
Camrelizumab and apatinib combined with chemotherapy in perioperative effective therapy for advanced gastric carcinoma with peritoneal metastasis: a case report
Highlight box
Key findings
• Camrelizumab plus apatinib in combination with chemotherapy showed favorable antitumor activity and manageable safety in perioperative therapy for gastric cancer (GC) patients with peritoneal metastasis (PM).
What is known and what is new?
• Despite the improved systemic chemotherapy, the long-term survival rate of patients with PM from GC is still very low. Although substantial progress has been made in various solid tumors, the efficacy and safety of camrelizumab plus apatinib used in combination with chemotherapy as the optional treatment in GC with PM remains unclear.
• The present study reports the effective treatment of camrelizumab plus apatinib combined with chemotherapy for peritoneal dissemination of GC.
What is the implication, and what should change now?
• This treatment regimen of immunotherapy and targeted therapy combined with chemotherapy has demonstrated good safety and efficacy, which may provide a new approach to improve the clinical prognosis of GC with peritoneal carcinomatosis.
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related deaths in the world (1). Around 42.6% of patients with newly diagnosed GC are from China (2). Currently, although early diagnosis and multimodal treatment strategies have improved the survival rate of GC patients, the prognosis of patients with advanced GC is still poor due to tumor recurrence and metastasis, with a mere 20% five-year survival rate (3,4). Patients with GC who undergo potentially curative resection (10–20%) exhibit peritoneal seeding on abdominal examination (5), and 17% of patients with GC will show peritoneal carcinomatosis at the time of diagnosis (6). Due to the poor prognosis of peritoneal dissemination of GC, surgery has not been the standard therapy for patients other thank those who need relief from symptoms such as bleeding or obstruction. The standard management for GC patients with peritoneal metastasis (PM) in the front-line setting has not yet been established. Despite advances in multimodal therapy including chemo-hyperthermia, early postoperative intraperitoneal chemotherapy, and peritonectomy, the prognosis of advanced GC remains poor and there is still room for improvement in survival rates. It is believed that combination therapy of intraperitoneal chemotherapy with systemic chemotherapy or the combination of intraoperative hyperthermic intraperitoneal chemotherapy with cytoreductive surgery is safe and efficient for GC patients with PM (7,8). Although combination therapy plays an important role in metastatic GC, the chemotherapeutic resistance and peritoneal-plasma barrier restrict its effectiveness (9).
With the development of new drugs and therapies, an improved understanding of the tumor immune escape and angiogenesis mechanism has revealed new possibilities for anti-cancer treatment. Over the years, the application of anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) antibodies has demonstrated their superior efficacy and safety (10,11), and PD-L1-negative tumors can still respond to anti-PD-1 therapy (10,12). Apatinib is a novel oral tyrosine kinase inhibitor (TKI) that selectively targets vascular endothelial growth factor receptor 2 (VEGFR2). Camrelizumab is a potent PD-1 inhibitor monoclonal antibody which has exhibited promising activity and tolerable toxicity in several kinds of solid tumors (13,14). Although the efficacy of PD-1 blockade or VEGFR inhibitor, or chemotherapy as monotherapy has been limited in the management of cancer patients, the combination of these three therapies may have synergistic effects. A phase II clinical trial of camrelizumab and apatinib plus chemotherapy in advanced esophageal squamous cell carcinoma has demonstrated improved survival outcomes (15), providing compelling evidence supporting the use of immune checkpoint inhibitors (ICIs) and molecular anti-angiogenic agents in combination with chemotherapy in patients with GC with PM. This may be because of the potential ability of chemotherapy to overcome immunosuppression, facilitate tumor antigen presentation, and modulate anti-angiogenesis activity; meanwhile, anti-PD-1 therapy may have enhanced the efficacy of the subsequent chemotherapy by an immune-mediated mechanism, and anti-angiogenic agents may modulate the tumor immunosuppressive microenvironment, as previously reported (16-18). Based on the promising anti-tumor activity of camrelizumab plus apatinib combined with chemotherapy in advanced malignant tumor patients that we observed in the previous study (15), and their potential synergy, we investigated the efficacy and safety of camrelizumab plus apatinib combined with chemotherapy as the front-line treatment for GC patients with PM.
Herein, we report the case of a GC patient with PM who had a favorable response and tumor shrinkage revealed by imaging and staging laparoscopy following 10 cycles of camrelizumab plus apatinib combined with SOX + IP-PTX. Apatinib combined with S-1 oral administration was continued as postoperative adjuvant therapy for 2 months. After recurrence was observed, the patient was treated with the regimen of camrelizumab plus apatinib combined with TAS-102 plus IP-PTX. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-741/rc).
Case presentation
A 39-year-old male was seen at the Peking University Cancer Hospital on 27 March 2019 due to upper abdominal discomfort for 7 months without a history of smoking and alcohol abuse. The patient had no family history and no specific social history. He was diagnosed with well-differentiated adenocarcinoma by gastroscopy, with a computed tomography (CT) scan suggesting thickening of the gastric wall at the gastric horn and multiple enlarged lymph nodes in the abdominal cavity (Table 1). On 10 April 2019, a laparoscopic examination was performed, and advanced peritoneal disseminated nodules were confirmed (CY1, P1). The patient underwent 2 cycles of chemotherapy on 13 April 2019 and 26 April 2019, respectively. The chemotherapy regimen comprised paclitaxel, 180 mg/dose, intraperitoneal infusion (days 1, 8), S-1 (120 mg/day, days 1–14), and the efficacy was assessed as stable-disease after chemotherapy. The chemotherapy regimen was later changed to paclitaxel 300 mg/dose, intravenous (day 1) + S-1, 60 mg/dose, twice/day, orally (days 1–14), with 1 cycle lasting for 21 days. The patient underwent 5 cycles of treatment from 15 May 2019 to 17 July 2019, after which his disease progressed. He also developed fourth degree leukopenia and was unable to tolerate further chemotherapy due to severe nausea and vomiting.
Table 1
| Characteristics | Patient |
|---|---|
| Sex | Male |
| Age, years | 39 |
| Body mass index, kg/m2 | 20.8 |
| Tumor size, cm2 | 4×3 |
| Tumor location | The lesser curvature, gastric body |
| TNM stage | T4aN1M1 |
| Operating time, min | 190 |
| Blood loss, mL | 60 |
| Prophylactic stoma | – |
| Anastomotic leakage | – |
| Anastomotic stricture | – |
TNM, tumor-node-metastasis.
On 2 August 2019, the patient visited the Department of Gastrointestinal Surgery of Ruijin Hospital, Shanghai Jiao Tong University for further consultation. The ECOG score was 1 and Nutritional Risk Screening (NRS) score was 0. The patient underwent four laparoscopic explorations during the treatment period for diagnostic purposes or surgical treatment (Figure 1). After hospitalization, the patient completed a preoperative examination and underwent a second laparoscopic exploration on 20 August 2019 to assess the stage of GC (Figure 1A). After laparoscopic exploration, the patient was diagnosed with positive PM (P1), and the cancer cells were found by the cytology of peritoneal washing fluids (CY1), so an intraperitoneal access port (PORT-A-CATH II implantable drug delivery system; Smiths Medical Corp., Minneapolis, MN, USA) was implanted to allow for chemotherapy administration. Similar to our previous study (19), the extent of peritoneal spread was assessed by the peritoneal cancer index (PCI), which was 4 (range, 0 to 39) in this case. We further ascertained the preoperative expression status of human epidermal growth factor receptor 2 (HER2) and PD-L1 and verified the negative protein expression of HER2 and PD-L1 in tumor tissues by immunohistochemical analysis. Tumor PD-L1 expression was evaluated by the PD-L1 22C3 pharmDx assay (Dako, Agilent Technologies, Santa Clara, CA, USA) and measured by using the combined positivity score (CPS), defined as the number of PD-L1 stained cells divided by the total number of surviving tumor cells, multiplied by 100. We defined PD-L1 positivity as CPS ≥1 and negative expression was judged as CPS <1. After a coordinated multidisciplinary discussion and obtaining the patient’s consent, we scheduled him for subcutaneous subabdominal chemotherapy with a treatment regimen of camrelizumab (200 mg, every 2 weeks) plus apatinib (250 mg, once daily) combined with systemic SOX (S-1 100 mg/body on day 1–14, followed by 7 days of rest, and then oxaliplatin 130 mg/m2 on day 1) + IP-PTX (50 mg/m2 on days 1, 8) for 5 cycles of conversion therapy from 23 August 2019 to 1 November 2019.

After confirmation of negative cytology, we performed a third laparoscopic exploratory surgery on the patient on 23 December 2019, and found that the peritoneal and greater omentum metastatic nodules had partially disappeared or were showing a white scar-like appearance (Figure 1B). Another 7 courses of the same conversion regimen were continued from 1 January 2020 to 12 April 2020. Under this therapeutic regimen, the patient experienced no significant adverse effects throughout the chemotherapy period, and his abdominal distention and appetite gradually improved. A CT scan revealed a reduction in the primary lesion of GC (Figure 2) and blood tests showed a decrease in the level of tumor markers, such as carbohydrate antigen 125 (CA125) (from 60 U/mL to normal range), but no significant trend in carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA199), and serum α-fetoprotein (AFP) values were observed (Figure 3). These data suggest that the new second-line therapy had achieved its anti-cancer effect. The clinical response was evaluated as partial response (PR).


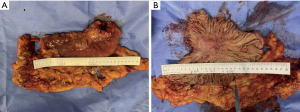
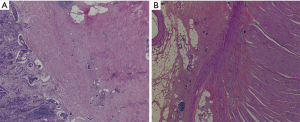
When the macroscopic disappearance of the peritoneal lesion was confirmed by staging laparoscopy (Figure 1C), along with negative peritoneal cytology on examination and the absence of other distant metastases, the patient was subjected to a total gastrectomy with D2 lymph node dissection on 13 May 2020. Gross findings of the excised specimen included an ulcerative and infiltrative (type 4) tumor (approximately 40 mm × 30 mm) with a serous surface infiltrate (Figure 4). We also performed “the intraperitoneal infuser port removal surgery” at the same time due to chemotherapy pump infection. The excised specimens were pathologically classified according to the Union for International Cancer Control tumor-node-metastasis (UICC TNM) classification of malignancies (8th edition) (20). The postoperative pathological diagnosis was ypT4aN1M1, ypStage IV. Histopathology showed that the GC cells mainly regressed to the mucosa and submucosa layer after preoperative combination therapy; the post-treatment tumor specimens also indicated extensive degenerative changes, and the tumor cells in the plasma muscle layer were replaced by granulomas or fibrous tissue of the lesion, showing a significant reduction in the number of cancer cells and a tumor regression rate of more than 90% (Figure 5). The patient recovered well after surgery, and 4 weeks later he was admitted for a total of 7 cycles of adjuvant therapy with camrelizumab (200 mg every 2 weeks) plus apatinib (250 mg, once daily) combined with S-1 oral administration (100 mg/body on days 1–14, followed by 7 days of rest) for 6 months, during which abdominal CT scans were performed every 3 months (Figure 6A-6D).
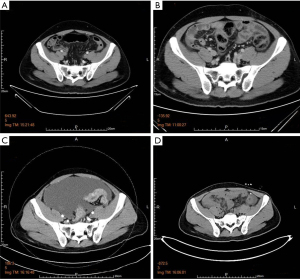
The patient visited the hospital for review on 9 January 2021 due to abdominal distension and loss of appetite, and a CT scan indicated PM with ascites (Figure 6C), and CA125 and CEA values were significantly elevated (Figure 3). On 12 January 2021, we performed the 5th laparoscopic exploration (Figure 1D) and inserted a subcutaneous subabdominal chemotherapy port in the lower abdomen. On 5 February 2021, the patient was treated with third-line chemotherapy with camrelizumab plus apatinib combined with TAS-102 (35 mg/m2/dose, twice daily for 5 days a week with 2 days rest for 2 weeks followed by a 14-day rest, repeated every 4 weeks) + IP-PTX. TAS-102 is a preconstituted drug combination comprising an oral fluoropyrimidine and a potent inhibitor of thymidine phosphorylase (21). The patient’s physical condition is currently significantly better than before, with less discomfort, and the recent CT scan shows a significant reduction in ascites (Figure 6D) and CA125 and CEA values gradually decreased (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PM and recurrence are a prevalent cause of death in patients with GC in clinical practice (22). The treatment options for patients with PM of GC are limited and include palliative systemic chemotherapy and/or best supportive care according to the National Comprehensive Cancer Network (NCCN) guidelines (23). As no treatment method has a sufficient level of evidence of success in the treatment of GC with PM, there is an urgent need to develop a new treatment that considers the pathophysiology of the disease. A novel multidisciplinary treatment approach combining IP-PTX, systemic chemotherapy, immunotherapy, targeted therapies, and gastrectomy is one of the promising treatment options, which may produce synergistic effects.
In this case, we firstly reported the efficacy and safety of camrelizumab and apatinib combined with chemotherapy in perioperative therapy for advanced GC with PM. This case demonstrated that multimodal treatment could improve the survival rate of patients with GC with PM by downgrading the primary site of GC and effectively controlling the PM through novel drugs and chemotherapy, and then providing a chance to accept radical resection of GC (R0 surgery).
As new class I drugs which have been independently developed in China, both camrelizumab and apatinib are effective. Several studies have demonstrated that apatinib could shorten the onset time and potentiate the anti-tumor effects of camrelizumab across various types of solid tumors, including advanced hepatocellular carcinoma, cervical cancer, lung cancer, and osteosarcoma (18,24-27). Peng et al. investigated the effects of camrelizumab in combination with CAPOX followed by camrelizumab plus apatinib therapy on advanced GC patients and revealed encouraging anti-tumor activity and manageable toxicity (28), Pan et al. also evaluated the efficacy and safety of apatinib in combination with camrelizumab and the SOX regimen as a first-line treatment for advanced gastric/gastro-oesophageal junction adenocarcinoma and the results showed a high objective response rate (ORR) (29). Therefore, camrelizumab in combination with apatinib has strong clinical data to support the treatment of GC. In a phase II clinical trial of camrelizumab combined with apatinib and chemotherapy for the first-line treatment of advanced esophageal squamous cell carcinoma, the ORR of the combined therapy reached 80%. The main endpoints of the study were median progression-free survival (PFS) of 6.85 months and overall survival (OS) of 19.43 months, which demonstrated that the combination treatment for advanced esophageal squamous cell carcinoma had already achieved higher response rate, disease control rate, and longer PFS (15). As the regimen of this trial was similar to the drug backbone in this case, the notable effect in ORR and long survival of the patients, consistent with the remarkable tumor regression in this case, strongly supports the hypothesis of synergy between immunotherapy, anti-angiogenesis, and chemotherapy. In this case, we explored the addition of a chemotherapeutic regimen to the combination of camrelizumab and apatinib, which also showed better results, and the patient converted successfully and was able to receive surgery. Although the patient eventually relapsed after surgery, his condition improved significantly after continued treatment with the combination of chemotherapy with camrelizumab and apatinib. We believe that this combination regimen has an improving effect for perioperative use in GC patients with PM.
In the case report presented here, combination treatment of camrelizumab and apatinib combined with chemotherapy resulted in significant regression of the malignant lesions, representing PR. The encouraging effect suggests the potential future application of this regimen not only in patients with GC with PM, but also in those who were initially considered as only marginally resectable or unresectable and may be convertible to a resectable status. However, the current research is mostly a case report, it is insufficient to screen outpatient characteristics that benefit from this combination therapy. Therefore, we established a multicenter, randomized, open-label, parallel-controlled trial to examine the potential benefits of camrelizumab or/and apatinib, either alone or in combination with chemotherapies (SOX), in the treatment of patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma (30). The results will contribute to optimal perioperative GC treatment.
In addition to efficacy, safety is an important factor to be considered in the treatment process. In this case, the majority of the adverse events (AEs) observed were consistent with the safety profiles of the individual drugs. The most common toxicities of this regimen were neutropenia, leukopenia, and peripheral sensory neuropathy. Hepatic toxicity, diarrhea, hypertension, or immune-related AEs were not reported in our combination therapy. All treatment-related AEs were managed with appropriate medical care. Due to the patient’s inability to tolerate the neurotoxicity of oxaliplatin and the fact that his chemotherapy pump had to be removed during gastrectomy due to infection, the oxaliplatin and intraperitoneal infusion chemotherapy was switched to a combination of camrelizumab plus apatinib and S-1 after surgery until recurrence. Compared with camrelizumab monotherapy, the skin capillary hyperplasia symptoms, which are the most common side effect of camrelizumab, were lower (31,32); the mechanism may be that apatinib inhibits the VEGFR2 related to vascular endothelial proliferation and blocks angiogenesis (32). Considering the efficacy and tolerability, the dose of camrelizumab 200 mg every 2 weeks and apatinib 250 mg once per day are recommended. This case indicates that camrelizumab plus apatinib combined with chemotherapy for the perioperative treatment of GC with PM has a good safety and anti-cancer effect, and the tumor shrinkage rate of this regimen is significant and worthy of further study. The continuous emergence of various therapeutic methods, such as molecular targeted therapy and immunotherapy, has made personalized treatment possible, and the selection of targeted individualized treatment based on patients’ clinical characteristics and biomarker expression may be the direction of the treatment of PM of GC in the future.
Conclusions
This case study reports a case of GC with PM, which progressed after the first-line chemotherapy and benefited from immunotherapy and targeted therapy combined with chemotherapy. To our knowledge, this case is the first report of advanced GC treated with camrelizumab plus the combination of apatinib and chemotherapy. The patient’s condition improved significantly after treatment, and no serious AEs greater than grade 3 were observed, thus confirming the favorable safety and efficacy profiles of this treatment regimen, which may provide a means to improve the clinical prognosis of patients with GC with PM. Therefore, the efficacy of this combination treatment in GC patients with PM resistant to conventional therapies should be further evaluated in a large cohort study.
Acknowledgments
We thank our staff for fruitful discussions and clerical support.
Funding: This project was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-741/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-741/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-741/coif). All authors report that this project was supported by the Scientific Research Foundation of Anhui Medical University of Anhui Province, China (No. 2021xkj101). The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Montori G, Coccolini F, Ceresoli M, et al. The treatment of peritoneal carcinomatosis in advanced gastric cancer: state of the art. Int J Surg Oncol 2014;2014:912418. [Crossref] [PubMed]
- Rau B, Brandl A, Thuss-Patience P, et al. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer 2019;22:1226-37. [Crossref] [PubMed]
- Shinkai M, Imano M. The clinical effect of conversion surgery for advanced gastric cancer patients with peritoneal metastasis. J Gastrointest Oncol 2022;13:2169-77. [Crossref] [PubMed]
- Canbay E, Canbay Torun B, Cosarcan K, et al. Surgery with hyperthermic intraperitoneal chemotherapy after response to induction chemotherapy in patients with peritoneal metastasis of gastric cancer. J Gastrointest Oncol 2021;12:S47-S56. [Crossref] [PubMed]
- Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer 2017;20:111-21. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Yang J, Zeng R, Zhou J, et al. Efficacy, prognosis and safety analysis of anti-PD-1/PD-L1 inhibitor rechallenge in advanced lung cancer patients: a cohort study. Transl Lung Cancer Res 2022;11:1038-50. [Crossref] [PubMed]
- Jing C, Wang J, Zhu M, et al. Camrelizumab combined with apatinib and S-1 as second-line treatment for patients with advanced gastric or gastroesophageal junction adenocarcinoma: a phase 2, single-arm, prospective study. Cancer Immunol Immunother 2022;71:2597-608. [Crossref] [PubMed]
- Song Y, Wu J, Chen X, et al. A Single-Arm, Multicenter, Phase II Study of Camrelizumab in Relapsed or Refractory Classical Hodgkin Lymphoma. Clin Cancer Res 2019;25:7363-9. [Crossref] [PubMed]
- Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol 2018;19:1338-50. [Crossref] [PubMed]
- Zhang B, Qi L, Wang X, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond) 2020;40:711-20. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185-204. [Crossref] [PubMed]
- Xie L, Xu J, Sun X, et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial. J Immunother Cancer 2020;8:e000798. [Crossref] [PubMed]
- Yan C, Yang ZY, Shi M, et al. Laparoscopic diagnosis of postoperative recurrence of peritoneal metastasis in gastric cancer patients and the clinical efficacy of bidirectional intraperitoneal and systemic chemotherapy. Zhonghua Wei Chang Wai Ke Za Zhi 2020;23:492-8. [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. John Wiley & Sons; 2017.
- Roviello G, Fancelli S, Gatta Michelet MR, et al. TAS-102 in gastric cancer: Development and perspectives of a new biochemically modulated fluroropyrimidine drug combination. Crit Rev Oncol Hematol 2020;152:102987. [Crossref] [PubMed]
- Dong C, Luan F, Tian W, et al. Identification and validation of crucial lnc-TRIM28-14 and hub genes promoting gastric cancer peritoneal metastasis. BMC Cancer 2023;23:76. [Crossref] [PubMed]
- Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2022;20:167-92. [Crossref] [PubMed]
- Chen D, Chen X, Xu L, et al. Camrelizumab combined with apatinib in the treatment of patients with hepatocellular carcinoma: a real-world assessment. Neoplasma 2023;70:580-7. [Crossref] [PubMed]
- Lan C, Shen J, Wang Y, et al. Camrelizumab Plus Apatinib in Patients With Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J Clin Oncol 2020;38:4095-106. [Crossref] [PubMed]
- Ren S, He J, Fang Y, et al. Camrelizumab Plus Apatinib in Treatment-Naive Patients With Advanced Nonsquamous NSCLC: A Multicenter, Open-Label, Single-Arm, Phase 2 Trial. JTO Clin Res Rep 2022;3:100312. [Crossref] [PubMed]
- Fan Y, Zhao J, Wang Q, et al. Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial. J Thorac Oncol 2021;16:299-309. [Crossref] [PubMed]
- Peng Z, Wei J, Wang F, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021;27:3069-78. [Crossref] [PubMed]
- Pan L, Tian Y, Wang K, et al. Low-dose apatinib combined with camrelizumab and the SOX regimen in the neoadjuvant treatment of locally advanced gastric/gastroesophageal junction adenocarcinoma (SPACE-neo): a protocol for an open-label, single-arm, clinical trial. J Gastrointest Oncol 2022;13:3300-13. [Crossref] [PubMed]
- Zheng Y, Wang Z, Yan C, et al. Protocol for a randomized controlled trial of perioperative S-1 plus oxaliplatin combined with apatinib and camrelizumab in patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma. Ann Transl Med 2020;8:1684. [Crossref] [PubMed]
- Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res 2019;25:515-23. [Crossref] [PubMed]
- Li W, Wei Z, Yang X, et al. Salvage therapy of reactive capillary hemangiomas: Apatinib alleviates the unique adverse events induced by camrelizumab in non-small cell lung cancer. J Cancer Res Ther 2019;15:1624-8. [Crossref] [PubMed]


