
Comparison of clinicopathological features and survival analysis between esophageal neuroendocrine carcinoma and esophageal squamous cell carcinoma based on the SEER database, alongside nomogram analysis for esophageal neuroendocrine carcinoma
Highlight box
Key findings
• Our study found significant clinicopathologic differences between esophageal neuroendocrine carcinoma (ENEC) and esophageal squamous cell carcinoma (ESCC). Patients with ENEC were associated with worse prognosis compared to those with ESCC. Our constructed nomogram predicted the disease-specific survival of patients with ENEC. The model could provide a comprehensive guidance in counseling for patients with ENEC and clinical treatment decision-making.
What is known and what is new?
• Earlier retrospective analyses with limited sample sizes have suggested that ENEC is a rare type of esophageal malignancy characterized by an unfavorable prognosis.
• In this study, we conducted a comprehensive analysis and comparison of the clinicopathological features among patients with ENEC and ESCC based on the Surveillance, Epidemiology and End Results database. Furthermore, we developed a prognostic prediction model specifically tailored for ENEC patients.
What is the implication, and what should change now?
• ENEC is a rare but devastating disease and more exploration is needed.
Introduction
Esophageal cancer (EC) ranks 7th in terms of global incidence and 6th in mortality (1). Esophageal squamous cell carcinoma (ESCC) is the most prevalent histological subtype among esophageal malignancies, followed by esophageal adenocarcinoma (EAC) and other pathological types. Esophageal neuroendocrine carcinoma (ENEC) is a rare malignancy of the esophagus, initially identified and reported by the pathologist Mckeown in 1952 (2). Subsequent reports on ENEC have been published, albeit primarily in the form of individual case reports.
Through years of clinical practice, we have gained profound insights into the clinicopathological features, survival, and prognosis associated with ESCC. Standardized treatment protocols have been established for ESCC (3-5). Conversely, the situation is markedly different for ENEC. Literature review reveals that the incidence of ENEC accounts for only 0.8–2.8% of all esophageal malignancies (6). Due to its exceptionally low occurrence, conducting prospective clinical studies or large-sample retrospective analyses on ENEC is challenging. Hence, the establishment of a standardized treatment for ENEC remains elusive.
Literature about ENEC predominantly consists of individual case reports or small sample studies and conspicuously lacks perspective or large-scale retrospective investigations validating the clinicopathological characteristics and prognosis of ENEC. ENEC is commonly regarded as an aggressive disease with poor differentiation, often diagnosed at an advanced stage, and associated with an unfavorable prognosis (7-10). Zhang et al. reported on 51 cases of ENEC, highlighting a poorer prognosis compared to ESCC (11). Zhang et al. conducted a review of 82 ENEC patients, identifying associations between disease stage, liver, and lung metastases with poor prognosis (12). Zhang et al. documented the clinicopathological features of 162 ENEC patients to establish a nomogram model (13). Cai et al. reported that the 3-year survival rate for non-metastatic ENEC was 5.95%, while that for metastatic ENEC was 0% (14). However, it is noteworthy that the sample size in the aforementioned studies is relatively small, limiting its representativeness.
Neuroendocrine carcinoma can originate from various systems throughout the body, and there are notable differences in pathogenesis, clinicopathological features, and prognosis among neuroendocrine carcinomas of distinct origins (15-17). Previous literature has indicated that ENEC is frequently coexistent with ESCC, potentially linked to the pathological origin of ENEC (18). Most scholars believe that ENEC originates from a pluripotent stem cell with differentiation potential in the endoderm. This pluripotent stem cell is considered the common precursor for all epithelial tumors of the esophagus (19). In order to comprehensively compare these two esophageal malignancies with the same pathological origin but significantly different clinical features and prognosis and to better understand the clinical features and prognostic factors of ENEC, we extracted and compared ENEC and ESCC patients from the Surveillance, Epidemiology and End Results (SEER) database and we built a nomogram model for ENEC patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-905/rc).
Methods
Patients
This is a retrospective cohort study. Patients diagnosed with ENEC and ESCC between 1975 and 2016 in SEER 18 registries were enrolled in this study. ENEC and ESCC were defined according to the International Classification of Diseases for Oncology, third edition (ICD-O-3). Morphology codes used in neuroendocrine tumors (NETs) included 8041/3, 8013/3, 8046/3, 8244/3, and 8070/3 for ESCC. The diagnostic criterion was histological positivity. Patients with incomplete survival data or secondary malignancies were excluded. The demographic features (sex, race, age, marital status, grade, primary site, SEER stage, metastatic sites), treatment modalities [surgery, radiotherapy (RT), chemotherapy], and survival data were obtained for each patient in the SEER database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Endpoint definition
The main endpoint of this study was disease-specific survival (DSS). DSS and overall survival (OS) were defined as the interval from diagnosis to death or last follow-up. However, in DSS, death was caused by the specific disease, whereas OS represented death due to any reason.
Statistical analysis
Patient data were extracted from SEER*Stat software version 8.3.8 (https://seer.cancer.gov/seerstat). Categorical variables were summarized as proportions and were compared by the Chi-squared test. The median DSS and OS were estimated using the Kaplan-Meier method. Multivariate analysis was conducted using a Cox proportional hazard model. All P values were two-sided, and statistical significance was considered at P<0.05, with confidence intervals (CIs) reported at the 95% confidence level. The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R software version 4.3.0 (http://www.r-project.org) were used to perform the statistical methods mentioned above.
Propensity score matching (PSM) was used to match different patients with NEC and ESCC. Variables used for matching were age, race, SEER stage, grade, surgery, RT, and chemotherapy, which were screened from the multivariate analysis.
Patients with definitive SEER stage were enrolled in the nomogram model. With a ratio of 7:3, all enrolled patients were randomly divided into a training and a validation group. Prognostic factors of DSS as well as age were adopted to establish the nomogram. The concordance index (C-index) was employed to assess the predictive accuracy and discrimination ability of each factor and the nomogram. Calibration curves, generated with 1,000 bootstrap resamples, were used to evaluate the calibration of the nomogram. Decision curve analyses (DCAs) were performed to assess the clinical utility of the nomogram. Additionally, a risk classification system was devised based on the total scores of each patient in both cohorts using the nomogram, categorizing all patients into two prognostic groups (low-risk or high-risk). Kaplan-Meier curves were employed to illustrate and compare the DSS of patients in the different risk groups.
Results
Different clinical characteristics between ENEC and ESCC
A total number of 30,157 patients were enrolled in this study, consisting of 29,420 and 737 patients with ESCC and ENEC, respectively. The pathological types of ENEC included small cell carcinoma (60.4%), large cell neuroendocrine carcinoma (5.7%), mixed neuroendocrine-non-endocrine neoplasms (MiNEN) (0.8%), and neuroendocrine carcinoma NOS (not otherwise specified) (33.1%). The baseline characteristics of both cohorts are summarized in Table 1.
Table 1
| Characteristics | ENEC (N=737), n (%) | ESCC (N=29,420), n (%) | P value |
|---|---|---|---|
| Age, years | 0.549 | ||
| ≤70 | 308 (41.8) | 11,729 (39.9) | |
| >70 | 429 (58.2) | 17,691 (60.1) | |
| Sex | 0.220 | ||
| Male | 500 (67.8) | 19,300 (65.6) | |
| Female | 237 (32.2) | 10,120 (34.4) | |
| Race | <0.001 | ||
| White | 96 (13.0) | 18,343 (62.3) | |
| Black | 595 (80.7) | 8,449 (28.7) | |
| Other | 46 (6.2) | 2,628 (8.9) | |
| Primary site | <0.001 | ||
| Cervical and upper | 51 (6.9) | 5,254 (17.9) | |
| Middle | 163 (22.1) | 10,158 (34.5) | |
| Lower | 365 (49.5) | 7,726 (26.3) | |
| Other | 158 (21.4) | 6,282 (21.4) | |
| Marital status | 0.001 | ||
| Married | 597 (81.0) | 22,054 (75.0) | |
| Unmarried | 95 (12.9) | 5,166 (17.6) | |
| Unknown | 45 (6.1) | 2,200 (7.5) | |
| SEER stage | <0.001 | ||
| Regional | 128 (17.4) | 8,325 (28.3) | |
| Localized | 97 (13.2) | 7,296 (24.8) | |
| Distant | 389 (52.8) | 7,919 (26.9) | |
| Unknown | 123 (16.7) | 5,880 (20.0) | |
| Grade | <0.001 | ||
| I–II | 17 (2.3) | 12,434 (42.3) | |
| III–IV | 501 (68.0) | 10,913 (37.1) | |
| Unknown | 219 (29.7) | 6,073 (20.6) | |
| Surgery | <0.001 | ||
| No | 667 (90.5) | 24,133 (82.0) | |
| Yes | 70 (9.5) | 5,287 (18.0) | |
| Radiation | <0.001 | ||
| No/unknown | 416 (56.4) | 11,054 (37.6) | |
| Yes | 321 (43.6) | 18,366 (62.4) | |
| Chemotherapy | <0.001 | ||
| No/unknown | 265 (36.0) | 14,656 (49.8) | |
| Yes | 472 (64.0) | 14,764 (50.2) |
PSM, propensity score matching; ENEC, esophageal neuroendocrine carcinoma; ESCC, esophageal squamous cell carcinoma; SEER, SEER, Surveillance, Epidemiology and End Results; grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated.
Compared with ESCC, ENEC was more common in Black patients (80.7% vs. 28.7%, P<0.001). The most common involved site in ENEC was the lower esophagus (49.5% vs. 26.3%, P<0.001). ENEC had worse differentiation (III–IV, 68.0% vs. 37.1%, P<0.001) and was more prone to distant disease (52.8% vs. 26.9%, P<0.001). In addition, a higher percentage of patients with ENEC received chemotherapy (64.0% vs. 50.2%, P<0.001), whereas fewer underwent surgery (9.5% vs. 18.0%, P<0.001) or RT (43.6% vs. 62.4%, P<0.001). Lastly, the common metastatic organs of ENEC and ESCC were extracted and analyzed. We noticed marked differences in involved organs between ENEC and ESCC. ENEC most frequently metastasized to the liver, bone, and lungs, whereas the most common metastatic site of ESCC was the lungs, followed by liver, and bone (Figure 1).

Survival of ENEC Patients
The DSS was significantly longer in ENEC patients who received either surgery [22.0 and 7.0 months, hazard ratio (HR) =0.44, P<0.001], RT (11.0 and 5.0 months, HR =0.54, P<0.001), or chemotherapy (11.0 and 2.0 months, HR =0.45, P<0.001). As far as OS was considered, a similar survival benefit was observed for surgery (19.0 and 6.0 months, HR =0.46, P<0.001), radiation (11.0 and 4.0 months, HR =0.56, P<0.001), and chemotherapy (10.0 and 2.0 months, HR =0.45, P<0.001). Besides, early SEER stage was positively related to DSS and OS (P<0.001, Figure 2).

Univariate and multivariate analysis
In univariate analysis, age, SEER stage, surgery, RT, and chemotherapy were related to DSS (P<0.05). The above factors as well as tumor grade and race were incorporated into multivariate analysis. Based on univariate and multivariate analysis, elements such as age, race, SEER stage, surgery, RT, and chemotherapy were identified as predictors of DSS and were included in the predictive model (Table 2).
Table 2
| Characteristics | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, years | |||||||
| ≤70 | Reference | Reference | |||||
| >70 | 0.833 | 0.701–0.990 | 0.038 | 0.850 | 0.713–1.014 | 0.071 | |
| Sex | |||||||
| Male | Reference | – | |||||
| Female | 0.961 | 0.799–1.157 | 0.676 | – | – | – | |
| Race | |||||||
| White | Reference | Reference | |||||
| Black | 0.882 | 0.687–1.133 | 0.327 | 0.840 | 0.649–1.086 | 0.183 | |
| Other | 0.902 | 0.602–1.352 | 0.618 | 0.637 | 0.420–0.965 | 0.034 | |
| Primary site | |||||||
| Cervical and upper | Reference | – | |||||
| Middle | 1.086 | 0.750–1.570 | 0.663 | – | – | – | |
| Lower thoracic | 1.025 | 0.726–1.447 | 0.887 | – | – | – | |
| Other | 1.422 | 0.980–2.062 | 0.064 | – | – | – | |
| Marital status | |||||||
| Married | Reference | – | |||||
| Unmarried | 1.209 | 0.933–1.567 | 0.151 | – | – | – | |
| Unknown | 1.182 | 0.820–1.702 | 0.370 | – | – | – | |
| SEER stage | |||||||
| Regional | Reference | Reference | |||||
| Localized | 0.980 | 0.723–1.328 | 0.895 | 0.799 | 0.587–1.088 | 0.154 | |
| Distant | 2.485 | 1.966–3.142 | <0.001 | 1.914 | 1.498–2.446 | <0.001 | |
| Grade | |||||||
| I–II | Reference | Reference | |||||
| III–IV | 1.573 | 0.839–2.950 | 0.158 | 1.590 | 0.842–3.002 | 0.152 | |
| Unknown | 1.655 | 0.873–3.138 | 0.123 | 1.652 | 0.863–3.160 | 0.130 | |
| Surgery | |||||||
| No | Reference | Reference | |||||
| Yes | 0.444 | 0.327–0.604 | <0.001 | 0.498 | 0.359–0.691 | <0.001 | |
| Radiation | |||||||
| No/unknown | Reference | Reference | |||||
| Yes | 0.514 | 0.431–0.613 | <0.001 | 0.650 | 0.528–0.784 | <0.001 | |
| Chemotherapy | |||||||
| No/unknown | Reference | Reference | |||||
| Yes | 0.444 | 0.369–0.533 | <0.001 | 0.410 | 0.338–0.497 | <0.001 | |
DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval; SEER, Surveillance, Epidemiology and End Results; grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated.
Unfavorable survival of ENEC patients
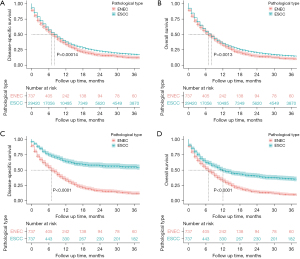
Significant differences were detected in demographic characteristics between patients with ENEC and ESCC. Here, a PSM was performed to balance the two groups. Independent prognostic factors that we found (age, race, etc.) were adopted for this purpose. After 1:1 PSM, 737 patients from each group were obtained; all the variables were well-balanced and comparable (Table S1). ENEC patients were associated with an unfavorable DSS (7.0 vs. 8.0 months, HR =0.86, P=0.00014) and OS (7.0 vs. 8.0 months, HR =0.89, P=0.0013) in contrast to ESCC before PSM. Notably, the tendency remained and became more obvious after PSM (DSS: 7.0 months vs. not reached, HR =0.34, P<0.0001; OS: 7.0 vs. 12.0 months, HR =0.54, P<0.0001) (Figure 3).
Nomogram building
A total of 614 patients with definitive SEER stage were enrolled the nomogram. With a ratio of 7:3, all enrolled patients were randomly divided into a training (n=430) and a validation (n=184) group. All the demographic and clinical features of patients for both groups were comparable (Table 3). The predictive model was demonstrated in the form of a novel nomogram (Figure 4A), and was validated by C-index, calibration curves, and DCA. The C-index of the novel nomogram was 0.751 and 0.706, respectively, in internal and external validation, which reflected the better discrimination capacity of the model. A good consistency in the probability of 1- and 3-year DSS was observed in calibration curves of either internal or external validation (Figure 4B-4E). In addition, DCA also showed great positive net benefits in the predictive model among almost all of the threshold probabilities at 1- and 3-year, indicating the favorable potential clinical effect of the predictive model (Figure 5).
Table 3
| Characteristics | Training group (N=430), n (%) | Validation group (N=184), n (%) | P value |
|---|---|---|---|
| Age, years | |||
| ≤70 | 239 (55.6) | 104 (56.5) | |
| >70 | 191 (44.4) | 80 (43.5) | 0.900 |
| Race | |||
| White | 59 (13.7) | 23 (12.5) | |
| Black | 344 (80.0) | 151 (82.1) | |
| Other | 27 (6.3) | 10 (5.4) | 0.833 |
| Sex | |||
| Male | 289 (67.2) | 135 (73.4) | |
| Female | 141 (32.8) | 49 (26.6) | 0.156 |
| Primary site | |||
| Cervical and upper | 37 (8.6) | 8 (4.3) | |
| Middle | 103 (24.0) | 33 (17.9) | |
| Lower | 205 (47.7) | 104 (56.5) | |
| Other | 85 (19.8) | 39 (21.2) | 0.059 |
| Marital status | |||
| Married | 363 (84.4) | 142 (77.2) | |
| Unmarried | 45 (10.5) | 29 (15.8) | |
| Unknown | 22 (5.1) | 13 (7.1) | 0.096 |
| SEER stage | |||
| Regional | 92 (21.4) | 36 (19.6) | |
| Localized | 77 (17.9) | 20 (10.9) | |
| Distant | 261 (60.7) | 128 (69.6) | 0.054 |
| Grade | |||
| I–II | 10 (2.3) | 3 (1.6) | |
| III–IV | 296 (68.8) | 124 (67.4) | |
| Unknown | 124 (28.8) | 57 (31.0) | |
| Surgery | 0.766 | ||
| No | 380 (88.4) | 170 (92.4) | |
| Yes | 50 (11.6) | 14 (7.6) | 0.177 |
| Radiation | |||
| No/unknown | 239 (55.6) | 100 (54.3) | |
| Yes | 191 (44.4) | 84 (45.7) | 0.847 |
| Chemotherapy | |||
| No/unknown | 143 (33.3) | 54 (29.3) | |
| Yes | 287 (66.7) | 130 (70.7) | 0.392 |
SEER, Surveillance, Epidemiology and End Results; grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated.


Risk classification system
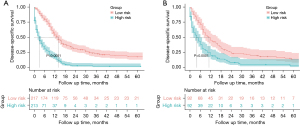
Based on the nomogram, a risk classification system for DSS was developed to divide patients into low-risk and high-risk groups, with a middle score value 276.2 and 232.7 in the training and the validation group, respectively. There were significant survival benefits in the low-risk group both in the validation group and the training group (P<0.0001, Figure 6).
Discussion
In this study, we observed distinct demographic features between patients with ENEC and ESCC. Poor differentiation, late SEER staging, tendency to liver metastasis, and dismal prognosis were observed in ENEC. We tried to predict the survival of patients with ENEC by constructing a nomogram model. The model indicates that in patients with ENEC, those with advanced stages, those who did not undergo chemoradiotherapy or surgery, individuals of Caucasian ethnicity, and patients under the age of 70 have a poorer prognosis. our model showed good accuracy and efficiency in prediction of DSS in these patients.
In our study, patients with ENEC exhibited an obvious discrepancy from those with ESCC in both clinical presentations and survival. Cai et al. reported that the liver was the most common site of metastasis in gastrointestinal neuroendocrine neoplasms (20), whereas in this study, we also observed a tendency to liver metastasis in ENEC compared with ESCC. A recent study reported that median OS in ENEC was 13 months, primarily influenced by the presence of distant metastases (12).
The optimal management of ENEC has not yet been established. For patients with limited stage, the role of surgery remains controversial, but there is increasing evidence that a comprehensive treatment based on surgery can provide remarkable survival benefits (21-23). The European Neuroendocrine Tumor Society suggests performing initial chemoradiation for localized ENEC, while platinum-based chemotherapy is advised after surgery with adjuvant intent (24). Zou et al. found that adjuvant chemotherapy or chemo-RT could improve the disease-free survival (DFS) and OS compared with surgery alone (25). Another analysis indicated that preoperative chemotherapy could significantly improve the OS and reduce the risk of distant migration in locally advanced ENEC (26). The median OS in the preoperative group reached 26 months. Combining literature review with our research results, we boldly speculate that for ENEC patients with limited stage, radical esophagectomy combined with perioperative treatment may bring more survival benefits. We plan to conduct a prospective, single-arm, phase II clinical study to explore the safety and effectiveness of the triple combination of neoadjuvant chemotherapy, immunotherapy, and radical esophagectomy in patients with localized ENEC. For patients with distant SEER stage, palliative chemotherapy is the dominant option.
In recent years, immunotherapy, especially immune checkpoint inhibitors (ICIs), has made a breakthrough in EC treatment. ICIs plus chemotherapy has been established as the first-line treatment for advanced EC (27,28). For those failing to achieve pathological complete response after neoadjuvant chemoradiotherapy (nCRT), adjuvant nivolumab has been recommended (29). Jiang et al. reported an advanced ENEC patient who received immunotherapy after chemotherapy resistance and achieved a long-time progression-free survival (30). A prospective clinical trial about chemotherapy plus JS001 in advanced ENEC is recruiting (NCT05173246). Another ongoing SWOG S2012 trial is exploring platinum/etoposide with or without atezolizumab in patients with poorly differentiated extrapulmonary small cell neuroendocrine carcinomas (NCT 05058651). Immunotherapy is promising in ENEC, but further evidence is needed.
Small cell carcinomas that originated from other organs have shown a poor prognosis (15-17,31,32). This study adopted a PSM algorithm to analyze the prognosis of ENEC. PSM helps to reduce the potential imbalance between groups and consolidates the comparative results. To the best of our knowledge, this was the first study to make this kind of comparison. Our study provided the strongest to-date data in support of inferior outcomes of ENEC.
At present, the mechanism behind the negative prognosis of ENEC has remained unclear. Absence of early symptoms and delayed diagnosis might be important reasons. SEER stage was shown to an independent prognostic factor in this study, which was in line with other preliminary studies (32,33). Pathology is also a very important prognostic factor. Especially, small cell carcinoma is well known for its aggressive behavior and poor prognosis, and the esophagus is the most commonly involved site in the gastrointestinal tract (34-36). In our study, small cell carcinoma was the predominant type in the total cohort. In addition, we found that ENEC was prone to liver metastases, which is believed to be related to inferior prognosis (37,38).
ENEC is characterized by poor differentiation and extensive neuroendocrine differentiation, accompanied by the worst prognosis when compared to their well-differentiated counterparts, NETs, or carcinomas with little to no neuroendocrine differentiation, such as conventional EAC or those displaying neuroendocrine differentiation (39). NECs and NETs share neuroendocrine differentiation features, however, they differ significantly in terms of histopathological morphology, and genetic characteristics (39,40). While some G3 NETs may exhibit diagnostic similarities to NECs, their prognosis and response to chemotherapy remain distinct (41). In certain cases, EAC can demonstrate neuroendocrine differentiation, resulting in a poorer prognosis compared to conventional EAC (42). Within the spectrum of neuroendocrine differentiation, NECs exhibit the most unfavorable survival outcomes when contrasted with conventional EAC and EAC displaying neuroendocrine differentiation.
Prognostic factors were proposed for ENEC from other studies (43-45). In one report, a multivariate model of competing-risks nomogram was established, but the power was restricted by both limited sample size (n=162) and absence of external validation (13). In this study, we built our nomogram based on the large-sized SEER database. The results revealed that age, race, SEER stage, surgery, RT, and chemotherapy were independent prognostic factors. The results were further verified by C-index, calibration curves, and DCA. Hence, our results might be more reliable.
This study had its limitations. Firstly, due to the retrospective nature, bias was inevitable. Secondly, all the data were obtained from the SEER database. Details including tumor size, Ki-67 index, or systemic chemotherapy were missing. This restricted our further research on ENEC.
Conclusions
In summary, our study found significant clinicopathologic differences between ENEC and ESCC. Patients with ENEC were associated with worse prognosis compared to those with ESCC. Our constructed nomogram predicted the DSS of patients with ENEC. The model could provide a comprehensive guidance in counseling for patients with ENEC and clinical treatment decision-making. This study helped to deepen our understanding of clinical and prognostic characteristics of ENEC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-905/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-905/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-905/coif). R.A.R. has been a paid consultant and accepted speaker bureau fees from Astra Zeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- MCKEOWN F. Oat-cell carcinoma of the oesophagus. J Pathol Bacteriol 1952;64:889-91. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- Qin J, Kang X, Li Y. Optimal management of locally advanced esophageal squamous cell carcinoma. Ann Esophagus 2023;6:36. [Crossref]
- Sohda M, Kuwano H, Saeki H, et al. Nationwide survey of neuroendocrine carcinoma of the esophagus: a multicenter study conducted among institutions accredited by the Japan Esophageal Society. J Gastroenterol 2021;56:350-9. [Crossref] [PubMed]
- Estrozi B, Bacchi CE. Neuroendocrine tumors involving the gastroenteropancreatic tract: a clinicopathological evaluation of 773 cases. Clinics (Sao Paulo) 2011;66:1671-5. [PubMed]
- Medgyesy CD, Wolff RA, Putnam JB Jr, et al. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer 2000;88:262-7. [Crossref] [PubMed]
- Huang Q, Wu H, Nie L, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol 2013;37:467-83. [Crossref] [PubMed]
- Dasari A, Mehta K, Byers LA, et al. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018;124:807-15. [Crossref] [PubMed]
- Zhang L, Yu B, Liu Z, et al. Analysis of the Clinicopathological Characteristics, Prognosis, and Lymphocyte Infiltration of Esophageal Neuroendocrine Neoplasms: A Surgery-Based Cohort and Propensity-Score Matching Study. Cancers (Basel) 2023;15:1732. [Crossref] [PubMed]
- Zhang Y, Liao JT, Lin Y, et al. Clinicopathological features, treatment modalities, and prognosis of esophageal neuroendocrine carcinoma: A single-center retrospective study. J Dig Dis 2023;24:472-9. [Crossref] [PubMed]
- Zhang G, Wu B, Wang X, et al. A competing-risks nomogram and recursive partitioning analysis for cause-specific mortality in patients with esophageal neuroendocrine carcinoma. Dis Esophagus 2019;32:doy129. [Crossref] [PubMed]
- Cai W, Ge W, Yuan Y, et al. A 10-year Population-based Study of the Differences between NECs and Carcinomas of the Esophagus in Terms of Clinicopathology and Survival. J Cancer 2019;10:1520-7. [Crossref] [PubMed]
- Yin Y, Han L, Chen Y, et al. Vulvar neuroendocrine carcinoma: a case report and literature review. Gynecol Pelvic Med 2022;5:30. [Crossref]
- Xia L, Lai J, Liu X, et al. Epidemiological and survival outcomes of neuroendocrine carcinoma of the breast: a SEER data analysis. Transl Cancer Res 2023;12:1951-62. [Crossref] [PubMed]
- Zhou H, Hu Y, Luo R, et al. Multi-region exome sequencing reveals the intratumoral heterogeneity of surgically resected small cell lung cancer. Nat Commun 2021;12:5431. [Crossref] [PubMed]
- Vos B, Rozema T, Miller RC, et al. Small cell carcinoma of the esophagus: a multicentre Rare Cancer Network study. Dis Esophagus 2011;24:258-64. [Crossref] [PubMed]
- Ishida H, Kasajima A, Kamei T, et al. SOX2 and Rb1 in esophageal small-cell carcinoma: their possible involvement in pathogenesis. Mod Pathol 2017;30:660-71. [Crossref] [PubMed]
- Cai W, Tan Y, Ge W, et al. Pattern and risk factors for distant metastases in gastrointestinal neuroendocrine neoplasms: a population-based study. Cancer Med 2018;7:2699-709. [Crossref] [PubMed]
- Situ D, Lin Y, Long H, et al. Surgical treatment for limited-stage primary small cell cancer of the esophagus. Ann Thorac Surg 2013;95:1057-62. [Crossref] [PubMed]
- Xie MR, Xu SB, Sun XH, et al. Role of surgery in the management and prognosis of limited-stage small cell carcinoma of the esophagus. Dis Esophagus 2015;28:476-82. [Crossref] [PubMed]
- Gu YM, Yang YS, Shi GD, et al. Limited-stage small cell carcinoma of the esophagus treated with curative esophagectomy: A multicenter retrospective cohort study. J Surg Oncol 2022;126:1396-402. [Crossref] [PubMed]
- Sorbye H, Grande E, Pavel M, et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J Neuroendocrinol 2023;35:e13249. [Crossref] [PubMed]
- Zou B, Li T, Zhou Q, et al. Adjuvant Therapeutic Modalities in Primary Small Cell Carcinoma of Esophagus Patients: A Retrospective Cohort Study of Multicenter Clinical Outcomes. Medicine (Baltimore) 2016;95:e3507. [Crossref] [PubMed]
- Cai G, Wang J, Zou B, et al. Preoperative Chemotherapy for Limited-stage Small Cell Carcinoma of the Esophagus. Ann Thorac Surg 2022;114:1220-8. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Jiang M, Zhang X. Antiangiogenesis Combined with Immunotherapy to Treat Advanced Small-Cell Carcinoma of the Esophagus Resistant to Chemotherapy: According to the Guidance of Next-Generation Sequencing. Onco Targets Ther 2021;14:1613-21. [Crossref] [PubMed]
- Hamilton K, Chiappori A, Olson S, et al. Prevalence and prognostic significance of neuroendocrine cells in esophageal adenocarcinoma. Mod Pathol 2000;13:475-81. [Crossref] [PubMed]
- Lee CG, Lim YJ, Park SJ, et al. The clinical features and treatment modality of esophageal neuroendocrine tumors: a multicenter study in Korea. BMC Cancer 2014;14:569. [Crossref] [PubMed]
- Babu Kanakasetty G, Dasappa L, Lakshmaiah KC, et al. Clinicopathological Profile of Pure Neuroendocrine Neoplasms of the Esophagus: A South Indian Center Experience. J Oncol 2016;2016:2402417. [Crossref] [PubMed]
- Brenner B, Tang LH, Klimstra DS, et al. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol 2004;22:2730-9. [Crossref] [PubMed]
- Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol 2013;20:4239-44. [Crossref] [PubMed]
- Ma Z, Cai H, Cui Y. Progress in the treatment of esophageal neuroendocrine carcinoma. Tumour Biol 2017;39:1010428317711313. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol 2015;3:217-21. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. Digestive system tumours. 5th edition. Lyon (France): International Agency for Research on Cancer; 2019.
- Yachida S, Totoki Y, Noë M, et al. Comprehensive Genomic Profiling of Neuroendocrine Carcinomas of the Gastrointestinal System. Cancer Discov 2022;12:692-711. [Crossref] [PubMed]
- Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013;24:152-60. [Crossref] [PubMed]
- Wang KL, Yang Q, Cleary KR, et al. The significance of neuroendocrine differentiation in adenocarcinoma of the esophagus and esophagogastric junction after preoperative chemoradiation. Cancer 2006;107:1467-74. [Crossref] [PubMed]
- Morita M, Taguchi K, Kagawa M, et al. Treatment strategies for neuroendocrine carcinoma of the upper digestive tract. Int J Clin Oncol 2020;25:842-50. [Crossref] [PubMed]
- Zhou Y, Hou P, Zha KJ, et al. Prognostic value of pretreatment contrast-enhanced computed tomography in esophageal neuroendocrine carcinoma: A multi-center follow-up study. World J Gastroenterol 2020;26:4680-93. [Crossref] [PubMed]
- Wu IC, Chu YY, Wang YK, et al. Clinicopathological features and outcome of esophageal neuroendocrine tumor: A retrospective multicenter survey by the digestive endoscopy society of Taiwan. J Formos Med Assoc 2021;120:508-14. [Crossref] [PubMed]


