Patients with PD-L1 positive tumors [combined positive score (CPS) ≥1] had superior ORR (15.5% vs. 6.4%) compared to PD-L1 negative tumors, however they had a similar CR (2.0% vs. 2.8%). These results led to accelerated approval of pembrolizumab for gastric tumors expressing PD-L1 with disease progression on at least two prior systemic therapies (70). KEYNOTE-061 is a randomized, open-label, phase III trial evaluating the efficacy of pembrolizumab compared to paclitaxel for the management of advanced gastric/GEJ adenocarcinoma in the second-line setting (71). The primary endpoints were OS and PFS in patients with PD-L1 CPS ≥1. Disappointingly this trial did not meet its primary endpoints, failing to show a significant improvement in OS or PFS with pembrolizumab. However, interestingly, in a post-hoc analysis there was a greater treatment effect for patients with a PD-L1 CPS ≥10 in which the mOS was 10.4 months (95% CI: 5.9–17.3) with pembrolizumab compared with 8.0 months (95% CI: 5.1–9.9) with paclitaxel (HR: 0.64; 95% CI: 0.41–1.02). KEYNOTE-062 is a randomized, controlled, partially-blinded phase III trial evaluating the safety and efficacy of pembrolizumab or pembrolizumab with chemotherapy vs. chemotherapy alone for advanced HER2-negative gastric/GEJ adenocarcinomas with a PD-L1 CPS ≥1 in the first-line setting (72). The addition of pembrolizumab to chemotherapy was not found to improve OS in patients with CPS ≥1 or even CPS ≥10. The disappointing results from KEYNOTE-061 and 062 trials lead the FDA to withdraw their approval of pembrolizumab for treatment of PD-L1 expressing gastric tumors in patients who had received at least two lines of chemotherapy. Off-label use should still be considered in patients with high levels of PD-L1 expression and no previous ICI treatment given potential benefits. Post-hoc analysis from the KEYNOTE trials 059, 061, and 062 confirmed better response rates (OS and PFS) for those with MSI-H tumors compared to those with MSS tumors, confirming that MSI-H status is an important predictor of response to pembrolizumab therapy in patients with advanced gastric cancers (73).
KEYNOTE-811 is a randomized, double-blind, placebo-controlled phase III study of pembrolizumab plus trastuzumab and chemotherapy for unresectable or metastatic, HER2-positive gastric or GEJ adenocarcinoma in the first-line setting. All patients were given a regimen of platinum-containing cytotoxic chemotherapy and trastuzumab and were randomized to receive either pembrolizumab or placebo. An interim analysis showed a 22.7% improvement in objective response rate in the pembrolizumab group over the placebo group (95% CI: 11.2–33.7%; P=0.00006). Additionally, the pembrolizumab group had more CRs (11% vs. 3%) and improved median duration of response (10.6 vs. 9.5 months) (74) when compared to the placebo group. Based on this interim analysis the FDA-approved pembrolizumab in combination with trastuzumab and fluoropyrimidine + platinum-containing chemotherapy for first-line treatment of locally advanced or unresectable/metastatic HER2-positive gastric or GEJ adenocarcinoma (75). A trend towards a greater degree of benefit in objective RR was seen in the subgroup of patients with CPS score >1 (74). Further research is needed to determine how PD-L1 expression impacts response to pembrolizumab in this setting.
KEYNOTE-859 was a double-blind, placebo-controlled phase III trial which evaluated the addition of pembrolizumab to chemotherapy (either fluorouracil plus cisplatin or CAPOX) in previously untreated HER2-negative, locally advanced or metastatic gastric/GEJ cancer (76). Across the entire study population, pembrolizumab + chemotherapy was superior to chemotherapy + placebo with improved OS (median 12.9 vs. 11.5 months; HR: 0.78; 95% CI: 0.70–0.87; P<0.0001) and PFS (mPFS 6.9 months vs. 5.6 months; HR: 0.76; 95% CI: 0.67–0.85; P<0.0001). Interestingly, OS and PFS were generally consistent across all subgroups including CPS >1, CPS >10, and MSI-H tumors (76). This is in contrast with KEYNOTE-062 where pembrolizumab with chemotherapy did not improve OS or PFS in CPS >1 patients or OS in patients with CPS >10 (72). Given the conflicting data, the precise degree of benefit in adding pembrolizumab to chemotherapy in patients with proficient MMR, CPS >1, HER2-negative tumors in the first-line setting is unclear, but is a reasonable option based on the KEYNOTE-859 results.
Nivolumab
Nivolumab is a fully human IgG4 monoclonal antibody that also targets PD-1. Nivolumab showed efficacy and safety in treating gastric cancer in the ATTRACTION-2 trial, a randomized, double-blind, placebo-controlled, phase III trial in gastric/GEJ adenocarcinomas refractory to two lines of therapy conducted in Japan, South Korea, and Taiwan (77). In this trial, mOS improved by 1 month with nivolumab over placebo (HR: 0.63; 95% CI: 0.51–0.78; P<0.001). The ATTRACTION-4 trial, performed in the same countries/region, included a phase II portion which showed that the combination of nivolumab with S-1 plus oxaliplatin (SOX) or CAPOX was well tolerated and had an objective RR of 57.1% for nivolumab + SOX and 76.5% for nivolumab + CAPOX for unresectable advanced or recurrent HER2-negative gastric/GEJ cancer (78). The phase III portion of ATTRACTION-4 in the first-line setting in HER2-negative gastric/GEJ cancer showed that the combination of nivolumab + SOX or CAPOX significantly improved PFS with a mPFS of 10.4 vs. 8.3 months for the placebo + chemo group (HR: 0.68; 98.51% CI: 0.51–0.90; P=0.0007). However, it did not show a statistically significant difference in OS (79).
CheckMate-649 is a randomized, global, open-label, phase III trial that assessed nivolumab in combination with ipilimumab or chemotherapy (FOLFOX or CAPOX) compared with chemotherapy alone for the management of HER2-negative gastric/GEJ and esophageal adenocarcinoma in the first-line setting (80). The primary endpoints were OS and PFS in the subgroup of patients with a PD-L1 CPS score ≥5 (although all levels of PD-L1 status, including <1, were included). A statistically significant improvement in survival was noted in the overall study population and the subgroup of patients with CPS >1. The most significant response was seen in patients with CPS >5 with a mOS of 14.4 vs. 11.1 months (HR: 0.71; 98.4% CI: 0.59–0.86; P<0.001) and a mPFS of 7.7 vs. 6.1 months (HR: 0.68; 98% CI: 0.56–0.81; P<0.001), favoring nivolumab + chemotherapy vs. chemotherapy alone. The positive response in the overall study population may be explained by the high proportion of patients with a PD-L1 CPS ≥5 (81). Based on these results, the NCCN guidelines include a category 1 recommendation for the use of nivolumab in combination with chemotherapy (FOLFOX or CAPOX) in the first-line treatment of advanced gastric cancers with CPS ≥5 and without HER2 overexpression (15). The 34 patients who had both PD-L1 ≥5 and MSI-H tumors had a dramatic improvement with combination therapy vs chemotherapy alone in survival (median: 44.8 vs. 8.8 months; HR: 0.32) and in ORR (55%, 95% CI: 32–77% vs. 39%, 95% CI: 17–64%) (81). This confirms the important role for ICIs in the treatment of patients with MSI-H or dMMR tumors.
AIO INTEGA is a phase two randomized clinical trial with two experimental arms in patients with untreated, metastatic HER2-positive esophagogastric adenocarcinoma. Patients were randomized to trastuzumab and nivolumab with either mFOLFOX6 or ipilimumab. The OS rate after 12 months was improved in the FOLFOX arm (70%; 95% CI: 54–81%) compared to the ipilimumab arm (57%; 95% CI: 41–71%). Additionally, PFS was higher in the FOLFOX arm (10.7, 95% CI: 6.6–13.1 vs. 3.2 months, 95% CI: 2.0–6.5) and ORR was also higher (56% vs. 32%). Compared to historical data from the ToGA trial, the FOLFOX arm showed significant improvement in the 12-month OS rate with a P value of 0.03. This improvement may be due to the difference in the line of therapy (first-line vs. subsequent line of therapy). The ipilimumab group showed a similar OS rate compared to historical data (82). Findings from this trial suggest that adding nivolumab to trastuzumab and cytotoxic chemotherapy in the first-line setting could be superior to trastuzumab and cytotoxic chemotherapy alone.
Dostarlimab
Dostarlimab is a new monoclonal antibody targeting PD-1 and gained its first FDA approval in treatment of endometrial cancer. The Garnet trial is an ongoing phase I, single-arm study evaluating the use of dostarlimab monotherapy in advanced solid tumors (83,84). New data from a cohort of 106 patients with dMMR non-endometrial cancers support its use in additional solid tumor types. In this cohort, the majority of patients had colorectal cancer (n=69) and eight patients had gastric cancer. ORR across all tumor types was 38.7% (95% CI: 29.4–48.6%), with a CR rate of 7.5%. Results were consistent across all tumor types, including gastric cancer, with an ORR of 37.5% (95% CI: 8.5–75.5%) (85). These findings led the FDA to grant accelerated approval in August 2021 for its use in patients with dMMR recurrent or advanced solid tumors that have progressed on or following prior treatment and who have no satisfactory alternative treatment options (85).
Management algorithm for advanced gastric/GEJ cancer
In Figure 3, we propose an algorithm for the management of metastatic or advanced gastric/GEJ adenocarcinoma. With the results of the recent trials, it is important to determine the HER2 status, PD-L1 CPS score, and MMR/MSI status in all patients with advanced disease. Patients who have a HER2-negative tumor and a PD-L1 CPS ≥5 should be considered for the combination of nivolumab + FOLFOX or CAPOX in the first-line setting. Similarly, patients with MSI-H or dMMR tumors are candidates to receive an ICI in combination with chemotherapy in the first-line setting. Upon progression on first-line agents, patients with a tumor showing MSI-H/dMMR could receive single-agent pembrolizumab or dostarlimab. For patients who are not candidates for ICI, those who are fit should receive combination chemotherapy. Patients who are not fit enough for combination chemotherapy, but who would benefit from therapy, should be considered for single-agent therapy. Patients with HER2 amplified tumors should have the addition of trastuzumab to first-line chemotherapy ± immunotherapy based on expected tolerance of therapy. At the time of progression on first-line anti-HER2 targeted therapy, tumors should ideally be re-biopsied to determine the current HER2 expression; if still amplified, T-DXd could be considered. Patients with NTRK-positive tumors with no other alternative therapies should be considered for entrectinib or larotrectinib. Patients with BRAF V600E mutated tumors should be considered for dabrafenib with trametinib. Patients with RET gene fusion-positive tumors should be considered for selpercatinib. Patients with a PD-L1 CPS >1 could receive pembrolizumab if an ICI was not used in prior lines of therapy. Patients with tumors that do not show these targets, may be candidates for trifluridine/tipiracil, apatinib or regorafenib. Further research needs to be done to elucidate the optimal sequencing of these therapies in patients who are a candidate for multiple targeted agents and to assess the role of sequential ICIs in patients who are good candidates for immunotherapy. In all lines of therapy, particularly after first-line therapy, clinical trial enrollment should be considered in fit patients.

Conclusions
In conclusion, advanced gastric cancer has historically been associated with a poor prognosis, however, recently there have been several new treatments for advanced disease. Targeted therapies and ICIs have emerged as important treatment options after progression on or in combination with cytotoxic chemotherapy. These new therapies have improved survival and outcomes in metastatic gastric cancer, however more research is needed to make even greater strides. Research continues to identify how to best utilize ICIs and identify patients who are most likely to benefit from them. Patients with dMMR/MSI-H tumors clearly benefit from the use of checkpoint inhibitors, but other studies are elucidating how patients with pMMR/MSS tumors can also benefit from the use of checkpoint inhibitors. In HER2-positive tumors, initial studies evaluating an investigational therapy, zanidatamab, appear promising. In HER2-negative tumors, investigational therapies targeting CLDN18.2 and FGFR2b have been encouraging. Further studies are ongoing to evaluate these novel targeted therapies. It is an exciting time in oncology with the hope that further advances in precision medicine will improve the survival and quality of life for patients with advanced gastric cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed Narrative Review reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-464/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-464/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-464/coif). A.D. received grant funding in collaboration with other investigators from her institution from the charitable Perritt Foundation for research in HCC. That work is not relevant to or included in this manuscript. Also, A.D. serves on her institution’s DSMB which did not impact this particular manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2022. Accessed March 12, 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html
- National Cancer Institute. Cancer Stat Facts: Stomach Cancer. Accessed Feb 07, 2023. Available online: https://seer.cancer.gov/statfacts/html/stomach.html
- Januszewicz W, Turkot MH, Malfertheiner P, et al. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers (Basel) 2023;15:664. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [Crossref] [PubMed]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Avital I, Nissan A, Golan T, et al. Cancer of the Stomach. In: DeVita VT, Lawrence TS, Rosenberg SA. editors. DeVita, Hellman, and Rosenberg's cancer: principles & practice of oncology. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2019:chapter 53.
- Klapper LN, Glathe S, Vaisman N, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A 1999;96:4995-5000. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273-8. [Crossref] [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Soularue É, Cohen R, Tournigand C, et al. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: a retrospective study. Bull Cancer 2015;102:324-31. [Crossref] [PubMed]
- Rivera F, Romero C, Jimenez-Fonseca P, et al. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol 2019;83:1175-81. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Gastric Cancer (Version 1.2023-March 20, 2023). Accessed August 18, 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005-20. [Crossref] [PubMed]
- Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018;19:1372-84. [Crossref] [PubMed]
- Tabernero J, Hoff PM, Shen L, et al. 1423MO end-of-study analysis from JACOB: a phase III study of pertuzumab (P)+ trastuzumab (H) and chemotherapy (CT) in HER2-positive metastatic gastric or gastro-esophageal junction cancer (mGC/GEJC). Ann Oncol 2020;31:S900-1. [Crossref]
- Makiyama A, Sukawa Y, Kashiwada T, et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). J Clin Oncol 2020;38:1919-27. [Crossref] [PubMed]
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640-53. [Crossref] [PubMed]
- Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020;382:2419-30. [Crossref] [PubMed]
- Van Cutsem E, di Bartolomeo M, Smyth E, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol 2023;24:744-56. [Crossref] [PubMed]
- FDA approves fam-trastuzumab deruxtecan-nxki for HER2-positive gastric adenocarcinomas. Accessed January 13, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-positive-gastric-adenocarcinomas
- Elimova E, Ajani JA, Burris HA III, et al. Zanidatamab+ chemotherapy as first-line treatment for HER2-expressing metastatic gastroesophageal adenocarcinoma (mGEA). J Clin Oncol 2023;41:347. [Crossref]
- Tabernero J, Shen L, Elimova E, et al. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol 2022;18:3255-66. [Crossref] [PubMed]
- Lieto E, Ferraraccio F, Orditura M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol 2008;15:69-79. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [Crossref] [PubMed]
- Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015;18:168-76. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Fuchs CS, Shitara K, Di Bartolomeo M, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:420-35. [Crossref] [PubMed]
- Klempner SJ, Maron SB, Chase L, et al. Initial Report of Second-Line FOLFIRI in Combination with Ramucirumab in Advanced Gastroesophageal Adenocarcinomas: A Multi-Institutional Retrospective Analysis. Oncologist 2019;24:475-82. [Crossref] [PubMed]
- Yi JH, Lee J, Lee J, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer 2012;106:1469-74. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Kang YK, Kang WK, Di Bartolomeo M, et al. Randomized phase III ANGEL study of rivoceranib (apatinib)+ best supportive care (BSC) vs placebo+ BSC in patients with advanced/metastatic gastric cancer who failed≥ 2 prior chemotherapy regimens. Ann Oncol 2019;30:v877-8. [Crossref]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Pavlakis N, Shitara K, Sjoquist KM, et al. INTEGRATE IIa: A randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—A study led by the Australasian Gastro-intestinal Trials Group (AGITG). J Clin Oncol 2023;41:LBA294. [Crossref]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2–positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. Accessed July 24, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid
- FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. Accessed July 27, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc
- FDA approves larotrectinib for solid tumors with NTRK gene fusions. Accessed July 27, 2023. Available online: https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions
- FDA approves selpercatinib for locally advanced or metastatic RET fusion-positive solid tumors. Accessed July 25, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-solid-tumors
- Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018;15:731-47. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018;15:151-67. Erratum in: Nat Rev Clin Oncol 2017. [Crossref] [PubMed]
- Salama AKS, Li S, Macrae ER, et al. Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J Clin Oncol 2020;38:3895-904. [Crossref] [PubMed]
- Subbiah V, Wolf J, Konda B, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol 2022;23:1261-73. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. [Crossref] [PubMed]
- Forsythe A, Zhang W, Phillip Strauss U, et al. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol 2020;12:1758835920975613. [Crossref] [PubMed]
- Shi M, Wang W, Zhang J, et al. Identification of RET fusions in a Chinese multicancer retrospective analysis by next-generation sequencing. Cancer Sci 2022;113:308-18. [Crossref] [PubMed]
- van Grieken NC, Aoyama T, Chambers PA, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer 2013;108:1495-501. [Crossref] [PubMed]
- Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14:7624-34. [Crossref] [PubMed]
- Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023;401:1655-68. [Crossref] [PubMed]
- Xu R, Shitara K, Ajani JA, et al. Zolbetuximab+ CAPOX in 1L claudin-18.2+(CLDN18. 2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin Oncol 2023;41:405736. [Crossref]
- Maia A, Wiemann S. Cancer-Associated Fibroblasts: Implications for Cancer Therapy. Cancers (Basel) 2021;13:3526. [Crossref] [PubMed]
- Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2022;23:1430-40. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018;8:1069-86. [Crossref] [PubMed]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. Accessed Nov 14, 2020. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication
- Abrha A, Shukla ND, Hodan R, et al. Universal Screening of Gastrointestinal Malignancies for Mismatch Repair Deficiency at Stanford. JNCI Cancer Spectr 2020;4:pkaa054. [Crossref] [PubMed]
- An JY, Kim H, Cheong JH, et al. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer 2012;131:505-11. [Crossref] [PubMed]
- Kim H, An JY, Noh SH, et al. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J Gastroenterol Hepatol 2011;26:585-92. [Crossref] [PubMed]
- Mathiak M, Warneke VS, Behrens HM, et al. Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization. Appl Immunohistochem Mol Morphol 2017;25:12-24. [Crossref] [PubMed]
- Pietrantonio F, Miceli R, Raimondi A, et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J Clin Oncol 2019;37:3392-400. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- FDA grants accelerated approval to pembrolizumab for advanced gastric cancer. Accessed August 20, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-advanced-gastric-cancer
- Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1571-80. [Crossref] [PubMed]
- Chao J, Fuchs CS, Shitara K, et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol 2021;7:895-902. [Crossref] [PubMed]
- Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021;600:727-30. [Crossref] [PubMed]
- FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer. Accessed January 14, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-her2-positive-gastric-cancer
- Rha SY, Wyrwicz LS, Weber PEY, et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol 2023;34:319-20. [Crossref]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019;30:250-8. [Crossref] [PubMed]
- Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234-47. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022;603:942-8. [Crossref] [PubMed]
- Stein A, Paschold L, Tintelnot J, et al. Efficacy of Ipilimumab vs FOLFOX in Combination With Nivolumab and Trastuzumab in Patients With Previously Untreated ERBB2-Positive Esophagogastric Adenocarcinoma: The AIO INTEGA Randomized Clinical Trial. JAMA Oncol 2022;8:1150-8. [Crossref] [PubMed]
- Oaknin A, Gilbert L, Tinker AV, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer 2022;10:e003777. [Crossref] [PubMed]
- Andre T, Berton D, Curigliano G, et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J Clin Oncol 2021;39:9. [Crossref]
- FDA grants accelerated approval to dostarlimab-gxly for dMMR advanced solid tumors. Accessed January 14, 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors


