
Oncological role of surgical resection in patients with pancreatic ductal adenocarcinoma with liver-only synchronous metastases in a single-center retrospective study
Highlight box
Key findings
• Median survival time from initial treatment was 9.9 months in the chemotherapy group, 10.4 months in the UpS/short NAC group (upfront surgery with or without short-term neoadjuvant chemotherapy), and 36.7 months in the conversion surgery group (conversion surgery vs. UpS/short NAC or chemotherapy, P<0.005). Only conversion surgery was a significant independent prognostic factor in a total cohort (hazard ratio; 0.173, P=0.002).
What is known and what is new?
• Systemic chemotherapy is the standard treatment in pancreatic ductal adenocarcinoma (PDAC) patients with liver metastasis.
• Surgical resection can be an option in the limited PDAC patients with liver metastasis who favorably responded to systemic chemotherapy.
What is the implication, and what should change now?
• Conversion surgery during multimodal treatments can prolong survival in the limited PDAC patients with liver metastasis.
• In addition to anatomical, biological, and conditional response, laparoscopic objective and metabolic complete responses (ABC-LM) criteria may lead to a better selection for a candidate of conversion surgery.
Introduction
Most patients with pancreatic ductal adenocarcinoma (PDAC) have a limited lifespan. Approximately half are initially diagnosed with distant organ metastatic disease, which is associated with poor survival. The most common site of metastasis is the liver, followed by the peritoneum, lungs and pleura, bones and adrenal glands. Although systemic chemotherapy is the standard treatment for patients with metastatic PDAC, median survival time (MST) and progression-free survival are generally limited to 8–12 and 5.5–6.4 months, respectively, even after administration of 5-fluorouracil (5FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) (1) or gemcitabine plus nab-paclitaxel (GnP) (2) in 84–87% patients who had liver metastasis of a total cohort in both studies (1,2). Several studies have revealed that MST after synchronous resection of liver metastases with or without short-term neo-adjuvant therapy ranged from 5.9 to 15.7 months (3-9). Recently, FOLFIRINOX or GnP provided clinical response rates of 30–40%, even in patients with metastatic PDAC (1,2).
Conversion surgery can be defined as an additional surgery during multimodal treatment in patients with initially unresectable (UR)-PDAC, but not planned surgery following neoadjuvant therapy in patients with resectable (R)/borderline resectable (BR) PDAC (10,11). Some studies have shown that conversion surgery after a favorable response to anti-cancer treatment in patients with metastatic PDAC might provide a potential survival benefit of an MST ranging from 26 to 56 months from initial treatment (12-19). To investigate the oncological role of conversion surgery in patients with liver-only metastases, clinical outcomes were compared among patients who underwent conversion surgery, upfront surgery with or without short-term neoadjuvant chemotherapy (NAC) for oligometastases and occult liver metastases, and chemotherapy only for patients with R/BR disease with occult liver metastasis. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-655/rc).
Methods
Patients
This retrospective analysis was limited to 49 patients with liver-only synchronous metastases which was pathologically diagnosed using staging laparoscopy or open laparotomy between January 2007 and March 2022. The 1,279 patients were diagnosed as PDAC, which contained metastatic PDAC in 561 patients and liver-only synchronous metastasis in 233 patients (Figure 1). Generally, all patients received four-phasic contrast-enhanced multi-detector computed tomography (CT) with a 1.0-mm × 64-detector configuration using the Aquilion CT system (Toshiba Medical Systems, Tochigi, Japan) which were evaluated by an experienced pancreato-biliary surgeon and two blinded consultant radiologists. When distant organ metastasis was suspected on CT imaging, positron emission tomography (PET) scanning and biopsy were performed from locations such as the liver, lung, cervical lymph nodes, and bone when appropriate. When liver metastasis was suspected on CT imaging, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging was additionally performed. Occult metastasis was evaluated and diagnosed surgically during staging laparoscopy or open laparotomy to observe the intraperitoneal cavity in all patients with UR-locally advanced (LA) disease and in the limited patients with R/BR (20,21). When liver metastasis was suspected, intraoperative ultrasonography was additionally performed. Resectability status of primary tumor such as R/BR/UR-LA was categorized in Table 1. Patients were divided into three groups as described below (Figure 1).
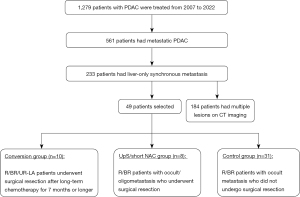
Table 1
| Pre-treatment factor | UpS/short NAC (n=8) | Chemotherapy (n=31) | Conversion surgery (n=10) | P value (CS vs. UpS/short NAC) | P value (CS vs. Chemo) | P value (UpS/short NAC vs. Chemo) |
|---|---|---|---|---|---|---|
| Gender (male:female) | 4:4 | 16:15 | 5:5 | 1.000 | 0.929 | 0.935 |
| Age, years | 65 [52–78] | 72 [51–82] | 64 [46–84] | 0.624 | 0.075 | 0.304 |
| PNI | 52.6 [36.5–57.3] | 48.1 [37.8–59.2] | 47.9 [39.3–63.5] | 0.534 | 0.927 | 0.578 |
| NLR | 2.30 [0.99–5.14] | 2.71 [0.75–13.43] | 2.86 [1.38–6.87] | 0.328 | 0.976 | 0.135 |
| Performance status (0:1) | 7:1 | 26:5 | 10:0 | 0.193 | 0.083 | 0.796 |
| BMI, kg/m2 | 20.9 [16.7–24.5] | 20.7 [15.5–29.9] | 22.2 [19.6–37.1] | 0.131 | 0.078 | 0.835 |
| mGPS (0:1:2) | 6:1:1 | 22:5:4 | 8:2:0 | 0.409 | 0.304 | 0.965 |
| Primary tumor site (Ph:Pbt) | 6:2 | 24:7 | 5:5 | 0.274 | 0.108 | 0.886 |
| Radiological tumor size (mm) | 29 [19–78] | 33 [19–55] | 36 [25–45] | 0.722 | 0.533 | 0.626 |
| Preoperative radiological tumor size, mm | 29 [18–78] | – | 12 [5–30] | 0.002 | – | – |
| Resectability status of primary tumor (R:BR:UR-LA) | 4:4:0 | 23:8:0 | 5:2:3 | 0.094 | 0.010 | 0.199 |
| CA19-9, U/mL | 148 [45–5,091] | 168 [1–15,380] | 569 [2–5,668] | 0.563 | 0.682 | 0.835 |
| Preoperative CA19-9, U/mL | 69 [22–5,091] | – | 15 [1–87] | 0.007 | – | – |
| Number of liver metastases (≤3:4–9:≥10) | 8:0:0 | 17:6:8 | 5:1:4 | 0.025 | 0.621 | 0.016 |
Data are presented as number or median [range]. UpS, upfront surgery; NAC, neoadjuvant chemotherapy; UpS/short NAC, UpS with or without short-term NAC; CS, conversion surgery; Chemo, Chemotherapy; PNI, prognostic nutritional index; NLR, neutrophil-to-lymphocyte ratio; BMI, body mass index; mGPS, modified Glasgow prognostic score; Ph, pancreas head; Pbt, pancreas body and tail; R, resectable; BR, borderline resectable; UR-LA, unresectable locally advanced disease.
Conversion surgery group
This group consisted of ten patients who underwent surgical resection with or without liver resection after anatomically, biologically and conditionally favorable responses to systemic chemotherapy for 7 months or longer from the initial treatment and with liver-only metastases. The criteria for surgical resection (defined as conversion surgery) were as follows: Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; marked primary tumor shrinkage in patients with LA PDAC; decreased (less than 150 U/mL) or normalization of CA19-9 level; and disappearance of liver metastases or oligometastases (3 or less) on imaging studies followed by staging laparoscopy.
UpS/short NAC group
These eight patients with R or BR PDAC who underwent pancreatectomy and concomitant liver resection with or without short-term NAC (less than 3 months) when oligo and occult metastasis was incidentally found on open laparotomy were categorized as the UpS/short NAC group.
Control group
This group contained 31 patients who received chemotherapy only for R or BR PDAC with occult liver metastasis who were diagnosed with staging laparoscopy or open laparotomy. The other 184 patients with liver-only synchronous metastasis showed apparent multiple lesions on CT imaging. Since overall survival (OS) in patients with radiologically-diagnosed liver metastases was reported to be significantly worse than that in patients with occult liver metastases, this cohort was excluded from this study (20).
Data were collected from the prospective pancreatic database of Kansai Medical University Hospital. All patients had pathologically confirmed presence of liver metastasis without microscopic and macroscopic peritoneal dissemination during open laparotomy, such as bypass procedure or staging laparoscopy. Selection bias seems to be present in patients who underwent surgical resection.
OS was compared among the three groups. Recurrence-free survival (RFS) was compared between the conversion surgery and UpS/short NAC groups. Prognostic factors were investigated in all 49 patients. Clinical background characteristics, including pre-operative parameters such as resectability status (22), radiological tumor size, CA19-9 level, modified Glasgow prognostic score (22,23), prognostic nutritional index (24), and objective tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1 (25), were compared among the three groups. Surgical and pathological parameters were compared between the conversion surgery and UpS/short NAC groups.
Statistical analysis
For categorical variables, the Chi-square test or Fisher’s exact test was used to examine differences between groups; for numerical variables and nonparametric independent samples, the Mann-Whitney U test was used. Survival curves were calculated using the Kaplan-Meier method, and differences were compared using the Wilcoxon test. OS was defined as the time from chemotherapy introduction to all-cause of death or last follow-up (1 September 2022). RFS was calculated from the date of surgery to the date of recurrence on imaging or last follow-up. Hazard ratios (HRs) with 95% confidence intervals (CIs) and two-sided P values are reported. HRs in subgroups according to baseline characteristics and two-tailed 95% CIs were calculated using the Cox proportional hazards model. A P value of less than 0.05 was considered statistically significant. There was no missing data. All statistical analyses were performed using JMP Pro version 14.0 (SAS Institute, Cary, NC, USA).
Ethical statement
The study was reviewed and approved by the Institutional Review Board of Kansai Medical University, Japan (No. 2020131). All procedures were performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent for participation in the study was waived due to the retrospective nature of the study, and patients were given opportunities to opt out of the study.
Results
Patients
The median follow-up period was 21.6 (9.5–122.3) months, and only one patient in the UpS/short NAC group, four patients in the conversion surgery group, and five patients in the chemotherapy group were still alive at the time of analysis. Table 1 shows the clinical background characteristics of the patients in each group, and Table 2 reveals the clinical characteristics of patients with liver metastasis who underwent surgical resection. The number of liver metastases was ≤3 in all patients in the UpS/short NAC group and >3 in 5 of the 10 patients in the conversion surgery group and 14 of the 31 patients in the chemotherapy group. Radiological primary tumor diameter on the first presentation was not different between groups. The resectability status of the primary tumor was R (n=5), BR (n=2), and UR-LA (n=3) in the conversion surgery group. The UpS/short NAC and chemotherapy groups did not contain patients with UR-LA. Most patients received modern chemotherapy, such as FOLFIRINOX or GnP (Table 2). Surgical resection was performed in 15 out of 18 patients in the latter phase [2015–2022] of this study.
Table 2
| No. | Group | Gender | Age (years) | Response | Tumor location/resectability | No. of liver metastasis | Pre-op therapy | RECIST | Baseline/pre-op CA19-9 (U/mL) | Changes in CA19-9 | Surgical procedure | Op time (min) | Extent of blood loss (mL) | pT | pN | Evans | Adjuvant chemotherapy | OS from surgery (months) | OS from initial treatment (months) | RFS (months) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Upfront | F | 78 | None | Ph/R | 1 | None | None | 5,091 | NE | PD + PV + Hep | 432 | 1,816 | 3 | 1b | NE | S-1 | 17.8 | 17.8 | 8.7 | Dead |
| 2 | Upfront | F | 70 | None | Pbt/R | 1 | None | None | 45.2 | NE | DP + Hep | 266 | 491 | 3 | 1a | NE | S-1 | 6.6 | 6.6 | 3.7 | Dead |
| 3 | Upfront | F | 52 | None | Ph/BR-A | 2 | None | None | 538.1 | NE | PD + PV + Hep | 309 | 368 | 3 | 1b | NE | GnP | 7.4 | 7.4 | 3.8 | Dead |
| 4 | Short NAC | M | 65 | None | Pbt/BR-PV | 1 | GS | SD | 74/53 | 0.72 | DP + PV + Hep | 241 | 553 | 3 | 1a | I | S-1† | 85.5 | 87.5 | 87.5 | Alive |
| 5 | Short NAC | M | 64 | A+B | Ph/BR-A | 1 | GnP | PR | 1,003/36.2 | 0.04 | PD + PV + Hep | 561 | 2,900 | 3 | 1b | IIA | None | 5.3 | 12.0 | 1.8 | Dead |
| 6 | Short NAC | F | 76 | None | Ph/BR-A | 1 | GnP-S-1 + RT | SD | 60.5/21.7 | 0.36 | PD + PV + Hep | 386 | 642 | 3 | 0 | IIA | None | 3.9 | 8.8 | 3.9 | Dead |
| 7 | Short NAC | M | 61 | None | Ph/R | 1 | GS | SD | 135/89.2 | 0.66 | PD + PV + Hep | 310 | 1,488 | 3 | 1b | IIB | S-1 | 4.9 | 7.2 | 3.0 | Dead |
| 8 | Short NAC | M | 55 | None | Ph/R | 1 | GS | SD | 161/84.4 | 0.53 | PD + Hep | 382 | 577 | 3 | 1b | I | GE | 15.6 | 16.4 | 3.0 | Dead |
| 9 | Conversion | F | 52 | A | Pbt/UR-LA | 1 | GS + RT-GS | PR | 119/86.6 | 0.73 | TP + PV + Hep | 557 | 1,872 | 3 | 0 | I | S-1 | 8.1 | 19.0 | 2.8 | Dead |
| 10 | Conversion | M | 73 | A+B | Ph/BR-A | 6 | GnP | PR | 5,668/30.3 | 0.01 | DP + PV + Hep | 395 | 835 | 3 | 1a | IIA | S-1† | 15.5 | 26.6 | 9.9 | Alive |
| 11 | Conversion | M | 62 | A | Ph/R | ≥10* | GS | PR | <4/<4 | 0.50 | PD +Hep | 483 | 1,286 | 1 | 1a | IIB | S-1† | 111.3 | 122.3 | 122 | Alive |
| 12 | Conversion | M | 68 | A | Pbt/BR-A | 1 | GnP-G | PR | <4/<4 | 0.50 | DP | 296 | 207 | 3 | 1a | III | S-1-GEM† | 74.0 | 84.8 | 16.0 | Dead |
| 13 | Conversion | F | 57 | A+B | Pbt/R | ≥10* | GnP | PR | 571/12.8 | 0.02 | DP + Hep | 228 | 1,132 | 0 | 0 | IV | S-1† | 20.1 | 36.7 | 5.8 | Dead |
| 14 | Conversion | F | 84 | A+B | Pbt/R | ≥10 | GnP | PR | 566/7.5 | 0.01 | DP | 318 | 261 | 3 | 0 | IIB | S-1 | 51.1 | 60.5 | 3.4 | Dead |
| 15 | Conversion | M | 52 | A+B | Ph/UR-LA | 1* | mFFX-GnP | PR | 836/23.4 | 0.03 | PD + PV + Hep | 402 | 1,337 | 1 | 0 | IIA | S-1 | 20.9 | 28.5 | 7.8 | Dead |
| 16 | Conversion | F | 65 | A+B | Ph/UR-LA | 2 | GnP-mFFX | PR | 1,367/43 | 0.03 | PD + PV | 385 | 756 | 3 | 0 | IIA | S-1 | 9.2 | 23.6 | 7.8 | Dead |
| 17 | Conversion | M | 46 | A+B | Pbt/R | 2 | mFFX-PARP | PR | 135/14 | 0.10 | Lap DP | 417 | 362 | 3 | 0 | III | PARP inhibitor† | 8.7 | 16.8 | 16.8 | Alive |
| 18 | Conversion | F | 66 | A+B | Ph/R | ≥10 | mFFX-nal-IRI | PR | 1397/16 | 0.01 | PD | 364 | 620 | 3 | 0 | III | S-1† | 2.8 | 9.5 | 9.5 | Alive |
“Upfront” indicates a patient who underwent upfront surgery without pre-operative chemotherapy; “Short NAC” indicates a patient who received short-term neoadjuvant chemotherapy followed by surgical resection; “Conversion” indicates a patient who underwent surgical resection after a favorable response to chemotherapy of 7 months or longer; “Study period” was divided into “former [2007–2014]” and “latter [2015–2022]”; “Response” consists of anatomical response (A) showing partial response and biological response (B) of CA19-9 level decrease of 50% or more. *, indicates radiological diagnosis of liver metastasis; †, indicates completed administration of adjuvant chemotherapy. TNM classification was defined using the 4th English edition of classification of pancreatic carcinoma by the Japan Pancreas Society (20). No., number; NAC, neoadjuvant chemotherapy; F, Female; M, Male; Ph, pancreas head; R, resectable; Pbt, pancreas body and tail; BR-A, BR-artery attachment; BR-PV, BR-portal vein attachment; BR, borderline resectable; UR-LA, unresectable locally advanced disease; pre-op, pre-operative; GS, gemcitabine + S-1; GnP, gemcitabine + nab-paclitaxel; RT, radiation therapy; G, gemcitabine; mFFX, modified FOLFIRINOX; PARP, poly ADP-ribose polymerase inhibitor; nal-IRI, nanoliposomal irinotecan with fluorouracil/leucovorin; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; PR, partial response; NE, not evaluated; PD, pancreaticoduodenectomy; PV, Portal vein resection; Hep, hepatectomy; DP, distal pancreatectomy; TP, total pancreatectomy; Lap DP, laparoscopic distal pancreatectomy; Op, operative; min, minutes; pT, pathological T stage; pN, pathological N stage; Evans, Evans classification; GE, gemcitabine + erlotinib; GEM, gemcitabine; OS, overall survival; RFS, recurrence-free survival.
Comparisons of clinical response to systemic chemotherapy between the UpS/short NAC and the conversion surgery groups
Median radiological primary tumor diameter in the pre-operative period was reduced from 36 to 12 mm in the conversion surgery group, which was significantly smaller than in the UpS/short NAC or the chemotherapy groups (P<0.05). A partial response [defined by RECIST criteria (25)] was found in all patients in the conversion surgery group and in 1 of 5 patients of the short-NAC group.
Similarly, the CA19-9 level decreased significantly after long-term chemotherapy. The CA19-9 level decreased to within normal limits in 6 of 8 patients with increased CA19-9 before chemotherapy and by 50% or more in 7 of 8 patients in the conversion surgery group. The UpS/short NAC group had only two patients with CA19-9 levels that decreased to within normal limits or by 50% or more. When the clinical response to chemotherapy was evaluated with the anatomical response showing a partial response and the biological response showing a decrease in CA19-9 level by 50% or more, both responses occurred in 1 of 8 patients in the UpS/short NAC group and in 7 of 10 patients in the conversion surgery group (Table 2). Thus, the conversion surgery group had more meaningful anatomical and biological responses to systemic chemotherapy than the UpS/short NAC group. The presence of liver metastasis was post-operatively confirmed in all patients of the UpS/short NAC group and in 1 of 10 patients of the conversion surgery group in the pathological specimens. Post-operative mortality was nil. Pathologically, T3 disease was diagnosed in all patients in the UpS/short NAC group, but T0 (complete response, n=1) or T1 disease was diagnosed in 3 of 10 patients. N0 was found in 1 of 8 patients in the UpS/short NAC group but in 7 of 10 patients in the conversion surgery group (P<0.003). Adjuvant chemotherapy was performed in 6 of 8 patients in the UpS/short NAC group and in all patients in the conversion surgery group.
Survival analysis
The median (range) observation time was 21.6 (9.5–122.3) months. As shown in Figure 2 and Table 2, the MST from the initial treatment was 9.9 months (95% CI: 8.3–10.9) in the chemotherapy group, and 10.4 months (95% CI: 6.6–17.8) in the UpS/short NAC group, and 36.7 months (95% CI: 19.0–84.8) in the conversion surgery group, which revealed a significant difference between the conversion surgery and the UpS/short NAC (P=0.002) or chemotherapy group (P<0.001). There was no significant difference in OS between the UpS/short NAC group and the chemotherapy group (P=0.554).
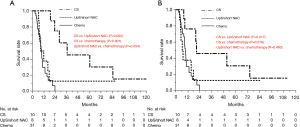
The MST from pancreatectomy was 7.0 months (95% CI: 3.9–17.8) in the UpS/short NAC group and 20.9 months (95% CI: 8.1–74.0) in the conversion surgery group. The MST in the conversion surgery group was significantly longer than that in the UpS/short NAC group (P=0.017) and the chemotherapy group (P=0.018). A significant difference in MST was not found between the UpS/short NAC group and the chemotherapy group (P=0.490). Five-year survival was observed in one patient without recurrence in the UpS/short NAC group, and in three patients in the conversion surgery group (two patients with recurrence and one patient without recurrence).
Figure 3 shows the RFS in the conversion surgery and UpS/short NAC groups. Median RFS was 3.8 months (95% CI: 1.8–8.7) in the UpS/short NAC group and 7.8 months (95% CI: 2.8–16) in the conversion surgery group (P=0.146). A significant difference in MST was not found between the conversion surgery and UpS/short NAC groups (P=0.146). Disease recurrence in the liver within 6 months after surgical resection was observed for six of eight patients in the UpS/short NAC group and 3 of 10 patients in the conversion surgery group. Patients with disease recurrence in the conversion surgery group received chemotherapy for a median (range) of 21 (16 to 57) months.

Univariate and multivariate analyses in the total cohort
As shown in Table 3, univariate analysis revealed that age (HR, 0.495), CA19-9 response (HR, 0.442) and conversion surgery (HR, 0.195) were significantly associated with survival (P<0.05). Multivariate analysis revealed that only conversion surgery was a significant independent prognostic factor for survival (HR, 0.173, P=0.002).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (male:female) | 0.702 (0.366–1.339) | 0.282 | – | – | |
| Age (years) (<70:≥70) | 0.495 (0.256–0.943) | 0.033 | 0.623 (0.317–1.203) | 0.159 | |
| Primary tumor site (Ph:Pbt) | 0.600 (0.286–1.187) | 0.145 | – | – | |
| Radiological tumor size (mm) (<33:≥33) | 0.982 (0.511–1.855) | 0.955 | – | – | |
| NCCN resectability status of primary tumor (R:BR/UR) | 1.318 (0.692–2.594) | 0.408 | – | – | |
| Number of liver metastases (≤3:>3) | 0.881 (0.459–1.760) | 0.713 | – | – | |
| Pre-treatment CA19-9 (U/mL) (<150: ≥150) | 0.965 (0.494–1.843) | 0.915 | – | – | |
| CA19-9 response (<50%:≥50%) | 0.422 (0.157–0.954) | 0.037 | 0.743 (0.249–2.576) | 0.743 | |
| Conversion surgery (−:+) | 0.195 (0.070–0.460) | <0.001 | 0.173 (0.047–0.562) | 0.002 | |
HR, hazard ratio; CI, confidence interval; Ph, pancreas head; Pbt, pancreas body and tail; NCCN, National Comprehensive Cancer Network; R, resectable; BR, borderline resectable; UR, unresectable.
Discussion
In this study, the oncological role of conversion surgery for liver-only synchronous metastases of PDAC was evaluated in comparison with upfront surgery and short-term NAC followed by surgery in patients with R/BR disease who had incidental oligo and occult metastasis found during surgery, and with systemic chemotherapy in patients with R/BR disease with occult liver metastasis diagnosed by staging laparoscopy. The MST from the initial treatment or surgical resection in the conversion surgery group was reached at 36.7 or 20.9 months, respectively, which was significantly better than 10.4 or 7.0 months, respectively in the UpS/short NAC group, or 9.9 months in the chemotherapy group. Three patients in the conversion surgery group and one patient in the UpS/short NAC group were 5-year survivors. No one survived beyond 24 months in the chemotherapy group. Significant differences were not found among the three groups except in the number of liver metastases and in clinical response, such as tumor shrinkage and decreased CA19-9 level. Subsequently, pathological tumor shrinkage and negative lymph node metastasis were more frequently found in the conversion surgery group. Pancreatectomy with partial liver resection was less frequently carried out in the conversion surgery group because of no visible tumor even in intra-operative US examination. The presence of liver metastasis was post-operatively confirmed in all patients of the UpS/short NAC group and in 1 of 10 patients of the conversion surgery group in the pathological specimens. Considering the survival benefit in the conversion surgery group, the presence of evident anatomical and biological responses to systemic chemotherapy, but not the number of liver metastasis, seems to be associated with improved survival in the patients with PDAC with liver-only synchronous metastases of PDAC. Most of patients who underwent surgical resection received adjuvant chemotherapy in this study. Although there has not been clear evidence showing the efficacy of adjuvant chemotherapy, Hank et al. (16) recently reported that adjuvant chemotherapy provided an additional survival advantage after surgical resection in patients with metastatic PDAC.
However, conversion surgery may provide better survival but is not a cure. the RFS did not differ between the conversion surgery and UpS/short NAC groups, and most patients suffered from early recurrence. Some articles reported RFS ranging from 5.0 to 16.5 months in patients with distant organ metastasis located primarily in the liver who underwent surgical resection (Table 4) (13,16-19). Wright et al. (13) reported that 7 of 23 patients (30.4%) experienced early disease recurrence (<6 months from surgery), mostly in the liver. The site of recurrence was the same as the initial site of metastatic disease in 76.5%, and was new in 23.5%. The conversion surgery group contained super-responders to systemic chemotherapy before surgical resection; therefore, the early recurrence rate was relatively low and they received chemotherapy for a median (range) of 21 (16 to 57) months, even after disease recurrence, leading to the improved OS. The other explanation may be raised that reduction of tumor burden by long-term chemotherapy followed by conversion surgery might be associated with prolonged survival. Among 173 patients with metastatic PDAC who received systemic chemotherapy and explored surgical resection, Hank et al. (16) clearly revealed that pancreatectomy and metastatic site resection were associated with favorable survival in patients with complete pathological response of metastasis (ypM0), but not in those with active metastases. However, the high proportion of early recurrence observed in the present study may reflect the difficulty in clinical diagnosis or confirmation of a complete response of liver metastasis using the imaging studies or macroscopic inspection. Other useful surrogate markers such as circulating tumor cells (26) or DNA (27), lymph node ratio (28), the microbiome (29) and so on for a complete response of liver metastasis should be explored in the future.
Table 4
| First author | Year | Study design | Study period | Category of UR-M | Regimen of chemotherapy | Number of patients | Number of resections (resectability) | Interval until surgery (months) | DFS (months) | MST, months from surgery [initial treatment] |
|---|---|---|---|---|---|---|---|---|---|---|
| Crippa (12) | 2016 | Retro | 2003–2013 | UR-liver | Multi-regimens | 127 | 11 (8.7%) | 12 | NA | NA [39] |
| Wright (13) | 2016 | Retro | 2008–2013 | UR-M | FFX/GEM base | 1,147 | 23 (2.0%) | 9.7 | 8.6 | 18.2 [34.1] |
| Satoi/Fujii (14) | 2017 | P-II | 2012–2015 | UR-PM | S-1 + iv/ip PTX | 33 | 8 (24.2%) | 9.0 | NA | NA [26] |
| Yamada (15) | 2020 | P-I/II | 2015–2018 | UR-PM | GnP + ip PTX | 46 | 8 (17.4%) | 8.8 | NA | Not reached |
| Frigerio (17) | 2017 | Retro | 2007–2015 | UR-liver | FFX/GnP/GEM | 535 | 24 (4.5%) | 10 | 13 | NA [56] |
| Hank (16) | 2023 | Retro | 2006–2019 | UR-M | FFX/GnP | 173* | 80 | ypM0: 8.1; ypM1: 5.9 | 8.7 5.0 |
25.5 [NA] 10.7 [NA] |
| Frigerio (18) | 2022 | Retro | 2008–2020 | UR-liver | FFX/GnP/GEM | NA | 52 (NA) | 10.2 | 16.5 | 23 [37.2] |
| Takeda (19) | 2023 | Retro | 2013–2020 | UR-liver | FFX/GnP/GEM | 243 | 13 (5.3%) | 9.2 | 14.0 | NA [54.6] |
| This study | – | Retro | 2007–2022 | UR-liver | Multi regimens | NA | 18 (NA) | 8.0 | 7.8** | 20.9 [36.7]** |
*, patients who explored surgical resection; **, indicates 10 patients who underwent conversion surgery. Retro, retrospective study; P-II, phase II; P-I, phase I; UR, unresectable; M, metastasis; PM, peritoneal metastasis; FFX, FOLFIRINOX; GEM, gemcitabine; iv, intravenous; ip, intraperitoneal; PTX, paclitaxel; GnP, GEM + nab-paclitaxel; DFS, disease-free survival; NA, not assessed; MST, median survival time.
Recent regimens such as FOLFORINOX (1) or GnP (2), in addition to gemcitabine + S-1 (30) as a key drug in Japan, provided a 20–30% clinical response on imaging studies, resulting in the increased trend of implementation of conversion surgery. Crippa et al. (12) reported that the MST in 11 patients with liver metastasis who underwent conversion surgery was 39 months. Frigerio et al. (17) reported that the MST after diagnosis was 56 months in 24 patients (4.5%) with liver metastasis among 535 patients with metastatic PDAC who underwent conversion surgery. Wright et al. (13) also reported an improved MST of 34.1 months from the time of diagnosis and 18.2 months from the time of surgery. As shown in Table 4, a long interval between initial treatment and surgery (approximately 9 months) and a strict surgical indication, such as evident tumor shrinkage and decreased levels of tumor markers, may provide improved survival in the limited numbers of patients with liver metastasis who had a favorable response to chemotherapy.
Our previous study revealed a surgical resectability rate of 16% in UR-LA and of 4.2% in metastatic disease among a total of 468 patients with UR-PDAC from 2006 to 2017 (31). A wide range of surgical indications for conversion surgery has been reported (11). Considering the relationship between surgical indication or resectability and MST, strict criteria, such as tumor shrinkage on imaging studies and decreases in tumor markers, was associated with low resectability but long MST. As shown in Table 4, the resectability rate in patients with liver metastasis has been reported to range from 4.5% to 8.7% (12,17,19), which was clearly lower than rates in patients with UR-LA PDAC. However, the MST ranged from 39 to 56 months from the initial treatment, which was similar to that in patients with UR-LA. The surgical indication of conversion surgery in this study was prospectively fixed as the ABC criteria, namely, anatomical objective response of disappearance of liver metastases on imaging studies and primary tumor shrinkage, biological objective response of CA19-9 level decrease to ≤150 U/mL, and conditional objective response of surgical fitness, as already proposed for BR PDAC (31,32). Meeting ABC criteria may mean suitable general condition of a candidate for surgical resection. In addition to the above ABC criteria, tumor disappearance at the liver was repeatedly confirmed using staging laparoscopy (laparoscopic objective response; L), and metabolic complete responses for the liver metastases with or without local tumor extension were confirmed using PET-CT (metabolic objective response; M). Meeting additional LM criteria may mean less activity of the tumor. As a result, meeting ABC-LM criteria may offer better surgical indication of conversion surgery in PDAC patients with liver metastasis. In this study, all ten patients in the conversion surgery group met the ABC-LM criteria. Based on the results of this study, a strict surgical indication of ABC-LM may be required for improving OS in candidates who responded favorably to systemic chemotherapy.
Recently, Ushida et al. retrospectively proposed the clinical significance of four prognostic factors of tumor shrinkage after chemotherapy (anatomical), normalized CA19-9 concentration (biological), modified Glasgow prognostic score of 0 (conditional), and chemotherapy duration ≥8 months in 454 consecutive patients with UR-PDAC who received modified FOLFIRINOX/GnP treatment (33). Considering OS according to prognostic factors, it was suggested that candidates for conversion surgery might have prognostic scores of 4 points (patients with distant organ metastasis) or 2–4 points (patients with UR-LA). The neutrophil-to-lymphocyte ratio and prognostic nutritional index as a nutritional parameter which can be correlated with prognosis in patients with PDAC did not differ among three groups in this study.
The consensus recommendations on oligometastatic disease classification and nomenclature defined it as the presence of a maximum of three to five metastases to the liver or lung on imaging studies (34). Takeda et al. (19) reported that patients with oligometastasis to the liver had a favorable survival duration of 13.2 months, which was significantly better than 8.2 months in patients with polymetastasis to the liver. The former underwent conversion surgery more frequently than the latter (12% vs. 1.3%, respectively), and the MST in patients who underwent conversion surgery reached 54.6 months. In this study, oligometastasis to the liver was found in 13 of 18 patients. All patients in the UpS/short NAC group were diagnosed with oligo and occult liver metastasis using open laparotomy or staging laparoscopy. OS in this group was only 10.4 months, which was similar to 9.9 months in patients with occult metastasis who were treated with chemotherapy alone. In contrast, polymetastases to the liver on CT imaging was found in 5 of 10 patients in the conversion surgery group.
The presence of a complete response on CT or PET-CT imaging with chemotherapy seems to be important for patient selection for surgical resection in this study. Even in patients with oligometastasis or occult metastasis, upfront surgery or surgical resection following short-term NAC may not be recommended. Regardless of the number of liver metastases, conversion surgery may be considered if chemotherapy provides favorable responses meeting ABC-LM criteria to systemic chemotherapy. Liver resection may be avoided in patients showing no viable tumor with liver biopsy. Further study will be required for investigating necessity of liver resection in patients with PDAC.
This study has several limitations. First, it was a single-center study carried out in a retrospective fashion, and it had a limited number of patients, leading to selection bias and limitations in the reliability of statistical analysis. Our findings should stimulate further inquiry into how to manage surgical resection in patients with PDAC with liver metastasis. Second, surgical resection in 15 out of 18 patients was performed in the latter phase [2015–2022] of this study, that may mean the changes in treatment strategy during the study period. Moreover, conversion surgery was performed for a limited number of patients who responded favorably to chemotherapy. It is difficult to determine if this is only the result of initial chemotherapy that selected a limited number of patients with favorable tumor biology or if conversion surgery may provide an actual survival advantage. However, 3 of 10 patients survived beyond 5 years after initial treatment in the conversion surgery group. Third, recent regimens such as FOLFIRINOX or GnP were used in approximately 50% of patients in each group, and no difference in chemotherapy regimen was found among groups. Since heterogenous groups were compared, caution should be used when making generalizations from the results of this study.
Conclusions
In conclusion, conversion surgery, but not upfront surgery or pancreatectomy following short-term NAC, should be performed in patients with liver metastasis who have a favorable response to systemic chemotherapy according to ABC-LM criteria. Although the early recurrence rate was high, long-term survivors were observed among the few patients who underwent surgical resection, and even in patients with initially multiple liver metastases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-655/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-655/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-655/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-655/coif). S.S. reports receipt of research funding from Nihon Servier and Amino-up co.jp. N.S. reports consulting or advisory roles for Kyowa Kirin Co., Ltd.; has received speaker’s bureau from Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Yakult Honsha Co., Ltd., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., MSD K.K., Merck Biopharma Co., Ltd., Bayer Yakuhin, Ltd., Eli Lilly Japan K.K., and Becton, Dickinson and Company; and has received research funding from Daiichi Sankyo Co., Ltd., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., and MSD K.K. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was reviewed and approved by the Institutional Review Board of Kansai Medical University, Japan (No. 2020131). All procedures were performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent for participation in the study was waived due to the retrospective nature of the study, and patients were given opportunities to opt out of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Sakaguchi T, Valente R, Tanaka K, et al. Surgical treatment of metastatic pancreatic ductal adenocarcinoma: A review of current literature. Pancreatology 2019;19:672-80. [Crossref] [PubMed]
- Hackert T, Niesen W, Hinz U, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol 2017;43:358-63. [Crossref] [PubMed]
- Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016;160:136-44. [Crossref] [PubMed]
- Seelig SK, Burkert B, Chromik AM, et al. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg 2010;2010:579672. [Crossref] [PubMed]
- Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007;14:118-27. [Crossref] [PubMed]
- Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 2007;110:2484-92. [Crossref] [PubMed]
- Takada T, Yasuda H, Amano H, et al. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival? Hepatogastroenterology 1997;44:567-73. [PubMed]
- Satoi S, Yamaue H, Kato K, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013;20:590-600. [Crossref] [PubMed]
- Satoi S, Yamamoto T, Yamaki S, et al. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg 2019;4:6-13. [Crossref] [PubMed]
- Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol 2016;42:1533-9. [Crossref] [PubMed]
- Wright GP, Poruk KE, Zenati MS, et al. Primary Tumor Resection Following Favorable Response to Systemic Chemotherapy in Stage IV Pancreatic Adenocarcinoma with Synchronous Metastases: a Bi-institutional Analysis. J Gastrointest Surg 2016;20:1830-5. [Crossref] [PubMed]
- Satoi S, Fujii T, Yanagimoto H, et al. Multicenter Phase II Study of Intravenous and Intraperitoneal Paclitaxel With S-1 for Pancreatic Ductal Adenocarcinoma Patients With Peritoneal Metastasis. Ann Surg 2017;265:397-401. [Crossref] [PubMed]
- Yamada S, Fujii T, Yamamoto T, et al. Phase I/II study of adding intraperitoneal paclitaxel in patients with pancreatic cancer and peritoneal metastasis. Br J Surg 2020;107:1811-7. [Crossref] [PubMed]
- Hank T, Klaiber U, Hinz U, et al. Oncological Outcome of Conversion Surgery After Preoperative Chemotherapy for Metastatic Pancreatic Cancer. Ann Surg 2023;277:e1089-98. [Crossref] [PubMed]
- Frigerio I, Regi P, Giardino A, et al. Downstaging in Stage IV Pancreatic Cancer: A New Population Eligible for Surgery? Ann Surg Oncol 2017;24:2397-403. [Crossref] [PubMed]
- Frigerio I, Malleo G, de Pastena M, et al. Prognostic Factors After Pancreatectomy for Pancreatic Cancer Initially Metastatic to the Liver. Ann Surg Oncol 2022;29:8503-10. [Crossref] [PubMed]
- Takeda T, Sasaki T, Okamoto T, et al. Outcomes of pancreatic cancer with liver oligometastasis. J Hepatobiliary Pancreat Sci 2023;30:229-39. [Crossref] [PubMed]
- Hashimoto D, Sakaguchi T, Satoi S, et al. Survival impact of occult liver metastasis and peritoneal dissemination compared with radiologically defined distant organ metastasis in pancreatic ductal adenocarcinoma. Pancreatology 2023;23:73-81. [Crossref] [PubMed]
- Sakaguchi T, Satoi S, Hashimoto D, et al. A simple risk score for detecting radiological occult metastasis in patients with resectable or borderline resectable pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci 2022;29:262-70. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003;89:1028-30. [Crossref] [PubMed]
- Toiyama Y, Miki C, Inoue Y, et al. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med 2011;2:95-101. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Yu J, et al. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann Surg 2018;268:408-20. [Crossref] [PubMed]
- Groot VP, Mosier S, Javed AA, et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin Cancer Res 2019;25:4973-84. [Crossref] [PubMed]
- Joliat GR, Labgaa I, Sulzer J, et al. International assessment and validation of the prognostic role of lymph node ratio in patients with resected pancreatic head ductal adenocarcinoma. Hepatobiliary Surg Nutr 2022;11:822-33. [Crossref] [PubMed]
- Wheatley RC, Valle JW, McNamara MG. The microbiome as a potential diagnostic biomarker for pancreatic ductal adenocarcinoma (PDAC). Hepatobiliary Surg Nutr 2022;11:752-4. [Crossref] [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [Crossref] [PubMed]
- Yanagimoto H, Satoi S, Yamamoto T, et al. Benefits of Conversion Surgery after Multimodal Treatment for Unresectable Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2020;12:1428. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Ushida Y, Inoue Y, Oba A, et al. Optimizing Indications for Conversion Surgery Based on Analysis of 454 Consecutive Japanese Cases with Unresectable Pancreatic Cancer Who Received Modified FOLFIRINOX or Gemcitabine Plus Nab-paclitaxel: A Single-Center Retrospective Study. Ann Surg Oncol 2022;29:5038-50. [Crossref] [PubMed]
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. [Crossref] [PubMed]


