
The prognostic value of the number of metastatic lymph nodes on the long-term survival of intrahepatic cholangiocarcinoma using the SEER database
Highlight box
Key findings
• The number of metastatic lymph nodes (LNs) appears to be a prognostic factor of intrahepatic cholangiocarcinoma (ICC) in the Surveillance, Epidemiology, and End Results (SEER) database.
What is known and what is new?
• American Joint Committee on Cancer 8th staging system suggested N stage as N0 and N1 in ICC, however, previous studies have reported that the number of metastatic LNs is associated with a long-term prognosis in ICC patients.
• This study reconfirmed the prognostic impact of the number of metastatic LNs on survival in the SEER database.
• It was difficult to accurately determine the prognosis of patients with less than six LN resection even by examining various values about LN status.
What is the implication, and what should change now?
• A revised N staging system that accounts for the quantity of metastatic LNs is needed for more accurate prognosis prediction in ICC patients.
• For accurate staging of ICC, six or more LNs should be harvested during surgery.
Introduction
Cholangiocarcinoma (CC) is a malignant tumor that occurs at various locations in the biliary tree and is classified according to the anatomic location of the occurrence. Intrahepatic cholangiocarcinoma (ICC) is a CC that occurs at the proximal level of the second-order bile duct (1,2). Globally, CC exhibits an average annual incidence of two cases per 100,000 individuals (3). However, marked regional disparities exist, particularly in certain Asian nations like Thailand, where the reported rates surpass the global average by over 40-fold, reaching an annual incidence of 96 cases per 100,000 people (3). Within the spectrum of CC, extrahepatic CC has displayed a relatively stable incidence pattern in recent decades (4,5). In contrast, ICC is experiencing a consistent and progressive increase worldwide, with notable rises observed in Europe, North America, Asia, Japan, and Australia over the past two decades (4).
Complete tumor resection is the only curative treatment option for CC (5-7). Nevertheless, the 5-year overall survival rate following surgery ranges from a modest 15% to 40%, with recurrence observed in approximately two-thirds of patients (8). Therefore, selecting the appropriate surgical treatment and predicting the prognosis of patients after surgery are crucial.
Since the introduction of the 7th edition of the American Joint Committee on Cancer (AJCC) staging system, significant endeavors have been undertaken to differentiate ICC from other intrahepatic tumors, aiming to refine prognosis prediction (9). Precise prognosis prediction plays a pivotal role in determining the necessity for supplementary adjuvant therapies, such as chemotherapy or radiotherapy. Furthermore, lymph node (LN) metastasis in ICC stands as a prominent prognostic determinant (10-14).
In the 8th edition of AJCC, although ICC is recommended to sample more than six LNs during the surgery, objective evidence is insufficient (9,15-17). Since it is a relatively rare disease in the field of hepato-biliary-pancreatic surgery, almost all ICC studies are single-institutional. The discussion of appropriate surgical treatment is ongoing, and especially the necessity of lymphadenectomy is still under discussion. In addition, debates on the appropriate number of LN retrieval and the appropriate extent of LN resection have not yet been concluded. Our institution also previously published a couple of studies on the appropriate number of LN sampling and the appropriate extent of LN dissection to reduce recurrence (11,12,18,19).
Moreover, nodal staging of ICC in the AJCC 8th guidelines is classified only into N0 without LN metastasis and N1 with LN metastases, unlike perihilar CC or extrahepatic CC (10,15,20-22). For the other biliary tract cancers, N2 is classified when four and more metastatic LNs are retrieved according to the AJCC 8th edition. There is still room for an improvement in the prognosis according to the metastatic state of the LNs in ICC. Several studies have reported that the number of metastatic LNs is associated with a long-term prognosis in ICC patients (10,12,23,24). These studies have been mainly published in East Asian countries. Zhang et al. reported a multicenter study involving hospitals in Western countries, demonstrating that the number of metastatic LNs does impact the prognosis (24). They also performed external validation using the Surveillance, Epidemiology, and End Results (SEER) program to support these results (24).
Since the SEER program collects cancer incidence data from population-based cancer registries and encompasses a diverse range of ethnicities, there is significance in fully utilizing this population database to reconfirm the prognostic impact of the number of metastatic LNs on survival. This study focused on efforts to reflect the details of treatment and tumor staging as much as possible. In addition, as recommended by the 8th edition of the AJCC, we decided to check how different long-term survival depends on the number of LN metastases when at least six LNs are retrieved. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-580/rc).
Methods
Subjects’ identification and inclusion criteria
This study was conducted with a database collected from the 18 registries from 2000 to 2018 among several databases registered in the SEER program. In the SEER database, the diagnostic name is coded according to the International Classification of Oncology Measures (3rd Edition, ICD-O-3) (25). To collect information on ICC patients, patients corresponding to ‘liver (22.0)’ in the topography code and ‘cholangiocarcinoma (8160)’ in the histology code were collected. Furthermore, patients with the topography code of ‘intrahepatic bile duct (22.1)’ and the histology code of ‘malignant neoplasm (8000)’, ‘malignant tumor cells (8001)’, ‘carcinoma (8010)’, ‘undifferentiated carcinoma (8020)’, and ‘cholangiocarcinoma (8160)’ were collected. A total of 11,744 patients diagnosed with ICC were included through this search result.
Among them, patients diagnosed after the age of 100 were excluded. Patients who underwent surgery with curative aim were included in the inclusion criteria using the ‘surgery of Primary Site Code’ and patients who received liver transplantation or only excision of bile duct were excluded. A total of 1,904 patients were included. Patients with accurate records of the number of LNs recovered after surgery and the number of metastatic LNs were selected using the ‘regional node examined’ and ‘regional node positive’ codes (n=1,112). In addition, patients with distant metastasis and stage IV according to AJCC 8th edition staging were excluded. A total of 1,025 patients were included. Finally, 658 patients were included in the inclusion criteria, excluding 367 patients who could not be classified as T-staging due to lack of information such as tumor size and degree of invasion of blood vessels. The flowchart of inclusion criteria was shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).

Identification of general characteristics and procedure
The SEER database collects information such as the gender of the patients, the age at the time of diagnosis, race, and year of diagnosis. In addition, the SEER database collects information about the surgery that patients have obtained with ‘surgery of Primary Site Code’. Using this code, the SEER database codes patients who did not undergo surgery, patients who received only local therapy, such as ablation, and patients who underwent surgery such as wedge resection, segmentectomy, hemihepatectomy, extended hemihepatectomy, excision of the bile duct (for an intra-hepatic bile duct primary only) with or without partial hepatectomy, and liver transplantation. In this study, the types of surgery were classified into wedge resection or segmentectomy, hemihepatectomy (right or left), extended hemihepatectomy, which is defined as the resection of a single lobe along with a segment of another lobe, and excision of the bile duct with partial hepatectomy. The information on chemotherapy and radiotherapy was also collected from the SEER database. The information such as CA19-9 level or tumor growth pattern for bile duct infiltration, which was known to be associated with prognosis, was collected under the code ‘CS site specific factor’ through the ‘Collaborative stage date set’, but the information was not collected adequately and excluded from this study.
Assessment of LN metastasis and LN dissection rate
The SEER database has collected the status of LN metastasis since 1988, and the LN was examined by a pathologist and the number of LNs recovered was coded as ‘Regional Nodes examined’. In addition, the SEER database used the ‘regional nodes positive’ code to record LN metastasis. LN positive is defined as the presence of a tumor measuring 0.2 mm or larger, confirmed through pathological examination in the SEER database.
During the study period, the AJCC staging classification was revised three times from the 6th edition to the 8th edition in 2004, 2010, and 2017. The AJCC staging of the patients was recorded according to the edition at the time of each diagnosis, so the ‘CS tumor size’ code and the ‘CS Extension’ code were referred to change it to AJCC 8th edition. Cases with insufficient information corresponded to the exclusion criteria and were excluded. Basis on these definitions, the rate of proper LN dissection accordance with AJCC (at least 6 LNs) was calculated by period.
Assessment of the impact of metastatic LN number and its survival
Patients diagnosed with ICC from 2000 to 2018 were followed, and the study cut-off date was December 2018. In the SEER data, there is an algorithm that collects information on the cause of death and obtains cause-specific survival (CSS) by excluding deaths from other causes than the disease.
The hazard ratio (HR) according to the increasing number of metastatic LNs was calculated. In addition, the enrolled patients were divided into three groups based on the number of metastatic LNs [N0: no metastasis; N1 (N+ <4): 1 to 3 metastatic LNs; N1 (N+ ≥4): 4 or more metastatic LNs], using the same nodal staging as other biliary tract cancers, such as perihilar CC or extrahepatic CC, consistent with the findings of a previous study conducted by our institution (12). CSS among the three groups was compared. Moreover, in the subgroup analysis, these groups were subdivided into two groups based on the harvested LN number (<6 vs. ≥6), and their CSS was also compared.
Statistics analysis
Statistical analysis was performed using SPSS Version 24 (IBM, Chicago, IL, USA) and R.3.6.3. Continuous variations are represented by median and interquartile range (IQR) and nominal variables in numbers and percentage. Univariate and multivariate survival analysis was conducted using the Cox proportional hazard model. Survival analysis according to the subgroup was conducted through the Kaplan-Meier method and log-rank test. In the subgroup survival analysis, CSS was compared with median and IQR in cases when death (event) occurred in more than 50% of patients, and medium and IQR in cases of less than 50%. In all tests, when the P value <0.05, it was set to be statistically significant. Akaike information criterion (AIC) and Harrell concordance index (C-index) were calculated by Stata software, version 12.0 (StataCorp, College Station, TX, USA).
Results
General characteristics of patients with ICC in SEER data
In this study, a total of 658 patients correspond to the inclusion criteria, and the general characteristics of the patients are shown in Table 1. The age of 60–69 years was the age of peak in ICC patients. The median number of harvested LNs was 3.0 [IQR, 1.0–5.0], and six or more LNs were retrieved in 159 (24.2%) patients. LN metastasis was not reported in 472 (71.7%) patients. One hundred and fifty-seven patients (23.9%) reported more than one and three or less LN metastases, and 29 patients (4.4%) reported more than four LN metastases. The median CSS was 36.000 [IQR, 30.755–41.245] months.
Table 1
| Variables | Values |
|---|---|
| Sex (male:female) | 295:363 (1:1.2) |
| Age (years), n (%) | |
| <40 | 28 (4.3) |
| 40–49 | 77 (11.7) |
| 50–59 | 159 (24.2) |
| 60–69 | 227 (34.5) |
| 70–79 | 142 (21.6) |
| >80 | 25 (3.8) |
| Race and origin, n (%) | |
| White | 446 (67.8) |
| Black | 47 (7.1) |
| Asian or Pacific Islander | 77 (11.7) |
| Hispanic all races | 82 (12.5) |
| American Indian/Alaska Native | 6 (0.9) |
| Types of surgery, n (%) | |
| Wedge resection or segmentectomy | 179 (27.2) |
| Hemihepatectomy | 261 (39.7) |
| Extended hemihepatectomy | 134 (20.4) |
| Excision of bile duct with partial hepatectomy | 84 (12.8) |
| Number of harvested LNs, median [interquartile range] |
3.0 [1.0–5.0] |
| Radiotherapy, n (%) | |
| Yes | 113 (17.2) |
| No/unknown | 545 (82.8) |
| Chemotherapy, n (%) | |
| Yes | 322 (48.9) |
| No/unknown | 336 (51.1) |
| LN sampling, n (%) | |
| <6 | 499 (75.8) |
| ≥6 | 159 (24.2) |
| Number of metastatic LNs, n (%) | |
| None | 472 (71.7) |
| 1–3 | 157 (23.9) |
| ≥4 | 29 (4.4) |
| T stage, n (%) | |
| T1a | 129 (19.6) |
| T1b | 118 (17.9) |
| T2 | 321 (48.8) |
| T3 | 19 (2.9) |
| T4 | 71 (10.8) |
| N stage, n (%) | |
| N0 | 472 (71.7) |
| N1 | 186 (28.3) |
| AJCC 8th stage, n (%) | |
| Ia | 110 (16.7) |
| Ib | 97 (14.7) |
| II | 213 (32.4) |
| IIIa | 9 (1.4) |
| IIIb | 229 (34.8) |
| Follow-up duration (months), median [interquartile range] |
36.000 [30.755–41.245] |
LN, lymph node; AJCC, American Joint Committee on Cancer.
Trend of LN dissection rate according to periods
The proportion of ICC patients with more than six LNs retrieved increased slightly over the years, but there was no statistical difference (P=0.120) (Figure 2).
Analysis of risk factors for survival in patients with ICC
The HR of each variable with CSS was investigated in the enrolled patients. In the univariate analysis, the number of LNs with metastasis increased by one and the HR was 1.300 [95% confidence interval (CI): 1.225–1.379], (P<0.001). In the multivariate analysis, the number of metastatic LNs was also a significant prognostic factor with T stage (metastatic LN: HR =1.245, 95% CI: 1.169–1.326, P<0.001; T stage: T1 vs. T2: HR =1.772, 95% CI: 1.378–2.278, P<0.001; T1 vs. T3: HR =2.220, 95% CI: 1.259–3.915, P=0.006; T1 vs. T4: HR =2.240, 95% CI: 1.525–3.291, P<0.001) (Table 2).
Table 2
| Variables | Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Male | 1.094 | 0.883–1.354 | 0.412 | ||||||
| Race and origin | |||||||||
| Hispanic | Reference | – | – | – | |||||
| Asian and Pacific islander | 1.221 | 0.785–1.898 | 0.376 | – | – | – | |||
| Black | 1.455 | 0.897–2.359 | 0.128 | – | – | – | |||
| White | 0.979 | 0.695–1.380 | 0.904 | – | – | – | |||
| American Indian/Alaska Native | 0.709 | 0.219–2.298 | 0.567 | – | – | – | |||
| LN sampling | |||||||||
| <6 | Reference | – | – | – | |||||
| ≥6 | 1.108 | 0.864–1.423 | 0.418 | – | – | – | |||
| T stage | |||||||||
| T1 | Reference | Reference | |||||||
| T2 | 1.902 | 1.483–2.440 | <0.001 | 1.772 | 1.378–2.278 | <0.001 | |||
| T3 | 2.616 | 1.489–4.597 | 0.001 | 2.220 | 1.259–3.915 | 0.006 | |||
| T4 | 2.952 | 2.045–4.260 | <0.001 | 2.240 | 1.525–3.291 | <0.001 | |||
| Metastatic LN (increment 1) | 1.300 | 1.225–1.379 | <0.001 | 1.245 | 1.169–1.326 | <0.001 | |||
HR, hazard ratio; CI, confidence interval; LN, lymph node.
Survival analysis of ICC patients according to number of LN metastasis
In the CSS analysis, the mean survival in the N0 group was 40.856 (95% CI: 38.806–42.919) months, the median survival in the N+ <4 group was 22.000 (95% CI: 18.283–25.717) months, and the median survival in the N+ ≥4 group was 15.000 (95% CI: 11.520–18.480) months and showed statistical difference (P<0.001). In addition, each subgroup also showed statistical differences (N0 vs. N+ <4, P<0.001; N+ <4 vs. N+ ≥4, P=0.004) (Figure 3A).
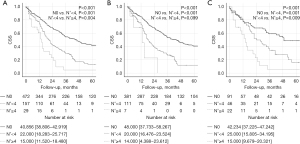
For the subgroup analysis, the survival analysis was performed to find out how the prognosis differs depending on the number of metastatic LNs in patients with more than six LNs retrieved and patients with less than six. For patients with less than six LNs retrieval, there was significant difference between the groups [N0 vs. N+ <4 vs. N+ ≥4: 48.000 (95% CI: 37.733–58.267) vs. 20.000 (95% CI: 16.476–23.524) vs. 14.000 (95% CI: 4.388–23.612) months, P<0.001]. In post hoc analysis, however, N+ <4 and N+ ≥4 group did not show statistically significant difference (P=0.099) (Figure 3B). For patients with six or more LNs retrieval, there was significant difference between the groups including post hoc analysis [N0 vs. N+ <4 vs. N+ ≥4: 42.234 (95% CI: 37.225–47.242) vs. 25.000 (95% CI: 15.805–34.195) vs. 15.000 (95% CI: 9.679–20.321) months, P<0.001] (Figure 3C).
Discussion
In this study, the association between the number of metastatic LNs and the prognosis of patients after surgery was investigated by the SEER database. Although the rate of retrieving six or more LNs was relatively low (24.2%), patients in the SEER database were also confirmed to have a worse prognosis depending on the number of metastatic LNs. Additionally, there was a statistically significant difference in prognosis when the LN status was classified according to the number of LN metastases of N0, N+ <4, and N+ ≥4, such as perihilar CC and distal CC. In addition, the HR according to the number of metastatic LNs increased in this study.
Discussion of the significance of lymphadenectomy in ICC patients is still ongoing (11,26-30). Several studies have been published that regional lymphadenectomy can improve survival after surgery (11,27,28). However, some studies reported that lymphadenectomy could not improve survival but also increase complication rate (30,31). Even though there are many controversies, it is a consensus that LN resection as a staging operation, so that it helps to accurate staging and planning further treatment (8,32,33).
The AJCC staging had been revised to the 8th edition and recommends sampling at least six LNs (15). However, according to Zhang et al., SEER database from 2000 to 2013 showed no significant change in the rate of LN resection in the United States, up to 50% (34). The percentage of patients who had more than six LNs increased slightly but it was still about 14.3% from 2009 to 2013 (34). Within the inclusion criteria of this study, although the rate of patients who had more than six LNs retrieved increased, only about 30% of ICC patients did from 2015 to 2018. The clinical significance of LN dissection in ICC had been suggested by several studies, but it was not until 2015 that it was officially endorsed in an expert consensus statement (33). The rate of appropriate LN dissection might have seen a gradual increase only following the establishment of a consensus on LN dissection in ICC. When including patients with inaccurate LN status who did not underwent LN dissection, it is expected that the actual proportion of patients with less than six LNs retrieved is much lower. On the other hand, a multicenter study from Korea and Japan reported 63.7% of patients underwent LN dissection (10). It was a relatively higher rate than the West.
In some studies, when dividing subgroups according to the number of metastatic LNs, more LN metastasis group had statistically worse prognosis (10,12,23,24). Furthermore, in this study, as the number of metastatic LN increased, it was associated with significantly increased risk for CSS in the multivariable analysis. However, among the patients who were harvested less than six LNs, there was no statistical difference in CSS between N+ <4 and N+ ≥4 group. There were two possible reasons for this. First, within the patients who retrieved less than six LN, N+ ≥4 group were only seven patients. Due to the lack of subjects, the difference between two groups could not be statistically significant. It was also possible that patients who should be included in the N+ ≥4 group were under-staged, as they did not receive sufficient lymphadenectomy. For this reason, the prognostic difference could be more evident in the group with sufficient LNs retrieved.
Since there are many patients who have less than six resected LNs, it was examined whether the prognosis could be analyzed with information on their LN status. In previous studies, some prognostic values about LN status were suggested to predict the prognosis of ICC (35,36). log odds of metastatic lymph nodes (LODDS) and lymph node ratio (LNR) already showed better prognostic performance than the AJCC 7th N stage in a previous study (35). Therefore, we investigated these values and AJCC 8th N stage in patients with less than six LNs retrieved. The cut-off value of LNR and LODDS were calculated by X-tile plot (37). Although dividing the subgroups according to the metastatic LN showed the lowest AIC; 2,902.5, its C-index was 0.58. Moreover, the other variables showed similar C-index that were under 0.6 (Table S1). That was to say, it was difficult to accurately determine the prognosis of patients with less than six LN resection even by examining various values about LN status. Retrieving more than six LNs is important to predict the patient’s prognosis.
In 2011, Clark et al. had already conducted a study on the effectiveness of lymphadenectomy in patients with ICC using the SEER database (38). Since the evaluation of lymphatic nodes was only performed in about 13.5% of all patients, there was a limit to the data to reach conclusions about the efficacy of lymphadenectomy. Zhang et al. also used the SEER database to validate the association between the number of metastatic LN and the prognosis (24). We have reaffirmed the significance of the number of metastatic LNs using the SEER database. Considering LN count as a continuous variable and demonstrating the increment-based HR with population-based data is, to the best of our knowledge, a novel aspect of this study. It might help to develop a new standard for LN staging for ICC. In addition, this study investigated alternative nodal status parameters, such as LNR and LODDS, for patients in whom fewer than six LNs were harvested during ICC surgery. However, statistically significant alternative parameters were not shown. Six or more LNs dissection must still be performed during ICC surgery for accurate staging.
This study has several important limitations. First, although this study used large-sized registry data, the number of patients corresponding to the inclusion criteria was relatively small compared to all ICC patients in the SEER database. Only about 5% of data were used for this study, and selection bias may have occurred in the process of identifying the patients in the inclusion criteria. In addition, subgroup analysis was limited by the small number of patients with less than six LNs retrieval and, above all, by the small number of patients with four or more confirmed LN metastases. Second, several parameters, such as the status of resection margin, histologic grade, and CA19-9, that were related to oncologic outcomes for ICC could not collected due to the limitation of retrospective registry data. In the SEER database, the coding for patients received chemotherapy included ‘None/Unknown’, referring to patients who did not receive treatment and those for whom it was unknown or not recorded. Similarly, for radiation therapy, there were categories of ‘None/Unknown’, ‘Refused’, and ‘Recommended, Unknown if Administered’. These limitations made it challenging to analyze the exact effect of chemotherapy or radiotherapy on survival. In addition, although the status of the resection margin is one of the significant prognostic factors, we were unable to confirm this information in the SEER database. Third, due to the lack of information about recurrence, we could not conduct the analysis about the association between the number of metastatic LNs and disease-free survival. Nonetheless, strength of this study is that it shows the prognostic value of the number of metastatic LNs itself with a long-term population-based database by using western public data that showed comparatively low rate of lymph node dissection.
Conclusions
In conclusion, the number of metastatic LNs appears to be a prognostic factor of ICC in the SEER database. However, for accurate staging, six or more LNs should be harvested during surgery. Further studies for detailed nodal staging of ICC in terms of a cut-off level for the adequate number of harvested LN metastasis needed in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-580/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-580/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-580/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was waived by the local ethics committee, as SEER data is publicly available and de-identified. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29. [Crossref] [PubMed]
- Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:22-34. [Crossref] [PubMed]
- Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77-82. [Crossref] [PubMed]
- Zhang H, Yang T, Wu M, et al. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198-205. [Crossref] [PubMed]
- Halder R, Amaraneni A, Shroff RT. Cholangiocarcinoma: a review of the literature and future directions in therapy. Hepatobiliary Surg Nutr 2022;11:555-66. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. [Crossref] [PubMed]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91. [Crossref] [PubMed]
- Sung YN, Song M, Lee JH, et al. Validation of the 8th Edition of the American Joint Committee on Cancer Staging System for Gallbladder Cancer and Implications for the Follow-up of Patients without Node Dissection. Cancer Res Treat 2020;52:455-68.
- Kang CM, Suh KS, Yi NJ, et al. Should Lymph Nodes Be Retrieved in Patients with Intrahepatic Cholangiocarcinoma? A Collaborative Korea-Japan Study. Cancers (Basel) 2021;13:445. [Crossref] [PubMed]
- Kim SH, Han DH, Choi GH, et al. Oncologic Impact of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma: a Propensity Score-Matched Study. J Gastrointest Surg 2019;23:538-44. [Crossref] [PubMed]
- Kim SH, Han DH, Choi GH, et al. Prognostic impact of the metastatic lymph node number in intrahepatic cholangiocarcinoma. Surgery 2022;172:177-83. [Crossref] [PubMed]
- Ma WH, Lei ZQ, Yu QS, et al. A novel nomogram for individualized preoperative prediction of lymph node metastasis in patients with intrahepatic cholangiocarcinoma. Zhonghua Wai Ke Za Zhi 2022;60:363-71. [PubMed]
- Lin Y, Chong H, Song G, et al. The influence of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography on the N- and M-staging and subsequent clinical management of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2022;11:684-95. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018;7:52.
- Sposito C, Ratti F, Cucchetti A, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol 2023;78:356-63. [Crossref] [PubMed]
- Kim SH, Han DH, Choi GH, et al. Recommended Minimal Number of Harvested Lymph Nodes for Intrahepatic Cholangiocarcinoma. J Gastrointest Surg 2021;25:1164-71. [Crossref] [PubMed]
- Kim SH, Han DH, Choi GH, et al. Extent of Lymph Node Dissection for Accurate Staging in Intrahepatic Cholangiocarcinoma. J Gastrointest Surg 2022;26:70-6. [Crossref] [PubMed]
- Gaspersz MP, Buettner S, van Vugt JLA, et al. Evaluation of the New American Joint Committee on Cancer Staging Manual 8th Edition for Perihilar Cholangiocarcinoma. J Gastrointest Surg 2020;24:1612-8.
- Jun SY, Sung YN, Lee JH, et al. Validation of the Eighth American Joint Committee on Cancer Staging System for Distal Bile Duct Carcinoma. Cancer Res Treat 2019;51:98-111. [Crossref] [PubMed]
- Kambakamba P, Linecker M, Slankamenac K, et al. Lymph node dissection in resectable perihilar cholangiocarcinoma: a systematic review. Am J Surg 2015;210:694-701. [Crossref] [PubMed]
- Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728-33. [Crossref] [PubMed]
- Zhang XF, Xue F, Dong DH, et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann Surg 2021;274:e1187-95. [Crossref] [PubMed]
- Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. 3rd edition. Geneva: World Health Organization; 2000.
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013;17:1917-28. [Crossref] [PubMed]
- Umeda Y, Mitsuhashi T, Kojima T, et al. Impact of lymph node dissection on clinical outcomes of intrahepatic cholangiocarcinoma: Inverse probability of treatment weighting with survival analysis. J Hepatobiliary Pancreat Sci 2022;29:217-29. [Crossref] [PubMed]
- Yang F, Wu C, Bo Z, et al. The clinical value of regional lymphadenectomy for intrahepatic cholangiocarcinoma. Asian J Surg 2022;45:376-80. [Crossref] [PubMed]
- Yoh T, Cauchy F, Le Roy B, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery 2019;166:975-82. [Crossref] [PubMed]
- Zhou R, Lu D, Li W, et al. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB (Oxford) 2019;21:784-92. [Crossref] [PubMed]
- Li DY, Zhang HB, Yang N, et al. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol 2013;19:9084-91. [Crossref] [PubMed]
- Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery 2009;145:411-6. [Crossref] [PubMed]
- Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg 2018;22:668-75. [Crossref] [PubMed]
- Kim Y, Spolverato G, Amini N, et al. Surgical Management of Intrahepatic Cholangiocarcinoma: Defining an Optimal Prognostic Lymph Node Stratification Schema. Ann Surg Oncol 2015;22:2772-8. [Crossref] [PubMed]
- Tamandl D, Kaczirek K, Gruenberger B, et al. Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg 2009;96:919-25. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, et al. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB (Oxford) 2011;13:612-20. [Crossref] [PubMed]



