
The “quadrant-sandwich” method in clockwise modular D2 lymph node dissection in laparoscopic total gastrectomy: a retrospective cohort study
Highlight box
Key findings
• This approach should make it easier for the chief surgeon to find the correct anatomical level. Additionally, the application of the “quadrant-sandwich” method in clockwise modular D2 lymph node dissection (LND) can ensure thorough LND, increase the safety of the operation and reduce the amount of bleeding during the operation.
What is known, and what is new?
• Some studies have examined the LND techniques in a certain area in laparoscopic total gastrectomy.
• This is a standardized technique and procedure for total LND in total gastrectomy.
What is the implication, and what should change now?
• With the application of the “quadrant-sandwich” method in clockwise modular D2 LND, laparoscopic total gastrectomy can be completed in an orderly, standardized and safe manner, and is thus worth popularizing in clinical practice.
Introduction
Gastric cancer is one of the most common malignant tumors worldwide (1). The incidence of gastric cancer in China is high (2). Currently, radical surgery offers the only chance for curing gastric cancer (2,3). According to the Japanese guidelines for the treatment of gastric cancer, in principle, for gastric cancer with cN (+) or above T2, D2 lymph node dissection (LND) is required (that is, No. 1–7, No. 8a, 9, 11p, 11d, 12a lymph nodes) (4); for locally advanced upper gastric cancer, the lymph node metastasis rate of No. 10 is 9.8–20.9% (5), thus, No. 10 is also included in the D2 lymphadenectomy for this group of patients. With the advancement of surgical techniques and the development of surgical instruments, laparoscopic radical gastric cancer surgery has rapidly gained popularity, as it is minimally invasive (6).
In radical gastric cancer surgery, laparoscopic surgery faces two major problems (7,8). The first problem is related to the specificity of the structure of the gastric anatomy itself; the complex and varied perigastric vascular anatomy requires higher levels of surgical design, and an extensive LND. The second problem is related to the inadequate lymph node sorting. Due to these issues, laparoscopic gastric cancer surgery is difficult to perform, which can easily lead to cumbersome operation details (sometimes, during the surgery, it is necessary to constantly adjust the patient’s position and the surgeon’s position, etc.), incomplete LND, and even intraoperative bleeding. Additionally, the operation time may have to be prolonged due to an inappropriate level in the process of dissection (this may cause damage to blood vessels or pancreas, etc.), or a failure to follow the principle of radical gastric cancer, and as a result, the operation may not achieve the expected therapeutic efficacy for the patients. Thus, LND has become an important measurement index and restricting the level of laparoscopic gastric cancer surgery.
The safety and efficacy of traditional laparoscopic surgery have been confirmed (9-11), but traditional laparoscopic radical gastrectomy has to first fully free the greater omentum and then deal with the splenic gastric ligament, followed by the duodenum, hepaticoduodenal ligament, left gastric vessels, and esophagus (12,13). Insufficient dissection of the mesenteric fat tissue on both sides of the blood vessels when operating in each area results in insufficient exposure of the blood vessels. In addition, insufficient dissection of the stomach pancreas ligament increases intraoperative bleeding due to insufficient exposure of the surgical field, making it difficult to locate and treat bleeding points during the process of freeing the splenic gastric ligament and clearing lymph nodes in each area.
Based on this, we have made adjustments to the surgical process: we adjusted the order of vascular treatment to right gastroepiploic vessel region → hepatoportal region → left gastric vascular region → splenic hilum region, and used a clockwise method to fully free the gastrocolic ligament, hepaticoduodenal ligament, hepatogastric ligament, and gastropancreatic ligament first, and finally dealt with the splenic gastric ligament, in order to expose the surgical field to a greater extent by changing the patient’s position; this would help reduce the difficulty of the operation. In addition, we optimized the lymph node clearance and vascular treatment: we first processed the mesentery on both sides of the blood vessels so that the blood vessels were fully exposed, and then ligated and cut the blood vessels to avoid unexpected bleeding and difficulty in finding the bleeding points; this made the surgery safer. This method is named as the “quadrant-sandwich” method. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-966/rc).
Methods
General information
A retrospective study was conducted to analyze the clinical data of patients who underwent laparoscopic total gastrectomy from January 2019 to January 2022 at the Affiliated Cancer Hospital of Zhengzhou University. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) undergone pre-operative gastroscopy and a pathologic biopsy clearly showing gastric adenocarcinoma; and (II) undergone laparoscopic radical total gastrectomy + D2 LND. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had imaging results that confirmed the presence of distant metastasis of the tumor; (II) had a pre-operative combination of obstruction, bleeding, and perforation; and/or (III) had other organ dysfunction and thus could not tolerate laparoscopic surgery. A total of 108 patients were selected according to the inclusion criteria, of whom 55 were allocated to the observation group, which received clockwise modular LND using the “quadrant-sandwich method”, and 53 were allocated to the control group, which received traditional LND. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Affiliated Cancer Hospital of Zhengzhou University (No. 2021-132-003) and individual consent for this retrospective analysis was waived. There were no statistically significant differences in the clinicopathological data of the patients in the two groups (Table 1).
Table 1
| Parameters | Observation group (N=55) | Control group (N=53) | χ2/Z/t value | P value |
|---|---|---|---|---|
| Age (years) | 66.0 (53.0–74.0) | 60.0 (55.5–70.0) | Z=0.704 | 0.481 |
| Gender | χ2=0.899 | 0.343 | ||
| Male | 38 | 32 | ||
| Female | 17 | 21 | ||
| BMI (kg/m2) | 21.8±2.785 | 20.8±2.840 | t=1.792 | 0.076 |
| cTNM stage | Z=−0.430 | 0.667 | ||
| I | 6 | 3 | ||
| II | 21 | 22 | ||
| III | 28 | 28 | ||
| Tumor location | ||||
| Upper | 20 | 18 | χ2=0.651 | 0.722 |
| Middle | 25 | 22 | ||
| Lower | 10 | 13 | ||
| Grade | Z=1.022 | 0.307 | ||
| High | 2 | 4 | ||
| Middle | 14 | 21 | ||
| Low | 30 | 21 | ||
| Other (mucinous carcinoma, neuroendocrine carcinoma, impression cell carcinoma) | 9 | 7 | ||
| American Society of Anesthesiologists Classification | Z=−0.166 | 0.868 | ||
| I | 27 | 27 | ||
| II | 25 | 23 | ||
| III | 3 | 3 | ||
| Neoadjuvant therapy | χ2=1.543 | 0.214 | ||
| Yes | 16 | 10 | ||
| No | 39 | 43 |
Data are presented as mean ± standard deviation, number or median (interquartile range). BMI, body mass index.
Surgeons
The same group of surgeons performed the laparoscopic radical total gastrectomy + D2 LND on all the patients.
Surgical position and trocar position
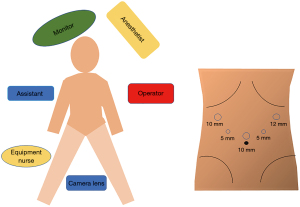
The patients were placed under general anesthesia and in the supine split leg position. A trocar was placed using the 5-hole method (Figure 1). Intra-abdominal pressure was maintained at 12 mmHg.
The “sandwich” method
The lymphatic adipose tissue around the blood vessels was viewed as the “bread layers” (Slices A and B), while the blood vessels in the core and the contouring lymphatic adipose tissue in the region of the blood vessels were viewed as the “middle layer” of the “sandwich” (14,15). The lymphatic adipose tissue on both sides was dissected first, and the vascular tissue in the middle was then processed.
Introduction of quadrants
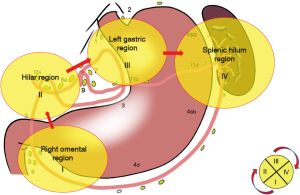
For the total gastrectomy, the perigastric lymph nodes were grouped, and the specific anatomical location of each group was determined. The following lymph nodes did not require special dissection, and were often resected along with the specimen removal: No. 1, No. 2, No. 3, No. 4 (No. 4sa, No. 4sb, and No. 4d), and No. 5. While, the following lymph nodes required fine anatomic clearance: No. 6, No. 7, No. 8a, No. 9, No. 10, No. 11 (No. 11p and No. 11d), and No. 12a. We divided the LND into four regions.: the right gastroepiploic vessel region (region I), the hepatoportal region (region II), the left gastric vascular region (region III), and the splenic hilum region (region IV). The order of surgical LND was as follows: region I → region II → region III → region IV. This method was known as the four-region clockwise modular LND designed by the authors (Figure 2). For each partition, we used the “sandwich” method for the LND.
LND process specific to each region
- Right gastroepiploic vessel region. We focused on clearing the lymph nodes in group No. 6, and the lymph nodes in group No. 4d were removed along with the specimen. Three subgroups of lymph nodes (i.e., No. 6v, No. 6a, and No. 6i) were removed from the root of the root of the right gastric omental vein, and the periphery of the subpyloric blood vessels, and the LND of group No. 6 was completed.
- Hepatoportal region. We then focused on the No. 8a and No. 12a lymph nodes. The No. 5 group of lymph nodes were removed along with the whole stomach specimen. Slice A was the lymphatic adipose tissue free from the angle between the gastroduodenal artery and the common hepatic artery (i.e., lymph node No. 8a). Slice B was free from the anterior part of the proper hepatic artery and the dissected right gastric blood vessel. In the middle layer, the No. 12a lymph node was completely resected from the left side of the common hepatic artery and the portal vein.
- Left gastric vascular region. We then focused on clearing the No. 7, No. 9, and No. 11p lymph nodes. The No. 1 and No. 3 groups of lymph nodes were removed along with the whole stomach specimen. At the upper edge of the pancreas, the left gastric artery root was exposed by freeing the celiac trunk from the pancreas. The No. 7 and No. 9 lymph nodes were removed. The left gastric artery was dissected. The proximal part of the splenic artery was dissected to the left. The No. 11p lymph node was removed. The lymph nodes were dissected upward to the esophageal hiatus.
- Splenic hilum region. We then focused on clearing the lymph nodes of groups No. 11d and No. 10. The lymph nodes of groups No. 2, No. 4sa, and No. 4sb were removed with the whole stomach specimen. Starting from the left side of the abdominal aorta, lymphadenectomy was freed to the left, with the superior border reaching the lower edge of the septum muscle, the lateral border reaching the posterior aspect of the splenic hilar, and the lower border reaching the upper edge of the splenic artery, splenic vein, and pancreas. The adhesions between the colonic splenic flexure, the pancreatogastric and lower pole of the spleen, and the greater omentum were then freed. The lymphadenectomy began with the colonic splenic flexure and ended with the dissecting of the left vessels of the gastric omentum. Finally, LND was performed along the splenic artery and vein toward the splenic hilum to complete the dissection of No. 11d and No. 10 lymph nodes. We then completed the LND of the entire splenic hilar region.
Statistical analysis
SPSS 26.0 software was used for the statistical analysis. The normally distributed measurements were expressed as the mean ± standard deviation (SD), and the independent sample t-test was used for comparisons between groups; the skewed measurements were expressed as median [inter-quartile range (IQR)], and the Mann-Whitney U test was used for comparisons between groups. The count data were expressed as absolute numbers, and the Chi-squared test or Fisher’s exact test was used for the intergroup comparisons. Grade information was compared between the groups using the Mann-Whitney U test, and a P value <0.05 was considered statistically significant.
Results
Intraoperative situation
Laparoscopic total gastrectomy was successfully completed in both groups of patients. There were significant differences in intraoperative bleeding, operation time, bleeding from perigastric vascular injury; anatomical level was correctly dissociated between the two groups. There were no statistically significant differences between the two groups in terms of intraoperative organ damage, the number of divisions, and total LND (P>0.05). The intraoperative conditions between the two groups were also compared (Tables 2,3). With the correct anatomical level, too deep dissociation may damage the surrounding organs (such as pancreas) and blood vessels (resulting in abnormal bleeding), yet too shallow may lead to incomplete lymphadenectomy. The blood loss was measured as follows: the gauze used during the operation was weighed and the blood was sucked out by the aspirator.
Table 2
| Parameters | Observation group (N=55) | Control group (N=53) | χ2/Z/t value | P value |
|---|---|---|---|---|
| Intraoperative bleeding (mL) | 100.0 (30.0–200.0) | 180.0 (130.0–245.0) | Z=3.179 | 0.001 |
| Surgical time (min) | 227.0±48.5 | 247±41.5 | t=2.363 | 0.020 |
| Bleeding from perigastric vascular injuries | χ2=14.85 | <0.001 | ||
| Yes | 18 | 37 | ||
| No | 37 | 16 | ||
| Anatomical level correctly dissociate | χ2=8.420 | 0.004 | ||
| Yes | 40 | 24 | ||
| No | 15 | 29 |
Data are presented as mean ± standard deviation, number or median (interquartile range).
Table 3
| Types of lymph nodes | Observation group (N=55) | Control group (N=53) | Z/t value | P value |
|---|---|---|---|---|
| Total number of lymph nodes (area I + II + III + IV) | ||||
| Positive | 2.0 (0.0–7.0) | 0.0 (0.0–5.5) | Z=1.117 | 0.264 |
| Total | 41.07±12.19 | 39.62±12.97 | t=0.599 | 0.550 |
| No. 4d + No. 6 (area I) | ||||
| Positive | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | Z=1.144 | 0.253 |
| Total | 7.00 (5.00–10.00) | 6.00 (5.00–8.00) | Z=−0.839 | 0.402 |
| No. 5 + No. 12a + No. 8a (area II) | ||||
| Positive | 0.00 (0.00–1.00) | 0.00 (0.00–1.00) | Z=−0.451 | 0.652 |
| Total | 6.85±3.841 | 7.79±4.765 | t=1.023 | 0.306 |
| No. 1 + No. 9 + No. 7 + No. 11p + No. 3 (area III) | ||||
| Positive | 1.00 (0.00–3.00) | 0.00 (0.00–3.00) | Z=0.399 | 0.690 |
| Total | 18.00 (14.00–22.00) | 18.00 (14.00–21.00) | Z=0.493 | 0.622 |
| No. 2 + No. 10 + No. 11d + No. 4sa + No. 4sb (area IV) | ||||
| Positive | 0.00 (0.00–2.00) | 0.00 (0.00–1.00) | Z=1.467 | 0.142 |
| Total | 10.00 (7.00–13.00) | 9.00 (6.50–13.00) | Z=0.666 | 0.505 |
Data are presented as mean ± standard deviation or median (interquartile range).
Post-operative results
In relation to the post-operative pathological results, there were no statistically significant differences between the two groups in terms of the maximum diameter of the tumor, incision margin, vascular infiltration/nerve invasion, pTNM stage, T stage, N stage, post-operative time of the first meal (d), post-operative complications, and post-operative hospitalization time (d) (P>0.05) (Table 4).
Table 4
| Parameters | Observation group (N=55) | Control group (N=53) | χ2/Z value | P value |
|---|---|---|---|---|
| Maximum diameter of tumor (cm) | 3.0 (2.0–4.5) | 3.5 (2.5–4.5) | Z=0.799 | 0.424 |
| Cutting margin | – | 1 | ||
| Negative | 54 | 52 | ||
| Positive | 1 | 1 | ||
| Vascular infiltration/nerve invasion | χ2=0.841 | 0.359 | ||
| Yes | 36 | 39 | ||
| No | 19 | 14 | ||
| pTNM stage | Z=0.908 | 0.364 | ||
| 0 | 3 | 1 | ||
| I | 16 | 14 | ||
| II | 18 | 17 | ||
| III | 18 | 21 | ||
| T stage | Z=0.536 | 0.592 | ||
| T0 | 3 | 1 | ||
| T1 | 9 | 8 | ||
| T2 | 10 | 21 | ||
| T3 | 25 | 3 | ||
| T4 | 8 | 20 | ||
| N stage | Z=0.903 | 0.366 | ||
| N0 | 25 | 23 | ||
| N1 | 11 | 5 | ||
| N2 | 6 | 6 | ||
| N3 | 13 | 19 | ||
| Time of first post-operative meal (days) | 4.0 (3.0–4.0) | 4.0 (4.0–5.0) | Z=−1.945 | 0.052 |
| Post-operative hospitalization (days) | 13.0 (12.0–16.0) | 15.0 (12.0–17.0) | Z=−1.229 | 0.219 |
| Postoperative complications | ||||
| Incision infection | 3 | 2 | χ2<0.001 | 1.000 |
| Pulmonary infection | 4 | 4 | χ2<0.001 | 1.000 |
| Anastomotic leakage | 1 | 1 | – | 0.743 |
| Lymphatic leakage | 2 | 1 | χ2<0.001 | 1.000 |
| Pancreatic leakage | 0 | 1 | – | 0.491 |
| Clavien-Dindo I + II | 10 | 8 | χ2=0.185 | 0.667 |
| Clavien-Dindo III + IV | 0 | 1 | – | 0.491 |
Data are presented as number or median (interquartile range).
Discussion
A Dutch (16) study in the Netherlands and the JCOG-950 (17) study in Japan established D2 LND as the standard treatment for locally progressive gastric cancer, and the safety of laparoscopic radical gastrectomy for gastric cancer was confirmed by the KLASS-03 study in Korea (18), the JCOG1401 study in Japan (19), and the CLASS02 study in China (20). Due to the complexity of the anatomical level of the perigastric region, the rich blood supply, and the specificity of the laparoscopic surgical instruments required for the operation, LND in laparoscopic radical gastric cancer surgery is more difficult and technically demanding than those in laparoscopic radical resection of colorectal cancer. For example, rectal cancer surgery only needs to deal with the inferior mesenteric artery and LND only needs to clear the lymph nodes in the inferior mesenteric artery supply area. However, stomach cancer involves more blood vessels and a wider range of lymph node clearance, making the entire surgical process not only complex, but also time-consuming and laborious, and these conditions make the laparoscopic gastric cancer surgery more difficult, which can easily lead to the occurrence of cumbersome operation details and unexpected bleeding, incomplete LND, and intraoperative complications, leading to the failure of achieving the expected post-operative curative effect.
In laparoscopic total gastrectomy for gastric cancer, some scholars have examined the use of LND technique in certain region (21,22), however, the standardized techniques and procedures for LND have not been reported locally or abroad. Our team subdivides LND into four zones, and then according to the anatomical location of the lymph node distribution, we list the lymph nodes that need to be dissected carefully, and then subdivide these lymph nodes into different zones. We refine the tedious workload of LND and focus on the key group of LND in each zone, so that the surgical process is progressive layer by layer, and having an integrity. There is also a partition module, which can save the operation time.
Under the “sandwich” method, the two sides of the meninges are treated first, and then the middle vascular area is treated. By meticulously dissecting the adipose tissue on both sides of the mesentery to the greatest extent possible, the blood vessels in the middle layer are more fully exposed, which reduces the chance of accidental bleeding of blood vessels during the surgical process. Even if blood vessels rupture and bleeding occurs accidentally, as both sides of the mesentery are sufficiently free, the bleeding point is more clearly exposed, making it easier to stop the bleeding under the luminal microscope, and thus reducing the possibility to open surgery.
In the comparison of the two groups of patients in this study, the bleeding in the observation group was less than that in the control group, which confirmed the safety of this method. The application of the “sandwich” method also made it easier for the attending surgeon to find the correct and proper anatomical level, which ensured the safety of the surgery and reduced the intraoperative bleeding while ensuring the thoroughness of the LND.
In addition, compared with the traditional operation method, we first deal with the bilateral mesangial tissue, and by dissociating the bilateral mesangial tissue, it is easier to guide us to the correct level of membrane anatomy, reducing the probability of surrounding organ injury and abnormal vascular bleeding. It can better ensure the thoroughness of lymphadenectomy. For gastric cancer surgery, vascular variation occurs from time to time. We can fully expose the blood vessels after dealing with the bilateral mesangial tissue with the “sandwich” method. For example, when there are two left gastric veins in the patient, if we cannot fully dissociate the bilateral mesangial tissue, we do not dare to ligate the two veins decisively. Therefore, the “sandwich” method has more advantages in dealing with patients with vascular variation. With the follow-up of the two groups of patients, there was no significant difference in patient survival rate, but the number of cases in this study is small, statistics and analysis on patient survival rate and its long-term effect would need the continuation to expand the sample size and increase the observation time.
At the same time, this study has the following limitations. Firstly, it is a retrospective single-center study, and this research findings need further validation through prospective studies in multiple centers. Secondly, the sample size in this study is relatively small, and the results of clinical trials need to be confirmed with a larger sample size. Finally, the research endpoint of this study primarily focuses on surgical-related indicators, and further follow-up of patients is needed to evaluate the research results fully.
Conclusions
In conclusion, using the “quadrant-clockwise” modular D2 LND technique, laparoscopic total gastrectomy can be completed in an orderly, standardized, and safe manner.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-966/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-966/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-966/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-966/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Affiliated Cancer Hospital of Zhengzhou University (No. 2021-132-003) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Nakamura K, Morisaki T, Sugitani A, et al. An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer 1999;85:1500-5. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023;26:1-25.
- Mönig SP, Collet PH, Baldus SE, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol 2001;76:89-92. [Crossref] [PubMed]
- Hu Y, Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol 2016;34:1350-7. [Crossref] [PubMed]
- Yang H, Zhang WH, Liu K, et al. Application of clockwise modularized laparoscopic lymphadenectomy in the suprapancreatic area, a propensity score matching study and comparison with open gastrectomy. Surg Endosc 2021;35:1465-75. [Crossref] [PubMed]
- The Laparoscopic and Endoscopic Surgery Group of the Surgical Branch of the Chinese Medical Association. Guideline for laparoscopic gastrectomy for gastric cancer (2023 edition). Chinese Journal of Digestive Surgery 2023;22:425-36.
- Wang Y, Lei X, Liu Z, et al. Short-term outcomes of laparoscopic versus open total gastrectomy after neoadjuvant chemotherapy: a cohort study using the propensity score matching method. J Gastrointest Oncol 2021;12:237-48. [Crossref] [PubMed]
- Wang F, Zhang S, Zhao W, et al. Open distal gastrectomy versus laparoscopic distal gastrectomy for early gastric cancer: a retrospective study. J Gastrointest Oncol 2021;12:2743-8. [Crossref] [PubMed]
- Feng Q, Zhang T, Xie M. Is D2 laparoscopic gastrectomy essential for elderly patients with advanced gastric cancer? J Gastrointest Oncol 2022;13:2703-4. [Crossref] [PubMed]
- Goh PM, Khan AZ, So JB, et al. Early experience with laparoscopic radical gastrectomy for advanced gastric cancer. Surg Laparosc Endosc Percutan Tech 2001;11:83-7. [Crossref] [PubMed]
- Uyama I, Kanaya S, Ishida Y, et al. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 2012;36:331-7. [Crossref] [PubMed]
- Li C, Zhang X, Li Z, et al. Application of the sandwich technique in porta-hepatis lymph-node dissection: A video vignette. Asian J Surg 2022;45:1615-6. [Crossref] [PubMed]
- Zhang X, Zhang J, Zhao Y. "Sandwich" infrapyloric lymphadenectomy in laparoscopic radical gastrectomy: A video vignette. Asian J Surg 2021;44:1079-80. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Yang HK, Hyung WJ, Han SU, et al. Comparison of surgical outcomes among different methods of esophagojejunostomy in laparoscopic total gastrectomy for clinical stage I proximal gastric cancer: results of a single-arm multicenter phase II clinical trial in Korea, KLASS 03. Surg Endosc 2021;35:1156-63. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 2019;22:999-1008. [Crossref] [PubMed]
- Liu F, Huang C, Xu Z, et al. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol 2020;6:1590-7. [Crossref] [PubMed]
- Shen J, Zhang B, Wei W, et al. Application of membrane anatomy in splenic hilar lymph node dissection in radical resection of gastric cancer. Chinese Journal of Gastrointestinal Surgery 2023;26:633-8. [PubMed]
- Zheng Z, Huang C, Zhong Q. “Huang's three-step method” splenic lymph node dissection. Chinese Journal of Gastrointestinal Surgery 2018;21:164.


