
The role of ramucirumab plus paclitaxel as second-line therapy after failure of nivolumab plus doublet chemotherapy in patients with advanced gastric cancer
Highlight box
Key findings
• In a cohort of 20 patients who received ramucirumab and paclitaxel as second-line treatment after failing first-line therapy with nivolumab plus doublet chemotherapy, this study revealed disappointing results in terms of efficacy, including median overall survival and progression-free survival.
What is known and what is new?
• Adding nivolumab to fluoropyrimidines and platinum-based chemotherapy has been considered a new standard first-line treatment in human epidermal growth factor receptor 2-negative advanced gastric cancer (AGC).
• The current study suggests that ramucirumab plus paclitaxel as second-line therapy might not be sufficient in AGC patients after failure of nivolumab plus chemotherapy.
What is the implication, and what should change now?
• A refined approach to novel second-line therapy is needed in patients with AGC who have progressed after nivolumab plus doublet chemotherapy.
Introduction
According to GLOBOCAN 2020 estimates, stomach cancer was the fifth most common malignancy in the world, with approximately 1.1 million new cases in 2020, and the fourth leading cause of cancer death with approximately 800,000 deaths (accounting for 7.7% of all cancer deaths) (1). For human epidermal growth factor receptor 2 (HER2)-negative advanced gastric cancer (AGC), a combination of fluoropyrimidines and platinum agents is the standard first-line treatment (2,3). Ramucirumab is a recombinant human immunoglobulin G1 (IgG1)-neutralizing monoclonal antibody (mAb) which is selective for the ectodomain of vascular endothelial growth factor receptor 2 (VEGFR-2) (4). As second-line chemotherapy, paclitaxel and paclitaxel/ramucirumab were compared in the phase III RAINBOW trial in 2014. The results showed that the combination group had significantly longer median overall survival (OS) (9.6 months) than the paclitaxel alone group (7.4 months), as well as a higher response rate (28% vs. 16%). As a result, the paclitaxel/ramucirumab combination is currently regarded as the second-line preferred standard care for treating metastatic gastric cancer around the world (5).
Recently, nivolumab has shown superior survival benefits and an acceptable safety profile when combined with fluoropyrimidines and platinum as first-line therapy in previously untreated patients with AGC. In the CheckMate 649 study, combining nivolumab with chemotherapy significantly improved OS [hazard ratio (HR): 0.71; 98.4% confidence interval (CI): 0.59–0.86; P<0.001] and progression-free survival (PFS; HR: 0.68; 98% CI: 0.56–0.81; P<0.001) (6) compared to the chemotherapy group in treatment-naive AGC patients with a programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of five or more (6). Therefore, nivolumab plus chemotherapy has become a new standard first-line treatment for patients with metastatic gastric cancer. However, the current standard second-line therapy for AGC has not been established based on this new first-line regimen of nivolumab plus chemotherapy. Angiogenesis and immune cells are important components of the tumor microenvironment (TME). The interaction of protumoral immune cells and tumor vasculature within the TME results in a vicious cycle that leads to increased inflammation and further damage to the tumor vessels, resulting in tumor progression and metastasis (7-9).
Herein, we analyzed the efficacy and safety of second-line ramucirumab plus paclitaxel in AGC patients refractory to nivolumab plus chemotherapy. We present this article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-598/rc).
Methods
We analyzed 20 AGC patients who received ramucirumab and paclitaxel as second-line therapy after failure of nivolumab, fluoropyrimidines, and platinum between December 2021 and September 2022 at Samsung Medical Center. The inclusion criteria for the study were as follows: (I) adult patients (18 years of age or older) with a pathologically confirmed diagnosis of adenocarcinoma in the stomach; (II) treated with nivolumab plus chemotherapy as the first-line treatment; and (III) patients refractory to nivolumab plus chemotherapy. This retrospective study was conducted according to the provisions of the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (No. 2022-12-064). Due to the retrospective nature of this study, the IRB waived the requirement for informed consent from the patients.
The following clinicopathologic characteristics were collected retrospectively from patient medical records. Clinicopathologic parameters included age, sex, European Cooperative Oncology Group performance status (ECOG PS), histology, microsatellite instability status, tumor differentiation, history of gastrectomy, stage, primary site, metastasis site, prior chemotherapy, survival and baseline laboratory findings.
Clinical outcomes were evaluated for objective response rate (ORR), disease control rate (DCR), PFS, OS, and safety. The ORR and DCR were assessed by investigators, according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 based on computed tomography (CT) or magnetic resonance imaging (MRI). The ORR is defined as the percentage of patients who achieve a response, which can either be complete response (CR; complete disappearance of lesions) or partial response (PR; reduction in the sum of maximal tumor diameters by at least 30% or more). The DCR is defined as the percentage of patients who have achieved CR, PR, and stable disease (SD). Treatment-related adverse events (TRAEs) were assessed and graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. PFS was defined as the time from the start of ramucirumab plus paclitaxel until the date of disease progression or death from any cause. Patients who remained alive at the analysis cutoff date were censored at that time. OS was defined as the time from the start of ramucirumab plus paclitaxel until death from any cause, with patients alive at the time of the last data cutoff and at the time of the last follow-up being censored. Responders were defined as patients whose CR or PR was confirmed in response evaluation performed during first-line nivolumab plus chemotherapy treatment, and non-responders were defined as patients whose best ORR was SD or progressive disease (PD).
Statistical analysis
Survival analysis between the two subgroups was analyzed using the Kaplan-Meier method, and the HR between the two subgroups was analyzed using Cox-proportional hazard models. All P values were two-sided, and statistical significance was set at P<0.05. Statistical analysis was performed using IBM SPSS Statistics 25 (Armonk, NY, USA).
Results
Patient characteristics
A total of 20 eligible patients with AGC who had progressed after first-line chemotherapy with nivolumab plus chemotherapy was treated with ramucirumab plus paclitaxel under routine clinical practice between December 2021 and September 2022. The median age was 56 (range, 41–76) years, and 65.0% of the patients were male. All patients had an ECOG PS of 1 at the date of second-line chemotherapy. Fifteen (75.0%) patients were stage IV AGC when first diagnosed, and 5 (25.0%) patients were presenting with recurrent disease. Fifteen (75.0%) patients received nivolumab plus capecitabine plus oxaliplatin (XELOX) as the first-line chemotherapy, while five (25.0%) patients received nivolumab plus 5-fluorouracil plus oxaliplatin (FOLFOX) as the first-line chemotherapy (Table 1).
Table 1
| Characteristics | Value (n=20) |
|---|---|
| Age (years) | 56 [41–76] |
| Sex | |
| Male | 13 (65.0) |
| Female | 7 (35.0) |
| ECOG PS at second-line start | |
| 1 | 20 (100.0) |
| Primary tumor site | |
| Gastric cancer | 20 (100.0) |
| Disease status | |
| Advanced | 15 (75.0) |
| Recurrent | 5 (25.0) |
| Histological type | |
| Tubular adenocarcinoma | |
| Moderate | 3 (15.0) |
| Poorly | 8 (40.0) |
| Poorly cohesive carcinoma | 9 (45.0) |
| Microsatellite instability status | |
| Microsatellite stable | 20 (100.0) |
| PD-L1 CPS | |
| <1 | 7 (35.0) |
| ≥1 | 8 (40.0) |
| N.E. | 5 (25.0) |
| Number of organs with metastasis | |
| 1 | 4 (20.0) |
| 2 | 9 (45.0) |
| 3 | 4 (20.0) |
| 4 | 3 (15.0) |
| Sites of metastases | |
| Lymph nodes | 15 (75.0) |
| Peritoneum | 13 (65.0) |
| Lung | 3 (15.0) |
| Liver | 3 (15.0) |
| Ovary | 3 (15.0) |
| Previous chemotherapy regimen | |
| Nivolumab + XELOX | 15 (75.0) |
| Nivolumab + FOLFOX | 5 (25.0) |
Data are presented as median [range] or n (%). ECOG PS, European Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; CPS, combined positive score; N.E., not evaluated; XELOX, capecitabine plus oxaliplatin; FOLFOX, 5-fluorouracil plus oxaliplatin.
Treatment outcomes
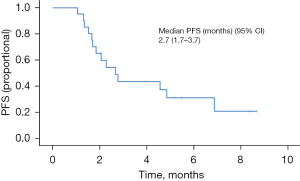
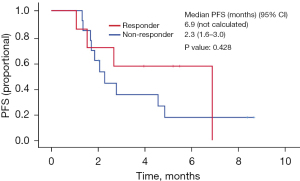
Twenty patients were evaluated for response to second-line ramucirumab plus paclitaxel. Three patients who expired within 1 month were not included in the analysis. A PR was achieved in 2 patients (10.0%), and SD was observed in 9 patients (45.0%). The ORR was 10.0%, and the DCR was 55.0% (Table 2). Over the median follow-up duration of 5.0 months (95% CI: 2.8–7.2), the median overall PFS was 2.7 months (95% CI: 1.7–3.7); 6.9 months (95% CI: not calculated) in responders (n=7) to the first nivolumab-containing chemotherapy and 2.3 months (95% CI: 1.6–3.0) in non-responders (n=13) (P=0.428) (Figures 1,2). At the time of data cutoff, six patients continued to receive ramucirumab plus paclitaxel. The median OS was 6.3 months (95% CI: 5.5–8.3); 6.9 months (95% CI: not calculated) in responders (n=7) to the first nivolumab-containing chemotherapy and 6.3 months (95% CI: 3.7–8.9) in non-responders (n=13) (P=0.401) (Figures 3,4).
Table 2
| Parameters | Total (n=20) | Responder (n=7) | Non-responder (n=13) |
|---|---|---|---|
| Best overall response | |||
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PR | 2 (10.0) | 2 (28.6) | 0 (0.0) |
| SD | 9 (45.0) | 3 (42.9) | 6 (46.2) |
| PD | 9 (45.0) | 2 (28.6) | 7 (53.8) |
| ORR | 2 (10.0) | 2 (28.6) | 0 (0.0) |
| DCR | 11 (55.0) | 5 (71.4) | 6 (46.2) |
Data are presented as n (%). CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.


Toxicity
TRAEs are summarized in Table 3. Anemia was the most common treatment-related toxicity. Grade 3 or 4 neutropenia was observed in 9 (45.0%) patients. Sensory neutropenia occurred in 1 patient (5.0%). No side effects related to the anti-angiogenic effect of ramucirumab, such as hypertension, bleeding, or thrombosis, were identified.
Table 3
| TRAEs | Total (n=20) | Grade 1–2 (n=20) | Grade 3–4 (n=20) |
|---|---|---|---|
| Hematological adverse events | |||
| White blood cell count decreased | 10 (50.0) | 8 (40.0) | 2 (10.0) |
| Neutrophil count decreased | 14 (70.0) | 5 (25.0) | 9 (45.0) |
| Anemia | 20 (100.0) | 17 (85.0) | 3 (15.0) |
| Thrombocytopenia | 6 (30.0) | 6 (30.0) | 0 (0.0) |
| Non-hematological adverse events | |||
| Sensory neuropathy | 1 (5.0) | 1 (5.0) | 0 (0.0) |
| Nausea | 1 (5.0) | 1 (5.0) | 0 (0.0) |
Data are presented as n (%). TRAE, treatment-related adverse event.
Discussion
This study examined the clinical outcomes of ramucirumab plus paclitaxel as a second-line treatment in AGC patients who had failed prior nivolumab-based combination chemotherapy. The results revealed an ORR of 10.0% and a DCR of 55.0%. The median PFS was 2.7 months (95% CI: 1.7–3.7), and the median OS was 6.3 months (95% CI: 5.5–8.3). Compared to the response and survival data from the RAINBOW trial, ramucirumab plus paclitaxel as a second-line treatment in the present study showed relatively worse outcomes. This raises the question of whether ramucirumab plus paclitaxel as a second-line regimen after first-line nivolumab plus chemotherapy is the best treatment for AGC patients (5).
The RAINBOW trial reported that the median PFS was 4.4 months (95% CI: 4.2–5.3) and the median OS was 9.6 months (95% CI: 8.5–10.8) for all enrolled patients. The present study showed relatively shorter survival outcomes compared to those in the RAINBOW trial. In the RAINBOW-Asia trial, the median PFS was 4.14 months (95% CI: 3.7–4.3) and the median OS was 8.71 months (95% CI: 7.98–9.49) in the ramucirumab plus paclitaxel group (10). However, patients achieving tumor response to nivolumab plus chemotherapy as the first-line had comparable survival outcomes to ramucirumab plus paclitaxel compared to those in the RAINBOW and RAINBOW-Asia trials (5,10). These findings suggest the possibility of selectively applying ramucirumab and paclitaxel to patients with AGC as second-line therapy, depending on tumor response before combining nivolumab and chemotherapy. However, with the small number of single-center studies, additional prospective studies are considered necessary.
Immunotherapy mainly targets PD-L1 or programmed cell death 1 (PD-1), as they regulate the immune activity of tumors. PD-L1 expression is regulated by various pathways, and tumors with low PD-L1 expression generally have less T cell infiltration than tumors with high PD-L1 expression (11,12). Ramucirumab, an anti-VEGFR mAb, inhibits angiogenesis and reduces tumor activity. It also causes hypoxia to increase the activity of effector T cells (13-15).
Immune cells have been found to play a role in tumor angiogenesis through both direct and indirect pathways, and immunotherapy has been shown to improve vascular function by influencing not only immune cells, but also non-immune stromal cells. This normalization of the vasculature allows increased tumor entry of immune cells, enhancing the effectiveness of immunotherapy (9,16,17). As a result, there is a current trend of combining anti-angiogenic agents with immunotherapy (18-21). The immune checkpoint inhibitor and the anti-angiogenic agents are important stromal agents. These stromal agents made changes in TME. Theologically, the effect of nivolumab plus cytotoxic chemotherapy influenced on the TME including immune cells and tumor vasculature. Also, the refractoriness to nivolumab and cytotoxic chemotherapy might make changes to the TME. This change at the time of refractoriness to nivolumab plus chemotherapy might affect the antitumor effect of ramucirumab and paclitaxel. Further studies for the TME after the immune checkpoint inhibitor and/or the anti-angiogenic agents are needed.
Sasaki et al. reported that anti-PD-1 therapy exposure in AGC enhanced the efficacy of ramucirumab plus paclitaxel. ORR of ramucirumab plus paclitaxel was 60.6% in the anti-PD-1 exposed group and 20.0% in the anti-PD-1 naïve group (P<0.001) (22). The study showed an unfavorable ORR compared to the present study and the RAINBOW study, however, the median PFS in this study was also 3.7 months in the anti-PD-1 exposed group and 3.3 months in the anti-PD-1 naive group. However, it is still unclear how Anti-PD-1 Therapy affects the subsequent chemotherapy in AGC patients (22). Further research for this area is needed.
There are a few limitations to this study. It was a retrospective study with a clinically diverse group which may have been biased. Second, the study included only a few patients, making it challenging to reach definitive conclusions on genomic biomarkers. Third, the study only included Asian patients with metastatic AGC, limiting the generalizability due to the different molecular profiles and clinical characteristics between Western and Eastern patients with AGC. Therefore, our findings must be interpreted with caution. Nevertheless, this analysis suggested that ramucirumab plus paclitaxel as second-line therapy might be further studied in AGC patients after failure of nivolumab plus chemotherapy.
Conclusions
Ramucirumab plus paclitaxel as second-line therapy might be selectively applied to AGC patients according to tumor response to prior nivolumab plus chemotherapy. In the future, a new innovative study for second-line therapy is needed in AGC patients after nivolumab plus chemotherapy.
Acknowledgments
Funding: This study was supported by a grant of
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-598/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-598/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-598/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-598/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted according to the provisions of the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. The study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (No. 2022-12-064) on December 27, 2022. The IRB waived the requirement for informed consent from the patients due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol 2022;28:1187-203. [Crossref] [PubMed]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904-12. [Crossref] [PubMed]
- Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 2020;18:59. [Crossref] [PubMed]
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475-85. [Crossref] [PubMed]
- Xu RH, Zhang Y, Pan H, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2021;6:1015-24. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Schoemig-Markiefka B, Eschbach J, Scheel AH, et al. Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 2021;24:1115-22. [Crossref] [PubMed]
- de Almeida PE, Mak J, Hernandez G, et al. Anti-VEGF Treatment Enhances CD8(+) T-cell Antitumor Activity by Amplifying Hypoxia. Cancer Immunol Res 2020;8:806-18. [Crossref] [PubMed]
- Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol 2020;11:598877. [Crossref] [PubMed]
- Yi M, Niu M, Xu L, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol 2021;14:10. [Crossref] [PubMed]
- Benavente S, Sánchez-García A, Naches S, et al. Therapy-Induced Modulation of the Tumor Microenvironment: New Opportunities for Cancer Therapies. Front Oncol 2020;10:582884. [Crossref] [PubMed]
- Petitprez F, Meylan M, de Reyniès A, et al. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front Immunol 2020;11:784. [Crossref] [PubMed]
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. [Crossref] [PubMed]
- Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol 2021;22:946-58. [Crossref] [PubMed]
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563-73. [Crossref] [PubMed]
- Socinski MA, Nishio M, Jotte RM, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 2021;16:1909-24. [Crossref] [PubMed]
- Sasaki A, Kawazoe A, Eto T, et al. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open 2020;4:e000775. [Crossref] [PubMed]


