
ACTB may serve as a predictive marker for the efficacy of lenvatinib in patients with HBV-related early-stage hepatocellular carcinoma following partial hepatectomy: a retrospective cohort study
Highlight box
Key findings
• The expression of beta-actin (ACTB) influences the efficacy of lenvatinib for patients with hepatitis B virus-related early-stage hepatocellular carcinoma (HCC) after surgery.
What is known and what is new?
• Lenvatinib effectively improves the prognosis of patients with HCC after surgery.
• HCC with low expression of ACTB is sensitive to lenvatinib, indicating low recurrence of patients after surgery.
What is the implication, and what should change now?
• It is recommended to detect the expression of ACTB in HCC tissue after surgery. The patients with low-level ACTB HCC should prioritize using lenvatinib to prevent recurrence.
Introduction
Hepatocellular carcinoma (HCC) is a prevalent malignancy worldwide, ranking fourth in cancer-related mortality globally (1,2). Liver resection remains the primary therapeutic option, despite the fact that less than 30% of patients meet the criteria for potentially curative treatment. However, due to high recurrence and death, survival after resection is unsatisfactory, with the 5-year overall survival (OS) of approximately 50%. The majority of patients experience recurrence following resection, with a postoperative recurrence rate ranging from 60% to 70% within a 5-year period (3). Factors contributing to recurrence include the presence of microscopic vascular invasion (MVI), elevated serum α-fetoprotein (AFP) levels, multiple tumors, higher grades of hepatitis activity and cirrhosis, as well as satellite nodules (4-7). Several randomized trials have investigated various adjuvant therapeutic interventions, such as transcatheter arterial chemoembolization (TACE), targeted therapy, interferon (IFN), internal radiation, and chemotherapy, with the aim of providing long-term recurrence or OS. However, as far as we are aware, there are currently no standardized treatment options available (3,8,9).
Lenvatinib is an orally administered multikinase inhibitor that selectively targets PDGF receptor α, fibroblast growth factor (FGF) receptors 1–4 (FGFR1–4), vascular endothelial growth factor (VEGF) receptors 1–3, KIT and RET. It has been authorized as a first-line systemic treatment for advanced HCC patients (10-12). This was the result of a randomized phase-III clinical trial in patients with advanced HCC, which demonstrated that lenvatinib had comparable OS to sorafenib while exhibiting superior progression-free survival (PFS) (13). Furthermore, lenvatinib was indicated with the efficacy of preventing the recurrence of HCC for patients after surgery (14,15). Despite numerous studies, no predictive factors for lenvatinib response have been identified. Given the limitations of the currently available clinicopathological biomarkers, a study suggests that molecular biomarker should be used in addition to clinicopathological marker (16). At present, most of the studies on biomarkers related to the prediction of HCC targeted therapy are still in the preliminary stage, and more large samples and prospective clinical studies are needed to verify them. Currently, some studies have successively reported various substances or cells as biomarkers for predicting the prognosis of patients with HCC after liver resection (16,17), but there are few biomarkers that predict the efficacy of lenvatinib. In a random phase III non-inferiority trial (REFLECT), there was a trend that lower VEGF, angiogenin-2 (Ang-2), and FGF21 serum levels at baseline were associated with better outcomes with lenvatinib; early changes in rehabilitation and treatment of contaminated Soil in the treatment of HCC, Ang-2 levels, AFP response, baseline albumin bilirubin (ALBI) score, change in the ALBI score, neutrophil to lymphocyte and platelet to lymphocyte ratios were early predictors of objective response (OR) in patients with HCC undergoing lenvatinib treatment (17,18). However, to date, none of the biomarkers have been validated for clinical use. Worse still, the status of lenvatinib biomarker expression in HCC and its correlation with lenvatinib response remain elusive. Therefore, it is imperative to investigate the correlation between biomarkers and its efficacy in treating HCC, as well as to develop innovative indicators for patient selection to optimize adjuvant treatment outcomes (19,20).
Given the widely held assumption that the beta-actin (ACTB) gene is constitutive and ubiquitous, performing housekeeping activities, recent research suggests a broader range of functional roles for this protein (21). Biological research suggests that a significant deficiency in ACTB protein levels can lead to detrimental effects on cell shape, migration, proliferation, and gene expression. These effects may hinder the proper development of vital organs such as the kidney, heart, and brain. Nevertheless, mounting evidence indicates that ACTB is expressed abnormally in various malignancies, disrupting the cytoskeleton and thereby impeding tumor invasion and metastasis (22-28). In the current research field of HCC, the expression of ACTB was downregulated in HCCs with varying degrees of invasiveness and Tumor-Nodes-Metastasis (TNM) stages, and the 3'-untranslated region (UTR) of ACTB played a critical role in HCC progression (29).
The current study investigated the correlation between ACTB and patient TTR in lenvatinib-treated individuals with high risk of recurrence, suggesting that ACTB may serve as a potential biomarker for predicting lenvatinib efficacy in HCC patients at high risk of recurrence. To the best of our knowledge, this is the first attempt to identify a predictive biomarker and build a development model in accordance with the CONSORT guidelines for patients with hepatitis B virus (HBV)-related HCC who have undergone partial hepatectomy and received lenvatinib treatment. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-942/rc).
Methods
Patients and treatment
In conducting this retrospective clinical cohort study, we employed a comprehensive research design and retrospectively enrolled a total of 256 HCC patients who had not received preoperative therapy at the Eastern Hepatobiliary Surgery Hospital (EHBH) to identify parameters associated with the benefits of lenvatinib in the adjuvant setting between 2009 and 2020. Between 2018 and 2020, a total of 76 patients received adjuvant lenvatinib treatment following surgical intervention and were prospectively monitored until the conclusion of this study (the lenvatinib group). Incorporating insights from previous research and a review of pertinent literature, we have included baseline characteristics of patients in our study. These characteristics have been determined based on the professional assessment of pathologists, outlining tissue and tumor features such as the presence of liver cirrhosis. Additionally, for specific data of particular interest, a separate and detailed examination has been conducted, as exemplified by our focused scrutiny on data related to ACTB. The control group comprised patients who underwent surgery between 2009 and 2011 but did not receive adjuvant therapy. During the first-year post-surgery, patients underwent follow-up visits every 2–3 months. After 1 year, they underwent follow-up assessments every 3–6 months to evaluate liver function and serum tumor markers. The duration between surgical resection and the day of recurrence (or the last follow-up) was utilized to ascertain the time to recurrence (TTR). Table S1 presents a comprehensive overview of their clinicopathological features. After propensity score matching (PSM), a cohort (n=225) was formed by conducting a propensity matching analysis on the patients who met the criteria, as shown in Table S2. The following selection criteria were employed: (I) patients with Child-Pugh A/B liver function and unilateral HCC, who exhibited at least two risk factors for recurrence, including the presence of MVI, high levels of AFP, multiple tumors and satellite nodules; (II) lenvatinib therapy was initiated within one-month post-surgery and continued for a duration of at least 1 year or until disease recurrence. The subjects were orally administered lenvatinib once daily in 28-day cycles, with a dosage of 12 mg/day (for body weight ≥60 kg) or 8 mg/day (for body weight <60 kg). Dose interruptions, followed by reductions to 8 mg/day or 4 mg/day (or 4 mg every other day), were permitted for lenvatinib-related toxicities; (III) hepatitis B surface antigen (HBsAg) and/or hepatitis B core antibody (HBcAb) were detected as positive, while hepatitis C antibody was identified as negative; (IV) refraining from preoperative therapies such as radiofrequency ablation, high-intensity focused ultrasound, or chemoembolization was the approach taken for HCC patients; (V) all patients underwent surgical treatment, and postoperative pathology confirmed that the tumor was R0 removed To conduct immunohistochemistry (IHC) staining, tissue slices were obtained from primary HCC patients. The procedural flow of HCC patients is illustrated in Figure 1A.
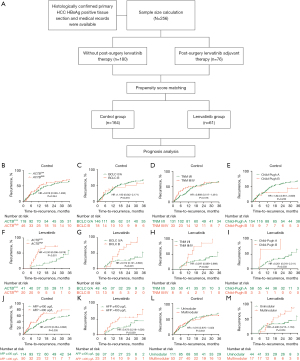
The TTR, which was the primary endpoint of this study, was defined as the duration from surgery to recurrence. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional ethics board of Eastern Hepatobiliary Surgery Hospital (No. EHBHKY2023-K036-P001) and informed consent was obtained from all individual participants.
Surgical procedure
The surgical team used conventional approaches to execute all surgical procedures. The surgical procedures were performed through a bilateral subcostal incision. The abdominal cavity was thoroughly examined to assess the extent of local disease and identify any extrahepatic metastases. Intraoperative ultrasonography was performed to determine the number, size, and vascular connection of the lesions. Pringle’s maneuver was employed to interrupt hepatic blood flow, utilizing cycles of 15 minutes occlusion followed by 5 minutes of reperfusion. The liver resection was performed using the clamp-crushing technique (30).
IHC analysis
The primary HCC and corresponding para-tumoral fresh specimens were promptly obtained post-surgery, fixed in neutral buffered formalin, and embedded in paraffin for subsequent analysis via hematoxylin and eosin (HE) or IHC staining as previously reported (31). For ACTB staining, the slides were incubated with a monoclonal antibody against human β-actin (D6A8, Cell Signaling Technology, Boston, USA) at a dilution of 1:100. The secondary antibody used was an horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulins/HRP (P0448, DAKO, Copenhagen, Denmark). The final steps involved the utilization of a colorimetric reagent solution containing diaminobenzidine (Liquid DAB + Substrate Chromogen System, K3468, DAKO), followed by counterstaining with hematoxylin. The specificity of ACTB antibody was assessed through western blotting. The slides stained in different batches exhibited minimal experimental variability, indicating excellent reproducibility. Simultaneous experimental controls were implemented. To observe the stained sections, we utilized an imaging system comprising of a Leica DFC420 CCD camera attached to a Leica DM IRE2 microscope procured from Leica Microsystems Imaging Solutions in Cambridge, UK. The images were captured using Leica QW in Plus v3 software at 200× magnification for each individual core (32). The degree of hepatic inflammation and fibrosis was evaluated utilizing the Glasgow University Score for Liver Pathology (GS) staging system. The quality of immunostaining was evaluated independently by two pathologists who were blinded to the clinical information and patient outcomes. The pathologists utilized the positive pixel count method provided by Image-Pro plus V6.0 (Media Cybernetics, Bethesda, MD, USA) to quantify and assess high-resolution digital images they gathered. The software automatically calculates the total intensity of positive (brown staining) pixels and measures the overall area of the image. The staining score for each sample was determined by calculating the sum of positive pixel intensities divided by the total area. To stratify patients in the adjuvant lenvatinib group based on their expression levels, we selected the upper tertile of IHC staining scores for lenvatinib target in HCC tissues. The X-Tile software was utilized to determine the cut-off point for ACTB protein and mRNA levels, which enabled the categorization of patients into two groups based on their high or low ACTB expression levels.
mRNA analysis
Real-time polymerase chain reaction (RT-PCR) analysis was performed using a SYBR Green PCR Kit (Roche, Switzerland, Basel) and a Light Cycler 480 System (Roche). Fifty pairs of HCC and corresponding peritumoral normal tissues were used for the analysis of β-actin mRNA. GAPDH was used as internal reference. The sequences of primers used are listed below:
- β-actin forward primer (5'-3'): AATCGTGCGTGACATTAAGGAG; reverse primer (5'-3'): ACTGTGTTGGCGTACAGGTCTT.
- GAPDH forward primer (5'-3'): ACCACAGTCCATGCCATCAC; reverse primer (5'-3'): TCCACCACCCTGTTGCTGTA.
Patient-derived xenograft (PDX) model
To establish the PDX model, primary HCC specimens were obtained from six independent patients and utilized for xenograft formation as previously reported (33). For this study, the primary tumor specimens were obtained post-surgery and subsequently washed with DMEM containing antibiotics. The specimens were then sliced into 20–30 mm3 sections and subcutaneously implanted bilaterally in male BALB/c nude mice. Once the original xenografts reached a volume of 500–800 mm3, they were retrieved and sliced into 30 mm3 cubes before being subcutaneously implanted into male BALB/c nude mice. Following size-matching of the xenografts (150–200 mm3), the mice were randomly assigned to two treatment groups (lenvatinib or vehicle). Mice bearing xenografts were orally administered daily doses of lenvatinib (10 mg/kg body weight) (34) or vehicle for 21 days, and the tumor volume was normalized at day 21. Each model was generated using the following number of mice: Patient #1, n=5 for both lenvatinib and vehicle groups; Patient #2, n=5 for both lenvatinib and vehicle groups; Patient #3, n=5 for vehicle group and n=4 for lenvatinib group; Patient #4, n=5 for both lenvatinib and vehicle groups; Patient #5, n=5 for both lenvatinib and vehicle groups; Patient #6, n=4 for vehicle group and n=5 for lenvatinib group. The tumor volumes were quantified as described, and Table S3 provides a comprehensive overview of ACTB expression in the six distinct primary HCCs used to develop the PDX model, along with relevant clinical features of the patients. IHC was conducted to evaluate the expression of ACTB in primary HCCs used for developing PDX models. The cutoff threshold for IHC staining score to distinguish between high and low HCCs was 88.4. In addition to obtaining written informed consent from each participant, the Institutional Review Board at EHBH authorized all the protocols and procedures of this study. The growth rate of tumors was calculated using the following equation:
Statistical analysis
Statistical analysis utilizing the SPSS V22.0 program (IBM, Chicago, IL, USA) was applied to identify risk variables. The baseline clinicopathological features of the patients were evaluated using the χ2 test. The categorical variables were categorized based on clinical outcomes, and the selection of groups was made prior to the modeling process. The Mann-Whitney test for abnormal distributed variables was utilized to compare the continuous variables. PASS 17 program was employed to compute the sample size, the median TTR time of lenvatinib and control group were used as basis, tests were two-sided and α is equal to 0.05. Propensity score matching analysis was used to match HCC patients who received treatment with or without lenvatinib employing psmatch2 tools, the hypothesis test for the balance of variables before and after matching employing pstest tools in STATA 17 version MP (https://www.stata.com). With the assistance of X-Tile statistical software (version 3.6.1, Yale University, New Haven, CT, USA), the optimal threshold points for TTR were determined. The presence of substantial HCC subpopulations was shown by an X-Tile plot, and a two-dimensional projection of each conceivable subpopulation was utilized to demonstrate the strength of the link between a biomarker and an outcome (34). X-Tile plots were constructed to determine the degree of quantitative elements including ACTB were present. For subgroup analysis, we employed a Cox proportional hazards regression model and the Kaplan-Meier technique to determine hazard ratios (HRs) and median recurrence time. An interaction term was included in the Cox proportional hazards regression model to explore interactions between therapy, biomarkers, or clinical factors. Based on R packages “rms”, Cox regression coefficients were also utilized to construct a nomogram, while calibration curves were employed to evaluate the concordance between observed and predicted outcomes derived from the nomogram (35). Multivariate analysis of TTR in matched patients was conducted using regression models (Table S4). All statistical evaluations were two-tailed, and a significance level of P<0.05 was considered statistically significant.
Results
Relationships between the expression of ACTB and patient outcomes were investigated in participants who underwent adjuvant lenvatinib therapy following surgery
The HBV infection status and utilization of antiviral therapy were succinctly summarized in Table S5. To investigate the correlation between ACTB expression and lenvatinib response as well as patient outcome in HCC samples, IHC staining was performed to measure and score ACTB expression in 76 post-surgical patients who received adjuvant lenvatinib treatment. According to the stratified factors affecting the efficacy of lenvatinib adjuvant therapy, the analysis was made (Figure 1). The patients were subsequently stratified into cohorts based on their ACTB expression levels. The control group did not exhibit a comparable level of performance (Figure 1B-1E) by Kaplan-Meier estimates. However, univariate analysis revealed that patients with high levels of ACTB (HR =5.014, P<0.001), BCLC stage B (HR =3.318, P=0.002), TNM stage III/IV (HR =3.485, P=0.004), Child-Pugh stage B (HR =5.265, P=0.008), AFP >400 μg/L (HR =2.160, P=0.045) and multinodular tumors (HR =2.427, P=0.023) were at an elevated risk for reduced TTR in the lenvatinib group (Table 1). Furthermore, patients in the lenvatinib group with low ACTB levels (HR =0.243, P<0.001) (Figure 1F), BCLC stage 0/A (HR =0.312, P=0.001) (Figure 1G), TNM stage I/II (HR =0.297, P=0.002) (Figure 1H), or Child-Pugh A (HR =0.201, P=0.003) (Figure 1I) exhibited a significantly longer TTR. Furthermore, upon subjecting these variables to multivariate analysis, the results indicated that elevated levels of ACTB (HR =5.879, P<0.001), BCLC stage B (HR =2.508, P=0.026) or Child-Pugh stage B (HR =5.416, P=0.015) were independent risk factors for TTR and associated with shorter TTR (Table 1). Simultaneously, we compared the baseline characteristics of patients in the lenvatinib group and control group based on the high and low expression of ACTB, and the results revealed no significant differences between the groups (Table S6). This suggests their potential in predicting recurrence among patients treated with adjuvant lenvatinib.
Table 1
| Variables | Subgroup | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Gender | Female vs. male | 1.931 (0.889–4.196) | 0.096 | – | – | |
| Age (years) | >50 vs. ≤50 | 1.176 (0.549–2.519) | 0.677 | – | – | |
| Tumor size (cm) | >3.5 vs. ≤3.5 | 2.709 (0.812–9.034) | 0.105 | – | – | |
| Tumor number | Multinodular vs. uninodular | 2.427 (1.129–5.217) | 0.023 | NA | NA | |
| MVI | No vs. yes | 0.522 (0.244–1.115) | 0.093 | – | – | |
| AFP (μg/L) | >400 vs. ≤400 | 2.160 (1.019–4.578) | 0.045 | NA | NA | |
| Cirrhosis | Yes vs. no | 1.093 (0.513–2.330) | 0.818 | – | – | |
| HBV-DNA (IU/mL) | Low vs. high | 0.975 (0.463–2.054) | 0.946 | – | – | |
| Child-Pugh | B vs. A | 5.265 (1.548–17.907) | 0.008 | 5.416 (1.390–21.104) | 0.015 | |
| TNM | III/IV vs. I/II | 3.485 (1.507–8.058) | 0.004 | NA | NA | |
| BCLC | B/C vs. 0/A | 3.318 (1.544–7.128) | 0.002 | 2.508 (1.116–5.639) | 0.026 | |
| ACTB | High vs. low | 5.014 (2.199–11.430) | <0.001 | 5.879 (2.424–14.259) | <0.001 | |
TTR, time to recurrence; MVI, macroscopic vascular invasion; AFP, α-fetoprotein; HBV, hepatitis B virus; TNM, Tumor-Nodes-Metastasis; BCLC, Barcelona Clinic Liver Cancer Staging; ACTB, beta-actin; HR, hazard ratio; CI, confidence interval; NA, not available.
The association between ACTB expression and lenvatinib efficacy in patients with HCC
According to the sample size calculation, a total of 173 patients were allocated (43 in the lenvatinib group and 130 in the control group), with a recorded power value of 0.8015 (Table S7). We subsequently conducted a retrospective analysis to assess the predictive value of ACTB in identifying HCC patients who would benefit from lenvatinib treatment. A total of 256 patients who underwent surgery for HCC at a single academic institution were included in the study. These patients were categorized into two groups: those who received treatment with lenvatinib and those who did not (Table S1). The variables to be matched were also screened using STATA software. Following PSM analysis, the control group comprised 164 participants who were matched with 61 participants in the lenvatinib group (Figure 1A). The results also indicated that there were no significant differences in case composition and variables between matched and original cases stratified by baseline parameters (Figure S1). Although there was no statistically significant difference between patients with and without cirrhosis, we further stratified cases without cirrhosis and found that those with low levels of inflammatory fibrosis had a longer time in therapeutic range (TTR) compared to those with high levels of inflammatory fibrosis and cirrhotic patients in both the lenvatinib and control groups (Figure S2A,S2B, Table S8). The expression of ACTB varied across different stages of inflammation and fibrosis, with a statistically significant correlation observed between ACTB expression and GS score in cancer tissue (r=0.228, P<0.001) (Figure S2C). However, no obvious correlation was found between ACTB expression and GS score in normal tissue (P=0.243) (Figure S2D). Patients with well-differentiated tissue in the lenvatinib and control groups exhibited a longer TTR compared to those with moderate and poor differentiation, as shown in Figure S2E,S2F. Subsequently, we performed IHC staining to assess the expression of ACTB in primary HCC specimens. The Cox proportional hazard model with an interaction term in the cohort was employed to evaluate the statistically significant interaction effect of continuous levels of ACTB and therapy on patient outcome. The results indicate a significant interaction (P=0.014) (Figure 2A). To determine the optimal cutoff threshold for ACTB levels, we employed X-Tile to traverse all ACTB expression values in ascending order and identify the cutoff point that best separates patient into low- or high-ACTB expression groups. We then evaluated the significance of lenvatinib benefit against control within each group. The groups exhibiting statistically significant variations in TTR were those with low expression of ACTB protein. A threshold point (88.4) was identified as having the greatest significance and used to further classify patients into two groups: ACTBHigh versus ACTBLow, comprising 29.3% and 70.7% of the total population, respectively (Figure 2A). ACTB levels in the cohort were compared with clinical characteristics of the patients, revealing a significant correlation between ACTB levels and cirrhosis as well as HBV-DNA (Table S9). HCC patients exhibiting low levels of ACTB demonstrated a superior TTR response to lenvatinib therapy (HR =0.502, P=0.001) (Figure 2B). However, our findings indicate that patients with low ACTB expression exhibited significantly improved TTR when treated with lenvatinib compared to those with high ACTB expression, while no significant improvement in TTR was observed in the control group (Figure 1B). These findings suggest that lenvatinib adjuvant therapy did not demonstrate a significant improvement in TTR for all patients, but may provide benefits for those with ACTBLow, BCLC 0/A and Child-Pugh A (Table 1).
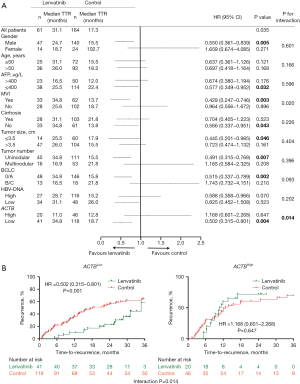
Additionally, we constructed a nomogram that combined the Child-Pugh stage, the BCLC stage, and the ACTB score in the lenvatinib group (Figure 3A). Calibration plots showed that the nomogram compared favorably with an ideal model in the lenvatinib group at 0.5, 1 and 2 years (Figure 3B-3D). The C-index of the nomogram for predicting survival was 0.76 [95% confidence interval (CI): 0.71–0.84].

The predictive value of ACTB in stratifying lenvatinib benefit among patients with different prognostic factors
A precise postoperative adjuvant treatment tailored to individual HCC patients heavily relies on the accurate classification of patients using an optimal combination of biomarkers and clinical factors (36). We evaluated the efficacy of lenvatinib in ACTBHigh and ACTBLow patients stratified by prognostic variables. The median dosage of lenvatinib was 12 mg/day in both the ACTBHigh and ACTBLow expression groups, with a median duration of administration of 13.6 months in the former group and 13.1 months in the latter. For patients with low ACTB levels, lenvatinib therapy improved their TTR, while for those with high ACTB levels, there was no significant improvement in most categories, as illustrated in Figure S3. Notably, a significant number of benefits from lenvatinib were observed in the ACTBLow cohort among male patients (Figure S3A), with MVI (Figure S3D), non-cirrhosis (Figure S3E), tumor size ≤3.5 or >3.5 cm (Figure S3G,S3H), uninodular (Figure S3I) and BCLC stage 0/A (Figure S3K). The results suggest that the integration of ACTBLow with any of these variables may facilitate the identification of individuals who are most likely to benefit from lenvatinib treatment.
An increase in the expression of ACTB was observed in HCC specimens
To determine the proportion of patients who may derive therapeutic benefits from lenvatinib based on their ACTB levels, we subsequently conducted an analysis of ACTB expression in both HCC and adjacent para-tumoral normal liver tissues. The results presented in Figure 4A,4B demonstrate a significant upregulation of ACTB mRNA transcripts in HCC patient samples compared to adjacent para-tumoral normal tissues. The downregulation of ACTB mRNA did not show a significant difference in TTR within the control group, whereas its downregulation in the lenvatinib group was associated with a prolonged TTR (Figure 4C,4D). Furthermore, IHC assessment of HCC sections and adjacent normal liver tissues revealed a significant elevation in the levels of ACTB protein within HCC specimens. Furthermore, IHC staining of corresponding HCC and adjacent normal liver tissues demonstrated that only 18.2% of patients displayed downregulation of ACTB in their HCC specimens (Figure 4E).

Low levels of ACTB are associated with increased sensitivity to lenvatinib in HCC PDX models
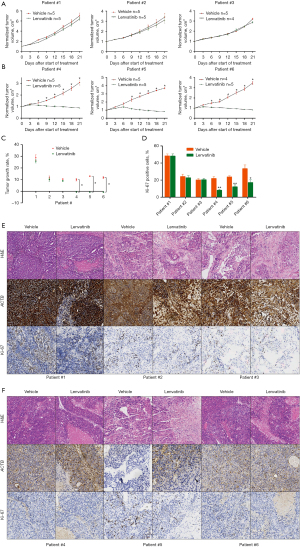
Subsequently, the correlation between ACTB levels and lenvatinib response in vivo was validated through PDX models (Figure 5). Primary HCCs collected from six distinct patients who were not part of the cohort are detailed in Table S3. The IHC staining score threshold of 90.5 was utilized to distinguish HCCs with high- and low-ACTB scores. After PDXs with primary HCC was treated with lenvatinib or vehicle for 21 days, it was intuitively demonstrated that the expression of ACTB had an influence on the sensitivity of lenvatinib in HCC PDX model (Figure 6). The results indicated that lenvatinib did not demonstrate any therapeutic efficacy on the PDXs derived from primary (HCCs of Patients #1–3, who exhibited elevated levels of ACTB (Figures 5A,6A,6C,6E). However, treatment with lenvatinib resulted in nearly complete inhibition of PDX development in tumors (Patients #4–6) exhibiting reduced ACTB levels, as compared to the vehicle control (Figures 5B,6B,6D,6F). Tumor growth rate analyses were performed on these PDXs, yielding consistent results (Figure 5C). Furthermore, a reduction in Ki-67 expression, which is a hallmark of proliferating cells, was only observed in PDXs obtained from tumors with low levels of ACTB when treated with lenvatinib (as shown in Figure 5D-5F). This implies that HCC patients with decreased ACTB expression may exhibit heightened responsiveness to lenvatinib therapy. These results additionally suggest that the levels of ACTB in patient tumors could serve as a valuable indicator of lenvatinib efficacy.

Discussion
This study aimed to predict HBV-related HCC recurrence post-partial hepatectomy and lenvatinib therapy. The focus was on identifying a predictive biomarker for personalized HCC treatment. In 2018, lenvatinib was approved as an alternative to Sorafenib and the first-line option for targeted therapy of advanced liver cancer. A multicenter phase III clinical trial published this year confirmed that lenvatinib combined with TACE can significantly improve the median OS of patients with advanced liver cancer, lenvatinib combined with immune checkpoint inhibitors (ICIs) can bring survival benefits to patients with liver cancer in second-line and second-line treatment (37,38). Based on the fact that there is no clear biomarker during the use of lenvatinib, propensity score matching analyzed patients with or without lenvatinib, using ACTB expression levels in HCC samples. The study employed a nomogram, C-index, and calibration curve for predictive modeling. Results showed that early-stage HCC patients with lower ACTB levels responded better to lenvatinib therapy. Reduced ACTB expression correlated with favorable lenvatinib outcomes. ACTB, along with Child-Pugh and BCLC stage, independently predicted TTR in the lenvatinib cohort. The nomogram’s predictions aligned well with actual observations. Lower ACTB levels in early-stage HCC patients indicate a more beneficial response to lenvatinib, suggesting its potential as a predictive biomarker for treatment stratification. Further validation in independent cohorts is essential for precision treatment in post-hepatectomy HCC patients.
Lenvatinib has been recently discovered to reduce tumor PD-L1 levels and inhibit Treg differentiation, thereby enhancing the efficacy of anti-PD-1 through FGFR4 inhibition (12,39). A stabilized inverse probability of treatment weighting (SIPTW)-weighted multivariate Cox regression analysis showed that continuous use of lenvatinib can prolong the OS and PFS in patients with unresectable HCC (u-HCC). The duration of lenvatinib was a protective factor of OS. In addition, the Subpopulation Treatment Effect Pattern Plot (STEPP) analysis showed that with the increase of anti-PD-1 treatment cycle, the 12-month survival rate increased slowly, and patients who had been treated with anti-PD-1 for more than five cycles may benefit most from the combined treatment (40). In other research studies, lenvatinib has demonstrated its ability to inhibit FGFR1–3 signaling and reduce cancer stem-like cells in HCC (13). Nonetheless, there is currently no clinically validated biomarker available for predicting response to lenvatinib or other targeted therapies, and none of the targets of lenvatinib have been demonstrated to be useful in patient stratification or anticipating prognosis. As demonstrated by this study, the correlation between ACTB, a cytoskeletal structural protein, and lenvatinib response as well as patient recurrence is significant. Our research has demonstrated that low ACTB expression is a predictive factor for improved adjuvant lenvatinib outcomes in specific subgroups of cases, including male patients with MVI, non-cirrhosis, tumor size ≤3.5 or >3.5 cm, unimodular and BCLC stage 0/A. This indicates that this marker is only applicable to certain cases rather than all. Besides, HCC patients with low levels of ACTB may benefit from lenvatinib therapy. However, in patients with multinodular, regardless of their ACTB levels, lenvatinib did not improve outcomes. This suggests that either ACTB is not a predictive factor for this patient group or that lenvatinib monotherapy should not be used as first-line adjuvant therapy for advanced HCC patients after surgery.
The protein ACTB, identified as a highly conserved structural component of the cytoskeleton, plays a crucial role in cell growth and migration (41). According to a recent research report, ACTB exhibits differential expression and plays a crucial role in various human diseases, particularly cancer (10,42-45). The levels of ACTB protein in renal cell carcinoma cell lines and tissues are significantly elevated compared to those observed in normal controls (46). In a recent study, Braoudaki et al. have discovered that the ACTB protein plays a pivotal role in leukemia prognosis and may serve as a biomarker for distinguishing leukemia aggressiveness or inhibitor proteins in patients with high-risk acute lymphoblastic leukemia (HR-ALL). ACTB molecules exhibit the ability to discriminate between high- and low-risk leukemias, irrespective of their tissue origins (47). The high expression of ACTB alone did not correlate with a shorter TTR. However, elevated ACTB expression following adjuvant lenvatinib treatment post-surgery was indicative of poor therapeutic response. Furthermore, our findings suggest that ACTB may serve as a useful biomarker for predicting the efficacy of lenvatinib adjuvant therapy, rather than directly predicting TTR in patients after surgery. The mRNA transcript levels of ACTB were found to be significantly higher in HCC samples compared to those detected in para-tumoral normal tissues. While the expression of ACTB mRNA did not serve as a reliable predictor for TTR among postoperative patients in the control group, downregulation of ACTB mRNA was associated with longer TTR in the lenvatinib group. In addition, immunohistochemical staining of paired HCC and adjacent normal liver tissues demonstrated that only 18.2% of patients exhibited decreased expression of ACTB in their HCC specimens. The modulation of tumor cell adhesion and motility, which are critical for tumor growth and metastasis, is well known to be influenced by the cytoskeletal structure and actin microfilament system (48). The localization, polymerization, cytoskeletal organization and ACTB overexpression are all implicated in the promotion of colon cancer cell motility and metastasis. Differential distribution of ACTB in the perinuclear region and at the leading edge of cells may impact tumor cell migration plasticity and polarity, thereby influencing tumor metastasis (49). Furthermore, the upregulation and aggregation of ACTB in pseudopodia may facilitate tumor cell invasion (50). The expression of ACTB was dysregulated in various stages of HCC invasiveness and TNM classification, with ACTB mRNA 3’-UTR promoting HCC proliferation and invasion by AGO2-involved miR-1 and miR-29a degradation, thus enhancing the expression of miR-1 target gene MET and miR-29a target gene MCL1 (51-53). Although the correlation between ACTB and tumor progression has been demonstrated in numerous studies, the impact of adjuvant therapy on ACTB expression levels remains unclear. This study highlights the evident advantages of lenvatinib in patients with ACTBLow. Nevertheless, PDX models derived from ACTBLow patients exhibited superior responsiveness to lenvatinib adjuvant therapy compared to those derived from ACTBHigh patients. Patients with low expression of ACTB in the TNM I/II or BCLC A groups of HCC patients exhibit a superior response to lenvatinib. However, in the PDX model, cases with TNM III or BCLC stage B also demonstrate favorable outcomes when ACTB expression is diminished. It is possible that the tumor tissue exhibiting low ACTB expression may be more responsive to lenvatinib, thereby resulting in a superior therapeutic outcome in the model. However, patients with advanced HCC may have a more intricate mechanism of drug resistance, resulting in limited efficacy of postoperative adjuvant therapy for lenvatinib monotherapy regardless of high or low ACTB expression. The drug resistance of Ranvartinib is caused by many reasons, involving many pathways, such as VEGF/VEGF receptor (VEGFR) pathway, RET signaling pathway, FGFR signaling pathway and so on. A combination of drugs is often used to overcome lenvatinib resistance in clinical practice, the combination of targeted and immune drugs, such as Ranvartinib combined with Pabolizumab, can accelerate the necrosis of tumor tissue and significantly reduce the level of tumor biomarkers such as AFP. The proportion of complete response (CR) and partial response (PR) in patients with PD-1 monoclonal antibody and Ranvartinib conversion therapy is also relatively high. (11,54). Meanwhile, biomarkers can help clinicians during treatment selection for individualized therapy, the classification of patients based on their low-ACTB levels could be a viable approach to enhance the response rates of lenvatinib in precision therapy for HCC.
Recent research has identified MVI, high levels of AFP, elevated HBV-DNA, multiple tumors, cirrhosis and satellite nodules as independent factors for predicting the risk of postoperative recurrence in HCC (6). A recent survey demonstrated that MVI is associated with aggressive biological features of HCC, lenvatinib can reduce postoperative recurrence and improve long-term survival in patients with HCC and MVI after curative hepatectomy, especially in cases with microvascular invasion where there is a higher risk of tumor spread (55). In our research, it was innovatively found that patients with HBV-related HCC used lenvatinib after radical surgery. Among people with microvascular invasion, the ACTBlow group often had a better long-term prognosis. Therefore, we suspect that MVI and ACTB may jointly predict the long-term survival rate of patients who use lenvatinib after operation. The natural history of human HBV infection varies greatly among different genotypes. This variability includes: the main mode of transmission, the time of hepatitis B early antibody (HBeAg) seroconversion, the speed and severity of liver disease progression (including the possibility of progression to HCC and serological clearance of HBsAg), and the diversity of HBV can lead to differences in the natural history, disease progression and treatment response of patients with chronic infection, especially in the treatment of IFN-α. A study on patients with chronic hepatitis B treated with tenofovir showed that HBsAg was almost eliminated. This is caused by HBV’s unique replication cycle and cell host factors. The mechanism of HBV-induced liver cancer usually includes direct induction of carcinogenic signal pathway, induction of chronic inflammation and subsequent cirrhosis. Therefore, the diversity of viruses must be considered when developing new treatment schemes (56). We further stratified cases without cirrhosis and found that patients with low levels of inflammatory fibrosis had longer TTR than patients with high levels and cirrhosis both in lenvatinib and control group (Figure S2A,S2B). The analyses also revealed no discernible differences in case composition between matched and original cases, stratified by baseline parameters. The implementation of postoperative adjuvant therapy represents a novel approach to reducing recurrence. According to a multicenter investigation, lenvatinib can be safely and effectively administered in patients with HCC regardless of their age (57). A retrospective exploratory analysis of the REFLECT trial revealed that patients who achieved an ORR demonstrated a significantly prolonged OS. It has been reported that a distinct trend in favor of lenvatinib over sorafenib (HR =0.82; 95% CI: 0.60–1.15) was observed among HBV-positive patients, indicating that lenvatinib may be the optimal treatment option for this patient population in approximately 59% of cases compared to only 1% of patients who received sorafenib therapy (58). The cost-utility analysis demonstrated that lenvatinib exhibited comparable clinical efficacy to sorafenib at a lower cost, suggesting that it may represent a cost-effective option for the treatment of u-HCC (59,60). The correlation between ACTB and objective radiological response was not established in this study. However, we observed a significant prolongation in recurrence time among adjuvant lenvatinib-treated ACTBLow patients. Additionally, our findings suggest that lenvatinib effectively suppresses the subsequent growth of ACTBLow PDXs rather than completely eradicating them. To minimize variations in tumor tissue characteristics, we endeavor to procure patient samples with consistent tissue structure types. Specifically, the HCC tissues under investigation exhibit a thick trabecular pattern and moderate differentiation. The aforementioned findings underscore the potential therapeutic efficacy of ACTB. Besides, the recurrence benefit observed in ACTBLow HCC patients may be attributed to lenvatinib’s ability to inhibit tumor growth and prevent HCC progression. Through Cox regression analysis, it was determined that Child-Pugh stage, BCLC and ACTB levels serve as independent risk factors, which have also been incorporated in this nomogram. Our proposed nomogram can efficiently predict the recurrence of HCC patients treated with lenvatinib post-surgery quite accurately.
There are certain limitations to the current study. Firstly, the decision-making process for Chinese patients regarding adjuvant treatment with lenvatinib in HCC setting is intricate. In addition to the doctor’s prescription, patient compliance is also influenced by various factors such as the cost of lenvatinib therapy. To be candid, this was an observational study, thus the participants were recruited retrospectively and there was limited information available regarding the specific reasons for patients’ receipt or non-receipt of lenvatinib treatment. Secondly, as lenvatinib has only been recently administered to HCC patients, we have solely obtained information on recurrence and not yet acquired data on OS of patients. Therefore, our study can only focus on the analysis of recurrence. Thirdly, the cohort consisted of patients with prior HBV infection while those with concurrent HCV infection were excluded from the study. Given the significant role of HCV in hepatocarcinogenesis, particularly in Western populations, it remains uncertain whether this biomarker is applicable to individuals of Western descent. Fourthly, the lenvatinib trial excluded patients who did not receive concurrent adjuvant therapy, and the control group was unable to select concurrent patients for comparison with the lenvatinib group. Finally, it should be noted that this study solely relies on a primary cohort and lacks a validation cohort, which may potentially introduce some limitations to the findings.
Conclusions
In summary, our study has demonstrated the predictive role of ACTB in identifying patients with high recurrence risk HCC who are likely to benefit from lenvatinib treatment. We propose that reduced levels of ACTB could serve as a novel and viable biomarker for personalized therapy with lenvatinib. To our knowledge, this is the first study to establish a correlation between a lenvatinib biomarker and its response to adjuvant therapy in early-stage HBV-related HCC. This has significant implications for personalized treatment with lenvatinib.
Acknowledgments
The authors would like to thank Zhenying Cao, Chao Yang and Anjie Tong in Department of Pathology, Eastern Hepatobiliary Surgery Hospital, the Second Military Medical University for their technical assistance.
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-942/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-942/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-942/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-942/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Eastern Hepatobiliary Surgery Hospital (No. EHBHKY2023-K036-P001) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345-62. [Crossref] [PubMed]
- Chen B, Wu JX, Cheng SH, et al. Phase 2 Study of Adjuvant Radiotherapy Following Narrow-Margin Hepatectomy in Patients With HCC. Hepatology 2021;74:2595-604. [Crossref] [PubMed]
- Liu SY, Yuan C, Tong XM. Antiviral therapy, HBsAg seroclearance and late recurrence of hepatitis B-related hepatocellular carcinoma. J Hepatol 2022;77:1471-2. [Crossref] [PubMed]
- Yoo S, Kim JY, Lim YS, et al. Impact of HBsAg seroclearance on late recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. J Hepatol 2022;77:939-46. [Crossref] [PubMed]
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-7. [Crossref] [PubMed]
- Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2019;154:209-17. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Ridola L, Bragazzi MC, Cardinale V, et al. Cholangiocytes: Cell transplantation. Biochim Biophys Acta Mol Basis Dis 2018;1864:1516-23. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Merle P. Lenvatinib applicability in the therapeutic strategy of patients with hepatocellular carcinoma. Hepatobiliary Surg Nutr 2023;12:248-51. [Crossref] [PubMed]
- Zou J, Huang P, Ge N, et al. Anti-PD-1 antibodies plus lenvatinib in patients with unresectable hepatocellular carcinoma who progressed on lenvatinib: a retrospective cohort study of real-world patients. J Gastrointest Oncol 2022;13:1898-906. [Crossref] [PubMed]
- Shigesawa T, Maehara O, Suda G, et al. Lenvatinib suppresses cancer stem-like cells in HCC by inhibiting FGFR1-3 signaling, but not FGFR4 signaling. Carcinogenesis 2021;42:58-69. [Crossref] [PubMed]
- Bai S, Hu L, Liu J, et al. Prognostic Nomograms Combined Adjuvant Lenvatinib for Hepatitis B Virus-related Hepatocellular Carcinoma With Microvascular Invasion After Radical Resection. Front Oncol 2022;12:919824. [Crossref] [PubMed]
- Zhou J, Sun HC, Huang ZY, et al. Adjuvant lenvatinib after radical resection in patients with hepatocellular carcinoma (HCC): Preliminary analysis of a prospective, multi-center, single-arm study. J Clin Oncol 2022;40:e16158. [Crossref]
- Zhang JS, Wang ZH, Guo XG, et al. A nomogram for predicting the risk of postoperative recurrence of hepatitis B virus-related hepatocellular carcinoma in patients with high preoperative serum glutamyl transpeptidase. J Gastrointest Oncol 2022;13:298-310. [Crossref] [PubMed]
- Zheng X, Ye B, Gou Y, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers to predict relapse and survival in posthepatectomy HBV-related hepatocellular carcinoma: a meta-analysis and preliminary immune perspective. Transl Cancer Res 2021;10:1261-72. [Crossref] [PubMed]
- Casco A, Gupta A, Hayes M, et al. Accurate Quantification of Overlapping Herpesvirus Transcripts from RNA Sequencing Data. J Virol 2022;96:e0163521. [Crossref] [PubMed]
- Sun W, Li SC, Xu L, et al. High FLT3 Levels May Predict Sorafenib Benefit in Hepatocellular Carcinoma. Clin Cancer Res 2020;26:4302-12. [Crossref] [PubMed]
- Gao C, Chang L, Xu T, et al. AKR1C1 overexpression leads to lenvatinib resistance in hepatocellular carcinoma. J Gastrointest Oncol 2023;14:1412-33. [Crossref] [PubMed]
- Ghosh T, Soni K, Scaria V, et al. MicroRNA-mediated up-regulation of an alternatively polyadenylated variant of the mouse cytoplasmic {beta}-actin gene. Nucleic Acids Res 2008;36:6318-32. [Crossref] [PubMed]
- Guo C, Liu S, Wang J, et al. ACTB in cancer. Clin Chim Acta 2013;417:39-44. [Crossref] [PubMed]
- Bunnell TM, Burbach BJ, Shimizu Y, et al. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol Biol Cell 2011;22:4047-58. [Crossref] [PubMed]
- Krzystek-Korpacka M, Diakowska D, Bania J, et al. Expression stability of common housekeeping genes is differently affected by bowel inflammation and cancer: implications for finding suitable normalizers for inflammatory bowel disease studies. Inflamm Bowel Dis 2014;20:1147-56. [Crossref] [PubMed]
- Aleshcheva G, Wehland M, Sahana J, et al. Moderate alterations of the cytoskeleton in human chondrocytes after short-term microgravity produced by parabolic flight maneuvers could be prevented by up-regulation of BMP-2 and SOX-9. FASEB J 2015;29:2303-14. [Crossref] [PubMed]
- Riwaldt S, Bauer J, Wehland M, et al. Pathways Regulating Spheroid Formation of Human Follicular Thyroid Cancer Cells under Simulated Microgravity Conditions: A Genetic Approach. Int J Mol Sci 2016;17:528. [Crossref] [PubMed]
- Liu K, Gao R, Wu H, et al. Single-cell analysis reveals metastatic cell heterogeneity in clear cell renal cell carcinoma. J Cell Mol Med 2021;25:4260-74. [Crossref] [PubMed]
- Latham SL, Ehmke N, Reinke PYA, et al. Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia. Nat Commun 2018;9:4250. [Crossref] [PubMed]
- Maehara O, Suda G, Natsuizaka M, et al. Fibroblast growth factor-2-mediated FGFR/Erk signaling supports maintenance of cancer stem-like cells in esophageal squamous cell carcinoma. Carcinogenesis 2017;38:1073-83. [Crossref] [PubMed]
- Huang G, Li PP, Lau WY, et al. Antiviral Therapy Reduces Hepatocellular Carcinoma Recurrence in Patients With Low HBV-DNA Levels: A Randomized Controlled Trial. Ann Surg 2018;268:943-54. [Crossref] [PubMed]
- Dong W, Yan K, Yu H, et al. Prognostic Nomogram for Sorafenib Benefit in Hepatitis B Virus-Related Hepatocellular Carcinoma After Partial Hepatectomy. Front Oncol 2021;10:605057. [Crossref] [PubMed]
- Dong W, Yu H, Zhu YY, et al. A Novel Pathological Scoring System for Hepatic Cirrhosis with Hepatocellular Carcinoma. Cancer Manag Res 2020;12:5537-47. [Crossref] [PubMed]
- Fang DD, Zhang B, Gu Q, et al. HIP1-ALK, a novel ALK fusion variant that responds to crizotinib. J Thorac Oncol 2014;9:285-94. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599-616. [Crossref] [PubMed]
- Peng Z, Fan W, Zhu B, et al. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol 2023;41:117-27. [Crossref] [PubMed]
- Yoo C, Kim JH, Ryu MH, et al. Clinical Outcomes with Multikinase Inhibitors after Progression on First-Line Atezolizumab plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma: A Multinational Multicenter Retrospective Study. Liver Cancer 2021;10:107-14. [Crossref] [PubMed]
- Yi C, Chen L, Lin Z, et al. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology 2021;74:2544-60. [Crossref] [PubMed]
- Chang X, Yu S, Pang J, et al. Population Sensitive to Lenvatinib Plus Anti-PD-1 for Unresectable Hepatocellular Carcinoma Infected with Hepatitis B Virus. J Hepatocell Carcinoma 2023;10:847-61. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010;59:953-62. [Crossref] [PubMed]
- Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 2014;6:18. [Crossref] [PubMed]
- Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol Res 2018;48:597-607. [Crossref] [PubMed]
- Schulze K, Gasch C, Staufer K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer 2013;133:2165-71. [Crossref] [PubMed]
- Lee TK, Castilho A, Cheung VC, et al. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 2011;9:50-63. [Crossref] [PubMed]
- Braoudaki M, Lambrou GI, Vougas K, et al. Protein biomarkers distinguish between high- and low-risk pediatric acute lymphoblastic leukemia in a tissue specific manner. J Hematol Oncol 2013;6:52. [Crossref] [PubMed]
- Maehara O, Sato F, Natsuizaka M, et al. A pivotal role of Krüppel-like factor 5 in regulation of cancer stem-like cells in hepatocellular carcinoma. Cancer Biol Ther 2015;16:1453-61. [Crossref] [PubMed]
- Shestakova EA, Wyckoff J, Jones J, et al. Correlation of beta-actin messenger RNA localization with metastatic potential in rat adenocarcinoma cell lines. Cancer Res 1999;59:1202-5. [PubMed]
- Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer 2010;126:2067-78. [Crossref] [PubMed]
- Li Y, Ma H, Shi C, et al. Mutant ACTB mRNA 3'-UTR promotes hepatocellular carcinoma development by regulating miR-1 and miR-29a. Cell Signal 2020;67:109479. [Crossref] [PubMed]
- Gao Q, Wang XY, Fan J, et al. Selection of reference genes for real-time PCR in human hepatocellular carcinoma tissues. J Cancer Res Clin Oncol 2008;134:979-86. [Crossref] [PubMed]
- Fu LY, Jia HL, Dong QZ, et al. Suitable reference genes for real-time PCR in human HBV-related hepatocellular carcinoma with different clinical prognoses. BMC Cancer 2009;9:49. [Crossref] [PubMed]
- Guo J, Zhao J, Xu Q, et al. Resistance of Lenvatinib in Hepatocellular Carcinoma. Curr Cancer Drug Targets 2022;22:865-78. [Crossref] [PubMed]
- Dai MG, Liu SY, Lu WF, et al. Survival Benefits From Adjuvant Lenvatinib for Patients With Hepatocellular Carcinoma and Microvascular Invasion After Curative Hepatectomy. Clin Med Insights Oncol 2023;17:11795549231180351. [Crossref] [PubMed]
- Revill PA, Tu T, Netter HJ, et al. The evolution and clinical impact of hepatitis B virus genome diversity. Nat Rev Gastroenterol Hepatol 2020;17:618-34. [Crossref] [PubMed]
- Tada T, Kumada T, Hiraoka A, et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol Res 2020;50:75-83. [Crossref] [PubMed]
- Chen Z, Xie H, Hu M, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 2020;10:2993-3036. [PubMed]
- Kobayashi M, Kudo M, Izumi N, et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol 2019;54:558-70. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]


