Immune checkpoint blockade induced sarcoid-like reaction mimicking progression of disease in a patient with microsatellite instable colorectal cancer: case report and review of the literature
Highlight box
Key findings
• Sarcoid-like reactions (SLRs) are uncommonly observed in patients receiving immune checkpoint blockade (ICB), and may be radiologically indistinguishable from progression of disease. If not recognized, SLRs may result in unnecessary treatment changes.
What is known and what is new?
• ICB may result in a variety of immune-related adverse events, including SLRs which are uncommon, and can generally be successfully managed with discontinuation of the immune checkpoint inhibitor.
• SLRs may be radiologically difficult to distinguish from progression of disease, but the index of suspicion should be high in patients who remain clinically well.
What is the implication, and what should change now?
• In patients who have received ICB, consideration of SLRs mimicking progression of disease on imaging should be considered, and worked up accordingly.
Introduction
The indications for the use of immune checkpoint blockade (ICB) in gastrointestinal malignancies is rapidly expanding, with tumor-specific indications across several gastrointestinal cancers, including colon, stomach, gallbladder, liver, and others, as well as tumor-agnostic approvals of immunotherapy in patients with microsatellite instable (MSI)-high tumors (1), and those with a tumor mutational burden (TMB) >10 mutations/megabase (2). We present the case of a young male patient who developed de novo metastatic MSI colon cancer to the liver, on a background of previously unknown Lynch syndrome. He developed a pulmonary sarcoid-like reaction (SLR) several months into treatment with pembrolizumab, which radiologically was indistinguishable from disease progression, and was proven by endobronchial ultrasound-guided lymph node biopsy. Excellent control of his malignancy was observed. After withholding pembrolizumab and observing ongoing disease stability for 8 months, the patient developed new parenchymal hepatic lesions and para-aortic adenopathy on imaging, concerning for progression of disease. Tissue biopsy of an inguinal lymph node again demonstrated the presence of non-necrotizing granulomatous inflammation, consistent with a SLR. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-435/rc).
Case presentation
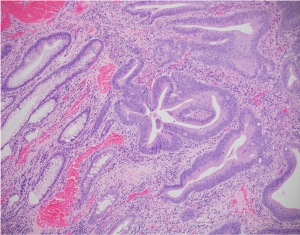
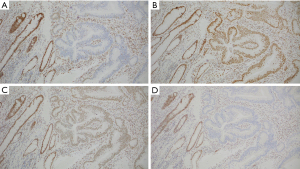
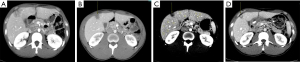
A previously well 26-year-old male, with an unremarkable past medical history, presented to the emergency room with abdominal pain, vomiting, and constipation for 5 days. Computed tomography (CT) scan demonstrated a high-grade large bowel obstruction due to a hepatic flexure mass, retroperitoneal lymphadenopathy, and numerous hepatic lesions consistent with metastases (Figure 1). Patient was taken emergently to the operating room where he underwent a right hemicolectomy and liver biopsy. Pathology from the hemicolectomy demonstrated a moderately differentiated adenocarcinoma of the hepatic flexure invading through the visceral peritoneum, and 6 of 40 lymph resected nodes notable for carcinoma (Figure 2). Liver biopsy confirmed the presence of metastatic adenocarcinoma of colorectal origin. Immunohistochemistry demonstrated deficient mismatch repair proteins, with loss of both MSH2 and MSH6 (Figure 3). Next-generation sequencing (NGS) with the MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets) multi-gene panel (3) confirmed MSI status, with a TMB of 50 mutations/megabase, with no alterations in KRAS or BRAF identified. The patient’s family history was significant for breast cancer in his mother in her 40s. The patient was unaware of any details of his paternal family history, including any history of malignancy. Germline testing demonstrated a pathogenic variant in MSH2 consistent with Lynch syndrome.
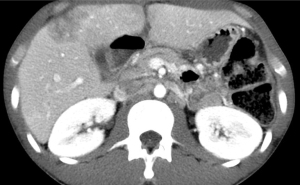
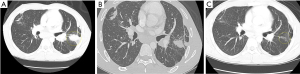
The patient recovered well from surgery and on the basis of bilobar liver metastases and retroperitoneal lymphadenopathy, he was commenced on front-line systemic therapy with single agent programmed cell death-1 (PD-1) inhibitor pembrolizumab, with a baseline carcinoembryonic antigen (CEA) of 25 ng/mL [upper limit of normal (ULN) =5 ng/mL]. After 8 weeks of systemic therapy his CEA had reduced to 6 ng/mL, and his performance status had improved to Eastern Cooperative Oncology Group (ECOG) 0. Restaging CT imaging demonstrated decreased liver metastases, an increase in the size of intra-abdominal lymph nodes, and a new left apical area of consolidation. Given his excellent clinical and CEA response, and in the absence of any respiratory symptoms pembrolizumab was continued, with a plan to closely observe these changes. Follow-up imaging after a further 10 weeks of pembrolizumab demonstrated ongoing disease response in the liver and reduced left apical consolidation, and pembrolizumab was continued. After a further 10 weeks of therapy imaging was repeated, with ongoing disease response in the liver and no further increase in intra-abdominal lymph nodes, but now demonstrating worsening left upper lobe consolidation, new and increasing pulmonary nodules, increased mediastinal and bilateral hilar lymph nodes of uncertain etiology. CEA remained stable at 5.9 ng/mL at this juncture. An endoscopic bronchial ultrasound was performed, and lymph node station 11L and station 7 sampling demonstrated non-necrotizing granulomatous inflammation, consistent with sarcoidosis (Figure 4). In the view of the patient’s treatment with ICB, and lack of personal or family history of autoimmune disease, the consensus was that the patient had developed a SLR secondary to pembrolizumab, and ICB therapy was withheld. As the patient remained very well and without any respiratory symptoms or exercise limitation, in consultation with the pulmonary service, the patient was kept under close surveillance and did not receive therapy for granulomatous disease.
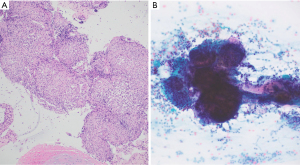
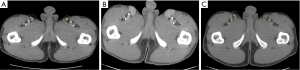
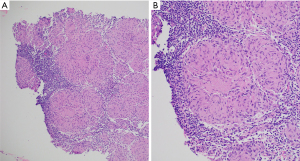
The patient remained well off all systemic therapy. His performance status remained excellent, and CEA remained stable between 5 and 6 ng/mL, and imaging demonstrated responding disease. He continued to have no respiratory symptoms, and restaging imaging showed ongoing disease response (Figure 5). However, after 8 months of surveillance off treatment, restaging imaging demonstrated new hypodense bi-lobar hepatic lesions (Figure 5), and increased bilateral supradiaphragmatic, abdominal and pelvic lymph nodes, with stable intrathoracic disease (Figures 6,7), concerning for possible progression of disease. The patient, however, remained asymptomatic, and his CEA remained stable. The case was discussed at our institutional multidisciplinary team meeting, and it was determined that based on the radiologic findings progression of disease was not distinguishable from an alternative diagnosis such as sarcoidosis, and the decision was made to pursue tissue biopsy of one of these sites of concern. Given its accessibility and safe access, a left inguinal lymph node was biopsied as a representative lesion, in place of pursuing a biopsy of the liver or other sites. Pathology demonstrated non-necrotizing granulomatous inflammation, consistent with sarcoidosis (Figure 8).
The patient is now 24 months since his last dose of ICB, and from the perspective of his malignancy we continue to observe a sustained clinical, radiological (Figures 5-7) and biochemical response, in the liver, despite no additional locoregional or systemic therapy. In addition, we observe radiological evidence of both pulmonary and extra-pulmonary SLR, however he remains clinically well and devoid of any respiratory or other symptoms, and has not required initiation of immunosuppressive therapy for the SLR.
All procedures performed in this study were in accordance with the ethical standards of the institution and/or national research committee(s), and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Pulmonary-related irAEs are described with ICB, with acute interstitial pneumonitis being the most acute and serious of the known pulmonary toxicities, however there is a growing appreciation for pulmonary irAEs beyond pneumonitis. To date, there have been fewer than 60 cases of sarcoidosis and SLRs attributable to checkpoint inhibitors in the literature. Those reported to date have most commonly been seen in patients with melanoma and non-small cell lung cancer, likely related to the frequency of ICB in these diseases, and they have been reported secondary to anti-PD-1, anti-programmed death-ligand 1 (PD-L1), and anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitors, as well as in combinations of anti-CTLA-4 and anti-PD-1 inhibitors (4).
The clinical presentation of a SLR is variable. The time between initiation of immunotherapy and onset of SLRs reported in previous reported cases is variable, with reports of early reactions in less than 1 month (5) and later onset up to 43 months (6). Intra-thoracic involvement with pulmonary or lymph node findings are the most frequently identified sites, other sites have been observed including skin (5,7), bone (8), and extra-thoracic adenopathy (6). As demonstrated in this case report, radiologically it can be near impossible to definitively distinguish SLRs from disease progression (9), and clinical correlation with symptoms or signs of disease progression, including tumor markers, may be of utility. Confirmation with tissue biopsy to evaluate the presence of granulomatous inflammatory changes, rather than active malignancy, is the definitive confirmatory test and should be considered if there is any ambiguity regarding the etiology of radiologic changes. Some published cases have highlighted situations where changes in systemic therapy were made due to interpretation of SLR radiologic changes as true progression of disease (10). Aside from progression of disease, other differential diagnoses include and other inflammatory processes (11), or potentially “nodal flare” which has been described in the neo-adjuvant setting (12), although the clinical index of suspicion for these processes was low in this case.
In general, international guidelines pertaining to the management of irAEs in many instances do not include algorithms specific to the management of SLRs associated with ICB. Based on the limited literature in this setting, there have been no reports to date of ICB-related SLRs which have been refractory to either discontinuation of the offending agent, or treatment with corticosteroids. In the case report described herein, following discontinuation of pembrolizumab, the disease course appeared to improve initially. However, 8 months after discontinuing pembrolizumab hepatic and intra-abdominal node involvement were identified, illustrating the waxing and waning course of a SLR, and furthermore demonstrating the often durable effects of ICB, even after a significant period off treatment after discontinuation for toxicity. In most reported cases, the SLR remained stable or improved after ICB discontinuation, and in most cases no further immunosuppressive therapy was commenced (4). Successful management with commencement of corticosteroids as the mainstay of treatment in irAEs remains the first-line treatment in patients who require treatment, however in some cases other immunosuppressive regimens were administered, namely combinations of hydroxychloroquine, methotrexate and infliximab (13-15). In a small minority of cases, the checkpoint inhibitor was continued (16). In the largest review of SLR cases to date, improvement or resolution was noted in 89% of cases, and the other 11% were stable, indicating that in general SLRs are manageable with multi-disciplinary team input to guide treatment (4).
Conclusions
In summary, we present herein in the case of a 26-year-old male with Lynch syndrome and metastatic MSI colon adenocarcinoma with an elevated TMB who received pembrolizumab and developed biopsy-proven pulmonary and extra-pulmonary sarcoidosis secondary to ICB, which masqueraded as progression of disease on imaging in both instances. The patient continues to have durable cancer control as well as stable SLR 24 months after discontinuation of ICB, and has not required immune suppressive therapy to manage this. This case highlights an important clinical conundrum underscoring the need for awareness and recognition of this irAE, and in particular, recognition of its propensity to mimic disease progression.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-435/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-435/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-435/coif). G.A.A. and E.M.O. both report research support from Agenus, Arcus, Astra Zeneca, BioNtech, BMS, Elicio, Genentech/Roche, Helsinn, Parker Institute, Pertzye, Puma, QED, Yiviva, and consulting support from Astellas, Astra Zeneca, Autem, Berry Genomics, BioNtech, Boehringer Ingelheim, BMS, Eisai, Exelixis, Fibriogen, Genentech/Roche, Helio, Incyte, Ipsen, Merck, Merus, Neogene, Newbridge, Novartis, QED, Servier, Tempus, Thetis, Vector, and Yiviva. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institution and/or national research committee(s), and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication
- U.S. Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. 2020. vailable online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors
- Mandelker D, Zhang L, Kemel Y, et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA 2017;318:825-35. [Crossref] [PubMed]
- Lin Y, Zhu W, Wu B, et al. Case Report: Hepatic Sarcoid-Like Reaction Associated With Checkpoint Inhibition in a NSCLC Patient and a Literature Review. Front Oncol 2022;12:824308. [Crossref] [PubMed]
- Hiraki T, Hatanaka M, Arimura A, et al. Granulomatous/sarcoid-like reactions in the setting of programmed cell death-1 inhibition: a potential mimic of disease recurrence. J Cutan Pathol 2020;47:154-60. [Crossref] [PubMed]
- Rodriguez EF, Lipson E, Suresh K, et al. Immune checkpoint blocker-related sarcoid-like granulomatous inflammation: a rare adverse event detected in lymph node aspiration cytology of patients treated for advanced malignant melanoma. Hum Pathol 2019;91:69-76. [Crossref] [PubMed]
- Ogawa T, Ishitsuka Y, Iwamoto K, et al. Programmed cell death 1 blockade-induced cutaneous sarcoid-like epithelioid granulomas in advanced melanoma: a case report. J Eur Acad Dermatol Venereol 2018;32:e260-1. [Crossref] [PubMed]
- Keukeleire S, Schwarze J, Awada G, et al. An atypical sarcoid-like reaction during anti-protein death 1 treatment in a patient with metastatic melanoma. Melanoma Res 2020;30:524-7. [Crossref] [PubMed]
- Garanzini EM, Scaramuzza D, Spadarella G, et al. Sarcoidosis-like disease mimicking metastases during adjuvant ipilimumab therapy in advanced melanoma patient: CT scan and MRI help in managing difficult clinical decision. BJR Case Rep 2020;6:20190065. [Crossref] [PubMed]
- Reddy SB, Possick JD, Kluger HM, et al. Sarcoidosis Following Anti-PD-1 and Anti-CTLA-4 Therapy for Metastatic Melanoma. J Immunother 2017;40:307-11. [Crossref] [PubMed]
- Gary PJ, Egan A. Sarcoid-like reaction after treatment with pembrolizumab. Chest 2022;162:A1669. [Crossref]
- Sirgi Y, Krochmal R, Fleury CM, et al. Pembrolizumab-Associated Cutaneous and Pulmonary Sarcoidosis in Non-Small Cell Lung Cancer Treatment. Clin Lung Cancer 2022;23:542-6. [Crossref] [PubMed]
- Tan I, Malinzak M, Salama AKS. Delayed onset of neurosarcoidosis after concurrent ipilimumab/nivolumab therapy. J Immunother Cancer 2018;6:77. [Crossref] [PubMed]
- Dunn-Pirio AM, Shah S, Eckstein C. Neurosarcoidosis following Immune Checkpoint Inhibition. Case Rep Oncol 2018;11:521-6. [Crossref] [PubMed]
- Kim C, Gao J, Shannon VR, et al. Systemic sarcoidosis first manifesting in a tattoo in the setting of immune checkpoint inhibition. BMJ Case Rep 2016;2016:bcr2016216217. [Crossref] [PubMed]
- Lainez S, Tissot C, Cottier M, et al. EBUS-TBNA Can Distinguish Sarcoid-Like Side Effect of Nivolumab Treatment from Tumor Progression in Non-Small Cell Lung Cancer. Respiration 2017;94:518-21. [Crossref] [PubMed]