Beyond RAS and BRAF: a target rich disease that is ripe for picking
Introduction
Despite the implementation of extensive population-wide screening and significant advancements in the understanding and treatment of colorectal cancer in recent decades, colorectal cancer remains the second-leading cause of cancer death in the United States. The American Cancer Society estimates that there will be 134,490 new cases of colorectal cancer and 49,190 deaths from colorectal cancer in 2016 (1). While the approach to early-stage colorectal cancer has been generally stable over many years, the management of metastatic colorectal cancer is growing increasingly complex and more personalized. In the last two decades, the FDA has approved ten new agents (irinotecan, oxaliplatin, capecitabine, bevacizumab, cetuximab, panitumumab, aflibercept, ramucirumab, regorafenib, TAS-102) for the treatment of metastatic colorectal cancer. However, despite rapidly accumulating data on the molecular profiles of colorectal cancer and the identification of dozens of potential targets for therapy which should eventually personalize treatment, the current treatment paradigm utilizes only rat sarcoma virus (RAS) mutational status to guide therapy (2).
RAS/rapidly growing fibrosarcoma (RAF)
RAS and RAF are both downstream effectors of many receptor tyrosine kinases, including the epidermal growth factor receptor (EGFR), in the RAS/RAF/[mitogen activated protein kinase (MAPK)/ERK kinase (MEK)]/extracellular-signal-related kinase (ERK) pathway, which ultimately promotes cell survival and proliferation (Figure 1). RAS encodes a family of membrane-bound small GTPases and includes Harvey-Ras (HRAS), neuroblastoma-Ras (NRAS), and the most well known, Kirsten-Ras (KRAS). Once mutated, RAS frequently becomes immune to GTPase-activating proteins (GAPs) and, in a constitutively active state, promotes tumorigenesis and proliferation via its many downstream effectors (3). RAS is mutated in approximately 30% of all tumor types, including cancers of the colon, pancreas, lung, and more (4-6). It was first implicated in the development in colorectal cancer when it was described by Vogelstein et al. in 1988. After evaluating 92 colon cancers, the authors found that 47% contained a RAS mutation (7). Attempts to target RAS specifically by a variety of mechanisms have generally failed in preclinical research. Mutation status is currently used clinically after trials of EGFR inhibition have convincingly shown that benefit exists only for patients with wild-type RAS (8,9).
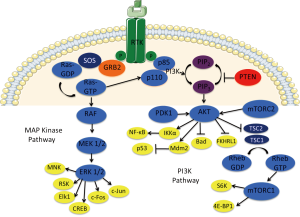
BRAF, the most well known in the RAF family which also includes ARAF and CRAF, is mutated in 5–10% of colorectal cancers. Like RAS, it promotes tumorigenesis through constitutive activation of the MAP kinase pathway. While resistance to EGFR in BRAF-mutant colorectal cancer has also been demonstrated, it is currently most valuable as a prognostic indicator (10,11). Independent of response to EGFR inhibition, it has been shown that BRAF mutations are associated with a more aggressive phenotype of colorectal cancer for which the prognosis is quite dismal, with a median survival of just 10.4 months versus the 34.7 months seen in metastatic colorectal cancer patients with wild-type BRAF (12,13). This is especially discouraging given that vemurafenib, a BRAF inhibitor which has shown such impressive results in BRAF-mutant melanoma and other malignancies, appears to be ineffective in colorectal cancer patients with the very same BRAF mutation (14-16).
It appears that redundancy in signaling and alternative pathways which circumvent targeted inhibitors are to blame for much of the resistance encountered so far with targeting EGFR and mutant BRAF (17-19). In particular, multiple studies have shown an apparent feedback up-regulation and activation of EGFR in the presence of BRAF mutation and treatment with vemurafenib (14). Though neither EGFR nor BRAF inhibition is itself active in BRAF-mutant colorectal cancer, they appear to have a synergistic and potent antitumor effect when used together (14). Another proposed mechanism for resistance to BRAF inhibition is the hypermethylated phenotype seen in BRAF-mutant colorectal cancer, supported by the finding that inhibition of methyltransferase augmented the antitumor activity of vemurafenib in colon cancer (20).
Lack of success with targeted therapies in the RAS and BRAF mutant population has generated a great deal of research into other targets and combinations of targets, within and outside of this intricate signaling pathway, in order to improve outcomes for patients with metastatic colorectal cancer.
Beyond RAS and RAF
MAPK
With direct inhibition of mutated RAS proving to be challenging, much attention has been directed at the direct downstream effectors. The MAPK/ERK kinase (MEK1/2) is tyrosine (Y-) and S/T-dual specificity protein kinases. They are rarely mutated but are constitutively active secondary to frequently mutated upstream activators.
There has been one FDA approved MEK inhibitor, trametinib, for unresectable BRAF mutant melanoma (21). MEK inhibition in combination with docetaxel has been studied in non-small cell lung cancer in another phase II trial which did show promising efficacy (22). Trametinib also has demonstrated activity in in KRAS-mutant melanoma, pancreatic, and non-small cell lung cancer patients in a phase I trial (23). Unfortunately this trial included 28 patients with colorectal cancer, none of whom had any objective response to MEK inhibitor monotherapy (23).
The concept of synthetic lethality, or two gene mutations leading to cell death when a mutation in one is viable, has driven a large amount of research with MEK inhibitors in combination regimens. Several preclinical trials, mostly in RAS-mutant cancers of many types, have screened thousands of genes and gene products for a therapy to pair with MEK inhibition and overcome resistance to it.
Using short hairpin RNA (shRNA) screening, Barbie et al. identified a synthetically lethal partner of mutated KRAS. TANK-binding kinase 1 (TBK1) is a tyrosine kinase downstream of RAS/RAL and regulates cell growth in KRAS-mutant cells via chemokine (C-C motif) 5 (CCL5) and interleukin-6 (24). An inhibitor of TBK1, momelotinib, is active in KRAS-mutant lung cancers when combined with MEK inhibition and the combination is currently in early clinical trials (NCT02258607).
Corcoran et al. also employed this strategy when they developed a pooled shRNA-drug screen to evaluate 1,200 genes that, when targeted, acted with the MEK inhibitor selumetinib to inhibit growth in KRAS-mutant cancers. B-cell lymphoma extra large (BCL-XL), a gene in the anti-apoptotic Bcl-2 homology (BH3) family, emerged as a prime target and studies using BCL-XL inhibitor ABT-263 in combination with MEK inhibition led to marked tumor regression in KRAS-mutant patient-derived xenografts and lung cancer mouse models (25).
Specifically studying KRAS-driven colorectal cancer, Spreafico et al. similarly evaluated the Wnt pathway as it was identified in synthetic lethality screens as possible mediator of MEK inhibitor resistance. Using shRNA to knock down parts of the Wnt signaling pathway, in combination with selumetinib, synergistic anti-tumor effects were observed, including tumor regression seen in patient-derived xenografts (26).
In a study evaluating resistance to vemurafenib in BRAF-mutant colorectal cancer, Corcoran et al. found that after an initial decrease in phosphorylated ERK (p-ERK) with vemurafenib, levels began to normalize again over 24 hours, suggesting reactivation of the MAP kinase pathway as a mechanism for vemurafenib resistance (27). Consistent with this finding, MEK inhibition has found impressive success in BRAF-mutant melanoma in combination with vemurafenib (28). In a recently reported trial in BRAF-mutant colorectal cancer, combination therapy with BRAF-inhibitor dabrafenib and trametinib did demonstrate decreased p-ERK levels and improve survival, though only modestly when compared to the results seen in melanoma (29).
Unfortunately responses to combination therapy with MEK and either EGFR or BRAF blockade have been susceptible to acquired resistance. A recent study used whole-exome sequencing before and after treatment with such combinations, and it appears that this acquired resistance is related to genetic alterations in the MAP kinase pathway including mutation of RAS and amplification of RAS and RAF (30). None of these resistance mechanisms were able to overcome treatment with ERK inhibition, which is prompting further investigation of ERK inhibitors for use in combination regimens (30). Recently the results of the first-in-class phase I study of the novel oral ERK 1/2 kinase inhibitor BVD-523 (ulixertinib) in patients with advance solid tumors has been reported. This study, in which 5 of 16 patients evaluated by PET had a metabolic response and 7 of 26 total patients had stable disease for at least 3 months, will lead to further investigation in clinical trials (31).
Phosphoinositide 3 kinase (PI3K)/protein kinase B (AKT)/PTEN
In parallel to the RAF-MEK-ERK pathway downstream of RAS is another survival-promoting pathway, PI3K-AKT-mechanistic target of rapamycin (mTOR), which has been implicated as a culprit in resistance to vemurafenib and offers an attractive therapeutic target. RAS activates PI3K, which then mediates the activity of Akt, leading to inhibition of apoptosis. Another part of this pathway is PTEN, which acts to inhibit Akt’s promotion of cell survival (Figure 1). Activation of PI3K/Akt secondary to loss-of-function PTEN mutation is known to be tumorigenic though has not shown any clinical predictive or prognostic utility as of yet (20,32,33). PI3K mutations, while not yet routinely targeted, are well known for conferring a survival benefit with aspirin therapy in colorectal cancer as inhibition of cyclooxygenase-2 attenuates PI3K activity (34).
In multiple trials evaluating direct PI3K inhibition in a variety of human cancer cell lines, RAS wild-type appears to be necessary for anti-tumor effect (35,36). It appears that the resistance to PIK3 inhibition in the setting of mutant RAS is related, again, to its ability to up-regulate alternative signaling pathways (35,37). This theory has been tested and confirmed in multiple preclinical trials in which combination therapy with PI3K and MEK inhibition had potent anti-tumor effects in murine lung and pancreatic models (38,39). A preclinical study using human lung and colon cancer cell lines demonstrated activity with dual inhibition of Akt/mTOR and MEK, and a recently reported phase I trial in advanced solid tumors including KRAS-mutant colorectal cancer suggested activity with a combination of MEK inhibitor pimasertib and dual PI3K/mTOR inhibitor SAR245409 (37,40).
Similar combination strategies have also shown promise in the BRAF-mutant colorectal cancer population. Yang et al. confirmed that increased PI3K activity is seen in BRAF-mutant CRC and seemed to be a driver for resistance to vemurafenib (32). Mao et al. studied PIK3 activation in both BRAF-mutant colorectal and melanoma and found that the PIK3 pathway was relatively more active in CRC, likely related to hypermethylation of PTEN promoter regions leading to PTEN downregulation (20). This increased activity from mutations in PIK3 or PTEN loss-of-function or down-regulation was associated resistance to vemurafenib. In both trials, inhibition of PI3K/AKT with either an AKT or PI3K inhibitor in addition to vemurafenib led to synergistic anti-tumor effects in BRAF-mutant CRC cell lines (20,32). Mao et al. also found increased anti-tumor activity with a methyltransferase inhibitor in addition to vemurafenib, strengthening the argument for hypermethylation and resultant PTEN activity loss conferring vemurafenib resistance (20).
HER2
HER2 is a member of the EGFR family of receptors that acts as an important driver of cancer and is a target of various therapeutics. This transmembrane tyrosine kinase receptor has no known natural ligand but instead functions through dimerization with other members of the HER, or EGFR, family to activate multiple cell survival pathways (41-44). Her2 structurally differs from other EGFR family members in that it has a fixed conformation that exposes the dimerization loop domain in an activated position allowing for regular heterodimerization (45).
Through amplification, not receptor mutation, HER2 is partially responsible for the progression from normal epithelia into invasive cancer in both breast and gastric cancer, and under exploration in additional tumor types such as urothelial cancers. Thus, this receptor is a target of many small molecule inhibitors both approved and in development. Approximately one third of breast tumors overexpress HER2 and when treated with the monoclonal antibody, trastuzumab, patients have an improved overall survival compared to standard therapy (46,47). The agent was approved in 1998 and is used in both the adjuvant and metastatic setting. It is also approved for the treatment of advanced HER2 overexpressing gastric cancer based on an overall survival benefit when given in combination with chemotherapy (48). Lapatanib is a small molecule inhibitor of the HER2 receptor and EGFR, which improved survival when used in combination with chemotherapy for the treatment of metastatic HER2 overexpressing breast cancer and in combination with trastuzumab (49-51).
Her2 amplification has been described in metastatic colorectal cancer, though the prevalence of 5% is significantly less than the described rates in breast and gastric cancer (52). Her2 amplification in colorectal cancer has been shown to associate with resistance to anti-EGFR monoclonal antibodies, and preclinical studies suggested that Her2 amplification may predict response to combined therapy with trastuzumab and lapatinib (53,54). A small phase II study (HERACLES) of trastuzumab and lapatinib in HER2 amplified in patients with refractory metastatic colorectal cancer met its primary endpoint with an overall response rate of 33.3% and several patients showing prolonged stable disease (55). Studies are currently being developed to test this target further in colon cancer.
Immunotherapy
The idea of harnessing the immune system to overcome RAS-mutant cancers is an old one but has been slow to develop. Decades ago researchers were able prepare vaccines against oncogenic RAS proteins capable of inducing a T-cell response in mice and human pancreatic cancer patients, though unfortunately this did not lead to a tumor response (56-58). This approach has also been tested in the adjuvant setting in resected pancreatic and colorectal cancers and has been well-tolerated with improved 10-year survival in one small study of just pancreatic cancer (59,60).
Much of the focus recently on immunotherapy has been on checkpoint inhibitors. There has been particular interest in targeting programmed death 1 (PD-1) and its ligand (PD-L1) which, when activated, serve to modulate the activity of T-cells and, as a result, decrease the immune response to a tumor. As with BRAF inhibition, melanoma has served as a model malignancy for this type of targeting, showing impressive tumor responses and now widespread of the use of PD-1 and PD-L1 agents (61,62).
In a familiar narrative, colorectal cancer treatment has unfortunately not yet showed the same promise in this strategy. In a phase 1 trial of PD-L1 inhibitor MDX-1106 in patients with multiple cancer types, only 1 of 33 patients with colorectal cancer exhibited a response, though this patient achieved a complete response durable to 21 months (63).
It was hypothesized that this single patient’s response was because of their mismatch-repair deficiency, something that is seen in approximately 5% patients with advanced colorectal cancer (39,64). Hereditary or acquired errors in mismatch repair are associated with microsatellite instability, yielding a phenotype of colorectal cancer that has a favorable prognosis in early-stage disease but does not confer an advantage in advanced disease (65,66). MSI-H cancers have orders of magnitude more somatic mutations and as a result should have more immunogenic potential for the patient’s immune system to recognize and attack (2,67,68).
Le et al. tested this hypothesis recently by treating 41 metastatic carcinoma patients, including colorectal cancer patients with and without deficient mismatch repair, as well as non-colorectal cancer patients with deficient mismatch repair, with PD-1 inhibitor pembrolizumab. The data demonstrated virtually no response to therapy in the cohort of patients with normal mismatch repair and significantly improved overall and progression-free survival, as well as increased tumor regression by RECIST and serum tumor markers, in the mismatch repair deficient cohorts (69). These data present a possible new treatment option in the near future for the MSI-H subset of metastatic colorectal cancer patients.
Conclusions
With the publication of the comprehensive metabolic characterization of human colon cancer in 2012, understanding of the molecular pathogenesis of colorectal cancer grew immensely (2). So, too, did the list of potential therapeutic targets. Despite this wealth of potential targets, routine molecular profiling currently includes just a few mutations, with only mutant RAS guiding therapy decisions.
RAS and RAF are centrally located in a complex network of signaling pathways from which many potential targets originate. Immediately downstream lies the remainder of the MAP kinase pathway, which has been the subject of many targeting studies. MEK inhibitors are already approved for use in other cancers and have shown some promising results when used in combination with other targeted therapies such as EGFR or BRAF inhibitors for colorectal cancer. Eventual acquired resistance to these has been an issue but may be overcome with ERK inhibition.
Similarly, the PI3K/AKT pathway has been implicated in resistance to certain targeted therapies in colorectal cancer. It appears that targeting this pathway in combination with BRAF or MEK inhibition in BRAF-mutant CRC is worthy of further investigation.
With EGFR inhibitors already in use for patients lacking mutations in RAS or BRAF, other receptor tyrosine kinases have been evaluated as potential targets, such as HER2. Though clinical data in colorectal cancer has been limited, research is ongoing in identifying patients who may benefit from inhibition of this receptor.
In addition to the aforementioned targets, countless more exist as others continue to emerge. Next-generation sequencing has led to the identification of more rare mutations in colorectal cancer, such as NTRK, ALK, and ROS1 (70,71). While these mutations are found in only 1–2% of the colorectal cancer population, they will certainly be the subject of future targeted trials.
In the era of personalized, genome-driven cancer therapy, the current treatment of metastatic colorectal cancer remains relatively simple and empiric. Fortunately as the molecular understanding of tumorigenesis and therapy resistance has grown, the field of colorectal cancer treatment stands on the brink of significant advances in the near future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol 2008;9:517-31. [Crossref] [PubMed]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003;3:11-22. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Singh H, Longo DL, Chabner BA. Improving Prospects for Targeting RAS. J Clin Oncol 2015;33:3650-9. [Crossref] [PubMed]
- Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525-32. [Crossref] [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [Crossref] [PubMed]
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. [Crossref] [PubMed]
- Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol 2013;14:749-59. [Crossref] [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [Crossref] [PubMed]
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623-32. [Crossref] [PubMed]
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98-9. [Crossref] [PubMed]
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483:100-3. [Crossref] [PubMed]
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma Proc Natl Acad Sci U S A 2008;105:3041-6. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- McDermott U, Pusapati RV, Christensen JG, et al. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Res 2010;70:1625-34. [Crossref] [PubMed]
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973-7. [Crossref] [PubMed]
- Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012;487:505-9. [Crossref] [PubMed]
- Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013;19:657-67. [Crossref] [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [Crossref] [PubMed]
- Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38-47. [Crossref] [PubMed]
- Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:773-81. [Crossref] [PubMed]
- Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 2009;462:108-12. [Crossref] [PubMed]
- Corcoran RB, Cheng KA, Hata AN, et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer cell 2013;23:121-8. [Crossref] [PubMed]
- Spreafico A, Tentler JJ, Pitts TM, et al. Rational combination of a MEK inhibitor, selumetinib, and the Wnt/calcium pathway modulator, cyclosporin A, in preclinical models of colorectal cancer. Clin Cancer Res 2013;19:4149-62. [Crossref] [PubMed]
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227-35. [Crossref] [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [Crossref] [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol 2015;33:4023-31. [Crossref] [PubMed]
- Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov 2015;5:358-67. [Crossref] [PubMed]
- Infante JR, Janku F, Tolcher AW, et al. Dose escalation stage of a first-in-class phase I study of the novel oral ERK 1/2 kinase inhibitor BVD-523 (ulixertinib) in patients with advanced solid tumors. J Clin Oncol 2015;33:2506.
- Yang H, Higgins B, Kolinsky K, et al. Antitumor activity of BRAF inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Cancer Res 2012;72:779-89. [Crossref] [PubMed]
- Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001;37 Suppl 4:S3-8. [Crossref] [PubMed]
- Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 2012;367:1596-606. [Crossref] [PubMed]
- Dan S, Okamura M, Seki M, et al. Correlating phosphatidylinositol 3-kinase inhibitor efficacy with signaling pathway status: in silico and biological evaluations. Cancer Res 2010;70:4982-94. [Crossref] [PubMed]
- Ihle NT, Lemos R Jr, Wipf P, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res 2009;69:143-50. [Crossref] [PubMed]
- Martinelli E, Troiani T, D'Aiuto E, et al. Antitumor activity of pimasertib, a selective MEK 1/2 inhibitor, in combination with PI3K/mTOR inhibitors or with multi-targeted kinase inhibitors in pimasertib-resistant human lung and colorectal cancer cells. Int J Cancer 2013;133:2089-101. [Crossref] [PubMed]
- Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 2008;14:1351-6. [Crossref] [PubMed]
- Alagesan B, Contino G, Guimaraes AR, et al. Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer. Clin Cancer Res 2015;21:396-404. [Crossref] [PubMed]
- Heist RS, Gandhi L, Shapiro G, et al. Combination of a MEK inhibitor, pimasertib (MSC1936369B), and a PR iI3K/mTOnhibitor, SAR245409, in patients with advanced solid tumors: Results of a phase Ib dose-escalation trial. J Clin Oncol 2013;31:abstr 2530.
- Chang JC. HER2 inhibition: from discovery to clinical practice. Clin Cancer Res 2007;13:1-3. [Crossref] [PubMed]
- Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol 2001;12 Suppl 1:S3-8. [Crossref] [PubMed]
- Le XF, Pruefer F, Bast RC Jr. HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle 2005;4:87-95. [Crossref] [PubMed]
- Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol 2008;44:831-54. [Crossref] [PubMed]
- Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003;421:756-60. [Crossref] [PubMed]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Yip AY, Tse LA, Ong EY, et al. Survival benefits from lapatinib therapy in women with HER2-overexpressing breast cancer: a systematic review. Anticancer Drugs 2010;21:487-93. [Crossref] [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012;30:2585-92. [Crossref] [PubMed]
- Cameron D, Casey M, Oliva C, et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 2010;15:924-34. [Crossref] [PubMed]
- Seo AN, Kwak Y, Kim DW, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One 2014;9:e98528. [Crossref] [PubMed]
- Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011;1:508-23. [Crossref] [PubMed]
- Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3:99ra86. [Crossref] [PubMed]
- Siena S, Sartore-Bianchi A, Lonardi S, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): The HERACLES trial. J Clin Oncol 2015;33:abstr 3508.
- Jung S, Schluesener HJ. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med 1991;173:273-6. [Crossref] [PubMed]
- Fenton RG, Taub DD, Kwak LW, et al. Cytotoxic T-cell response and in vivo protection against tumor cells harboring activated ras proto-oncogenes. J Natl Cancer Inst 1993;85:1294-302. [Crossref] [PubMed]
- Gjertsen MK, Bakka A, Breivik J, et al. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 1995;346:1399-400. [Crossref] [PubMed]
- Toubaji A, Achtar M, Provenzano M, et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother 2008;57:1413-20. [Crossref] [PubMed]
- Wedén S, Klemp M, Gladhaug IP, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer 2011;128:1120-8. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol 2014;25:1032-8. [Crossref] [PubMed]
- Lothe RA, Peltomäki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993;53:5849-52. [PubMed]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816-9. [Crossref] [PubMed]
- Timmermann B, Kerick M, Roehr C, et al. Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PloS one 2010;5:e15661. [Crossref] [PubMed]
- Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res 2008;68:889-92. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Aisner DL, Nguyen TT, Paskulin DD, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res 2014;12:111-8. [Crossref] [PubMed]
- Lee SJ, Li GG, Kim ST, et al. NTRK1 rearrangement in colorectal cancer patients: evidence for actionable target using patient-derived tumor cell line. Oncotarget 2015;6:39028-35. [PubMed]


