Usefulness and feasibility of endoscopic submucosal dissection for colorectal tumor: a nationwide multicenter retrospective study in Korea
Introduction
Early superficial gastrointestinal cancer is defined as cancer invasion limited to mucosal or submucosal layers. Until recently surgery was the cornerstone treatment for superficial/early gastrointestinal tumors. Diagnosis rate of precursor lesion of colorectal cancer and early stage colorectal cancer have been increasing rapidly because of increased screening gastrointestinal endoscopies in Korea (1). This trend has also resulted in advances in the therapeutic endoscopy, that is less invasive than surgery. More recently, endoscopic submucosal dissection (ESD) is feasible method in treating early gastric cancer (2).This technique enables en bloc resection of larger than 20mm, leading to exact histologic evaluation of the specimen and low recurrence rate (3-5).However, this technique in colorectal area is not widely used because of technical difficulty, a protracted procedure time, and the risk of complications accompanying the procedure such as perforation, bleeding (4). In Korea, colorectal ESD is performed as actively as in Japan. Although there are some single-center studies about feasibility of colorectal ESD came from Japanese experts (4,6-9), nationwide multicenter study for effectiveness and feasibility of colorectal ESD is still limited. The aim of this study is to investigate the usefulness and feasibility of ESD in colorectum performed by various experienced endoscopists from multiple university hospitals in Korea.
Methods
ESD database performed for colorectal neoplasia from January 2009 to October 2015 at six university hospitals in Korea were investigated retrospectively under IRB approval.
All the lesions that were node-negative cancer or premalignant neoplasia and were technically unsuitable for en bloc resection by conventional endoscopic mucosal resection (EMR) were considered suitable for ESD. We investigated medical records, including demographic, clinical, technical data, and data of immediate complications. The rates of en bloc resection, the incidence of complications, and procedure times were analyzed. The factors analyzed in relation to procedure complications were the tumor location and size, and the final histological data (tumor type and stage).
Endoscopic ultrasound and/or computed tomography (CT) scan examinations were carried out before treatment whenever they were considered to be useful, particularly in cases of cancer. CT scans were carried out in cancer patients (n=47) and carcinoid patients (n=25). EUS were carried out in only over 1cm sized carcinoid tumor (n=8). All patients gave informed consent before undergoing ESD.
ESD
A single-channel video colonoscope (CF type Q260AI with variable hardness; Olympus Optical Co, Ltd, Tokyo, Japan) usually with air rather than CO2 insufflation was used in this study. Transparent hood was attached to the tip of endoscopy to apply tension to the submucosal layer to enable easy entry of the endoscope into the submucosal layer and to stabilize knife handling during dissection. Peripheral marking using argon plasma coagulation was done after identification of the lesion with chromoendoscopy with indigo carmine.
Glycerol mixture solution (10% glycerol 15 mL + 1% hyaluronic acid 5 mL + 1% epinephrine 0.2 mL + 0.4% indigo carmine 0.2 mL) injected to submucosal layer to elevate the lesion from muscle layer. Flex-knife or Dual-knife was used for circumferential incision, and then submucosal dissection was performed with a variety of knives, including: Flex-knife, IT-knife, Dual-knife, Hook-knife (all Olympus Corp., Tokyo, Japan). Mucosal incision was done with the endocut mode (e.g., endocut I, effect 2, duration 3, interval 2 in VIO300D), and then submucosal dissection was done with coagulation mode (e.g., Forced coagulation, Output 40W, effect 2 in VIO300D). During ESD, either various knives used for dissection or hemostatic instrument such as Coagrasper (Olympus Corp., Tokyo, Japan) was used for hemostasis. If perforation was developed during procedure, hemoclip was applied. After the ESD, preventive endoscopic hemostasis was undertaken when deemed useful for any oozing or exposed vessel by using the same instruments as used during intra-operative hemostasis. If there was lateral resection involvement or deep resection margin involvement, the additional resection were made in later sitting.
Statistical analysis
Differences in categorical variables were evaluated by Fisher’s exact test or chi-square test. For comparison of continuous variables, Mann-Whitney U test were used. Univariate analysis was performed to analyze factors (location, tumor size) related short term clinical outcomes (En bloc resection, complication, procedure time). Multivariate analysis was performed using a logistic regression model [En bloc resection, location of tumor (Other colon), Macroscopic type (protruding), ESD only]. Odds ratios and 95% confidence intervals were calculated to evaluate predictors of perforation. P value of <0.05 were considered statistically significant.
Results
Clinicopathological features
During the study period, a total of 189 patients were included with 191 lesions resected by ESD. The mean age of the patients was 61.1±11.8 years, and the male/female ratio was 1.62 (118/73). The mean size of the tumors was 21.1±10.4 mm. The majority of the lesions were located in rectum (n=88, 46.1%). The others were located in right colon (n=45, 23.6%), sigmoid colon (n=33, 17.3%), transverse colon (n=17, 8.9%), and descending colon (n=8, 4.2%). In 46 patients (24.1%) the indication for ESD was adenocarcinoma. Data on tumor location and size, indication for ESD, histological type, and tumor infiltration are presented in Table 1.
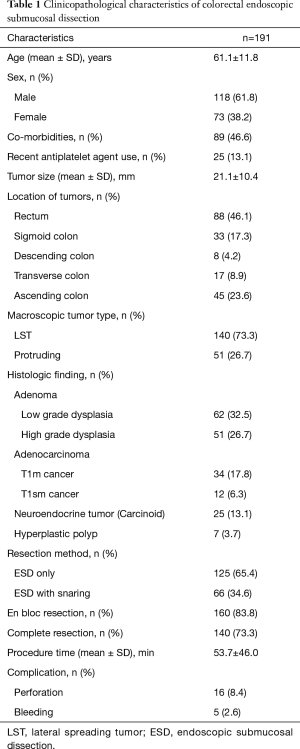
Full table
Curability
En bloc resection was achieved for 160 of the 191 lesions (83.8%). The lesions for which en bloc resection could not be achieved located in right colon/transverse colon 17/62 (27.4%) vs. other location 14/129 (10.9%) (Tables 2,3). The final pathology results showed low grade dysplasia in 32.5% (62/191), followed by high grade dysplasia in 26.7% (51/191), adenocarcinoma in 24.1% (46/191), neuroendocrine tumor in 13.1% (25/191). The overall complete resection rate was 73.3% (140/191) (Table 4). Lateral resection involvement was 22.0% (42/191), managed by additional resection or ablation with argon plasma coagulation. Deep resection margin involvement was 4.2% (8/191), two are cancers, others are carcinoids.
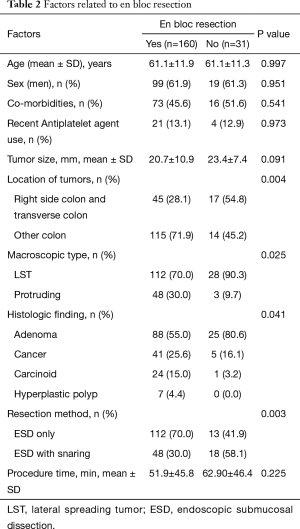
Full table
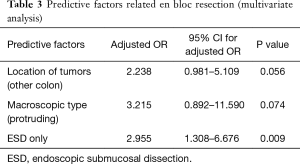
Full table
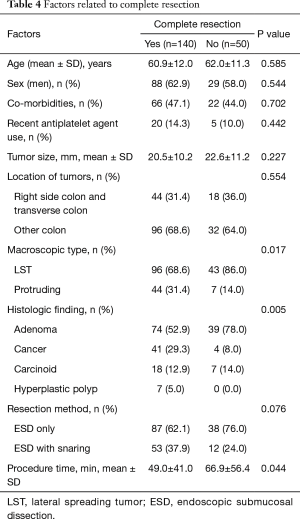
Full table
Procedure time
The median procedure time was 53 minutes, with a mean of 53.7±46.0 minutes. Small lesions (<20 mm) required less time for resection compared with larger ones (>20 mm) (mean 35.7±22.6 vs. 94.8±66.2 minutes; P<0.0001). Also, complicated ESDs had a significantly longer procedure time than uncomplicated ESDs (mean 96.8±57.6 vs. 48.7±41.8 minutes; P=0.002).
Complication
There were no deaths in patients who were enrolled this study. We assessed predictive factor of perforation using variables as tumor location, tumor size, procedure time. Perforation were observed in 16 of 191 cases (8.4%). All perforations were treated by endoscopic hemoclipping during initial ESD procedures. Eleven out of 16 cases of perforation needed surgical intervention due to ineffective clipping. Two cancer cases were underwent segment. 9 high grade dysplasia or low grade dysplasia cases underwent primary suture. The other 5 perforations (31.3%) were treated supportively, without surgical treatment, by NPO and giving antibiotics intravenously, with favorable outcomes (Table 5).
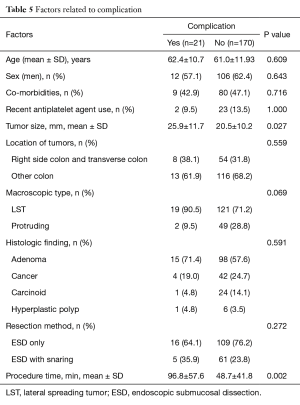
Full table
Significant ESD-related bleeding needed follow up endoscopy and endoscopic hemostasis involving endoclipping and electrocautery occurred in 5 cases (2.6%). No case required surgical intervention for post-ESD bleeding.
Factors related to short term clinical outcomes
The relationship among tumor location, tumor size, en bloc resection rate, complication rate, and procedure time was investigated. Multivariate logistic regression analysis was performed to evaluate predictive factor in en bloc resection. The analysis showed that proximal location was possible predictive factor (OR: 2.238, 95% CI: 0.981–5.109, P=0.056).
Discussion
Development of endoscopic technology and skill enables minimally invasive strategy in managing a superficial large gastrointestinal tumor. ESD is the one of the minimally invasive treatment for gastrointestinal tumor. ESD technique has higher en bloc resection rate than conventional EMR methods, facilitates accurate histopathological evaluation and reduces tumor recurrences (10,11). ESD has recently been reported to be useful and safe in the treatment of large superficial gastrointestinal tumor in Japan, because it provides a higher en bloc resection rate and is less invasive than surgical resection (7,11-13). However, ESD in colorectal tumor has not been widely performed because technical difficulty of colon ESD is very high because of characteristic of the colon (thin wall and existence of peristalsis, fold, flexion, fecal fluid) and the risk of complication such as perforation is higher than stomach. Although there were several single center studies about ESDs for colorectal neoplasm in Korea, this is the first nationwide Korean multicenter study for colon ESD.
En bloc resection rate in colon ESD has been reported about above 90% in Japan (14-16), 70% in Europe (17). In our multicenter study, the en bloc resection rate was 83.8%, similar to Japanese study. En bloc resection rate was lower in right colon and transverse colon than other colon location. It may result from operational difficulty in proximal colon. However, perforation rate is slightly higher than previous study in Japan.
The bleeding rate of 2.6%, was similar to most published series (3,7). We performed hemostasis on all vessels likely to bleed or actively bleeding, regardless of the location, if we could possibly do so, as it has already been shown that preventive coagulation of visible vessels in the resection area after ESD might decrease bleeding (18). All cases were successfully managed by conservative medical treatment with no need for surgery, and it can be considered a minor matter of concern that is likely to improve with experience.
The perforation is a trappy complication, especially in colon ESD, even in the hands of an expert endoscopist. Our study showed that perforation rate was 8.4%, slightly higher than previous study (3,7). Reluctance to perform colon ESD results from mostly perforation which may need emergent surgery. However, iatrogenic perforation during ESD procedure can be managed by endoscopic intervention and conservative management including fasting and antibiotics. Fujimoto reported that nonsurgical method including immediate endoclip closure and conservative strategy during ESD is feasible option treating iatrogenic perforation. In our study, 16 iatrogenic perforation was developed during ESD and 5 perforations were managed successfully by endoscopic intervention and conservative management while 11 case needed emergent surgery. Tumor location was an important factor predicting perforation during ESD in our study. In ESD at proximal colon, we should perform ESD carefully paying attention to perforation.
ESD procedure time is one important drawback of ESD. The procedure time in ESD is usually longer than conventional EMR, especially in colon. In our study, median procedure time was 53 minutes, shorter than European group, 105 minutes (17). We found that patient that take longer procedure time in ESD had more complication and large lesion tended to take longer time.
In summary, ESD as a new method shows promise or potential as a useful, potentially feasible procedure in colorectal superficial tumor because of high en bloc resection rate and low short term morbidity rate, especially in small lesions located from descending colon to rectum. However, as the study by Fujiya et al., in large lesion of proximal colon, the ESD procedure was longer, and the rate of additional surgery and perforation was higher, suggesting that indications for ESD should be rigorously determined in order to avoid such problems (19). In case of early colorectal cancer, it would be better to consider ESD prior to operation since ESD is less invasive than operation. Long-term outcome remains to be elucidated by a large-scale, prospective study.
Acknowledgements
The authors would like to acknowledge the work of Kyo Sang Yoo of the Department of Internal Medicine, Guri Hanyang University Hospital, Hanyang University College of Medicine, Guri, Korea; Min Ho Choi of the Department of Internal medicine, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea; Woon Geon Shin of the Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea; Yeon Soo Kim of the Department of Internal Medicine, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea; Sung Won Jung and Jin Bae Kim of the Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: ESD database performed for colorectal neoplasia from January 2009 to October 2015 at six university hospitals in Korea were investigated retrospectively under IRB approval and written informed consent was obtained from all patients.
References
- Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993-2007) in Korea. Seoul: Ministry for Health, Welfare and Family Affairs, 2009.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006;63:243-9. [Crossref] [PubMed]
- Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 2008;43:641-51. [Crossref] [PubMed]
- Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 2003;35:690-4. [Crossref] [PubMed]
- Hisabe T, Nagahama T, Hirai F, et al. Clinical outcomes of 200 colorectal endoscopic submucosal dissections. Dig Endosc 2012;24 Suppl 1:105-9. [Crossref] [PubMed]
- Saito Y, Uraoka T, Matsuda T, et al. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc 2007;66:966-73. [Crossref] [PubMed]
- Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007;66:100-7. [Crossref] [PubMed]
- Tanaka S, Terasaki M, Kanao H, et al. Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc 2012;24 Suppl 1:73-9. [Crossref] [PubMed]
- Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331-6. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010;24:343-52. [Crossref] [PubMed]
- Wang J, Zhang XH, Ge J, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol 2014;20:8282-7. [Crossref] [PubMed]
- Onozato Y, Ishihara H, Iizuka H, et al. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy 2006;38:980-6. [Crossref] [PubMed]
- Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol 2005;3:S71-3. [Crossref] [PubMed]
- Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 2006;38:987-90. [Crossref] [PubMed]
- Farhat S, Chaussade S, Ponchon T, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011;43:664-70. [Crossref] [PubMed]
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy 2008;40:179-83. [Crossref] [PubMed]
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015;81:583-95. [Crossref] [PubMed]


