Retrospective study of combined pelvic exenteration in the treatment of primary and recurrent pelvic malignant tumors
Highlight box
Key findings
• Pelvic exenteration (PE) is a viable option for pelvic malignancies. Aggressive radical resection of lesions and reduced postoperative complications can effectively improve patient outcomes.
What is known and what is new?
• The perioperative and long-term prognosis of patients with pelvic malignancies treated with PE in China has been rarely reported.
• We retrospectively studied the clinical data of PE patients in our hospital in the past 7 years.
What is the implication, and what should change now?
• PE is a viable option for pelvic malignancies.
• This study requires large-scale multicenter research validation.
Introduction
With the continuous progress in surgical techniques and preoperative comprehensive tumor treatment, great progress has been made in the treatment of pelvic malignancies. Population-based medical screening has reduced the incidence of locally advanced pelvic tumours, because tumours can be detected at an earlier stage by routine physical examination (1). Pelvic malignant tumors often originate in the rectum, bladder, uterus. Patients with severe pelvic symptoms and no distant metastases need to undergo multi-organ resection to achieve R0 radical treatment (2). Pelvic exenteration (PE) can achieve en-block resection and has become the popular option for the management of bulky pelvic malignancies which has multiple organ and structure involved. Although it was first used by Brunschwig in 1948 for palliative surgery of gynecological cancers, its widespread adoption has been slow due to the severe trauma caused by surgery, implementation difficulty, and risk of the procedure (3). Brintnall et al. reported the first case of PE for locally progressive rectal cancer in 1950 (4). PE surgery is complex, and although the morbidity and mortality of acceptable complications have been reported, the perioperative and long-term prognoses of patients with pelvic malignancies treated with PE in China have been rarely reported. In addition, the potential relationship between clinical histogenetic status and prognosis is unclear (5). We retrospectively studied the clinical data of PE patients in Affiliated Cancer Hospital of Zhengzhou University in the past 7 years to investigate the safety and efficacy of PE in the treatment of primary and recurrent pelvic malignancies, and the factors affecting prognostic survival. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-973/rc).
Methods
Patient characteristics
This study retrospectively analyzed 59 patients with pelvic malignancies who underwent PE at Affiliated Cancer Hospital of Zhengzhou University, between January 2015 and July 2021. Every patient undergoes a multidisciplinary expert consultation before undergoing treatment, a team that includes imaging specialists, radiotherapists, oncologists, surgeons, gynaecologists and nutritionists. They were divided into two groups according to the primary site: a rectal cancer group (n=40, 29 primary; 11 recurrent) and a cervical cancer group (n=19, 11 primary; 8 recurrent), and all diagnoses were confirmed by preoperative pathology. The medical histories and pathology reports were reviewed for basic information, clinical data and tumor characteristics [intraoperative blood loss, duration of surgery, adjuvant radiotherapy, postoperative pathology, lymph node metastasis, mortality, microsatellite instability (MSI) status, and survival, among others].
The inclusion criteria were as follows: (I) preoperative imaging suggesting more extensive rectal and cervical cancer invasion in the pelvis, which could be evaluated for R0 resection; (II) no distant organ metastases such as lung, liver, and bone; (III) bone destruction below the S2 level without invasion to the external iliac vessels; (IV) good physical condition and able to tolerate surgery.
The exclusion criteria were as follows: (I) distant metastases such as lung, liver, and bone; positive peritoneal cytology; (II) preoperative evaluation for R1/R2 resection; (III) tumor invasion above the level of S2–S3 sacral junction; (IV) tumor invasion of the lateral wall of the pelvis; (V) tumor invasion of the external iliac vessels; (VI) lower limb edema due to venous or lymphatic vessel compression; (VII) Those patients who received the Nutritional Risk Screening 2002 (NRS-2002) scores ≥3.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committees of the Affiliated Cancer Hospital of Zhengzhou University (No. 2016ct083). Due to the retrospective nature of the study and because no patient specimens were used, the requirement for individual consent for this retrospective analysis was waived by the ethics committees. The patient selection flow chart is shown in Figure 1.
Preoperative abdominal computed tomography (CT) and pelvic magnetic resonance imaging (MRI) as well as rectal ultrasound endoscopy should be routinely performed, and whole-body enhanced CT is used for 3D reconstruction of critical vessels to assist in preoperative surgical planning and to rule out distant metastases. Positron emission tomography/CT (PET-CT) can assist in the diagnosis of patients with distant metastases and in the determination of whether the suspected invasion is malignant. R1 evaluation criteria of surgical radicality: according to the postoperative pathological findings, the degree of radicality of pelvic tumour surgery was classified into three grades: R0 (pathological findings of negative margins), R1 (complete resection of the lesion by the naked eye, with tumour cell residues at the margins of the microscope) and R2 (tumour residues by the naked eye).
Resection
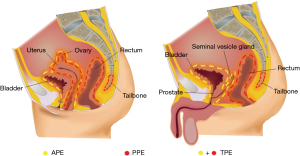
Total PE (TPE): the resection includes the rectum, anus, bladder, prostate (in men), lower ureter, and pelvic lymph nodes, and in women, the uterus, vagina, and/or external genitalia.
Anterior PE (APE) is indicated for patients who have preserved the anus and/or part of the rectum and require total cystectomy, which includes the upper rectum, bladder, prostate (in men), lower ureter and pelvic lymph nodes, and may include the uterus and both adnexa and vagina in women.
Posterior PE (PPE), for which bladder preservation is indicated (partial resection is possible), includes the rectum (or anus), uterus, vagina, and pelvic lymph nodes. In the case of rectal cancer invading the presacral fascia and the lower sacrum, sacral resection with proctectomy is performed (Figure 2).
For PE surgery, there is a complete procedure for lymph node dissection. Lymph node dissection begins at the aortic bifurcation and encompasses the lymph nodes of the main-iliac vascular bifurcation, the common iliac vessels, and the external iliac vessels up to the root of the internal iliac vessels, and lymph nodes in the region of the internal iliac vessels may be subsequently removed as a whole, along with the vessels and tumour specimen. Ligating and dissecting the internal iliac arteries and veins at the beginning effectively reduces the risk of pelvic haemorrhage during or after surgery.
Follow-up
Survival time was calculated from the day of surgery to the last follow-up visit or death, with the last follow-up visit as of October 2022. The follow-up included assessment of disease control (whether relapse, co-morbidities) and survival prognosis. Patients in both groups were followed up on an outpatient basis, every 3 months for the first three years postoperatively, every 6 months for 4–5 years postoperatively, and annually after 5 years. Routine follow-up of patients in both groups included whole-body CT and tumour markers which include carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), cancer antigen 19-9 (CA199). Rectal cancer patients underwent colonoscopy in the 1st, 3rd, and 5th postoperative years. Patients with cervical cancer had one additional Squamous Cell Carcinoma Antigen (SCCA) test for tumour markers at each follow-up. MRI and PET-CT were not included as routine follow-up items, and additional tests were performed when CT examination suggested the presence of suspected recurrent foci. We classified the complications according to the Clavien-Dindo classification (6).
Statistical analysis
The statistical software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was applied for statistical analysis. The quantitative data conforms to a normal distribution expressed as mean ± standard deviation (SD), and independent sample t-test is used for comparison between the two groups; the skewed distribution is represented by quartile (P25, P75), and the Mann-Whitney U test is used for inter group comparison. Count data are expressed in absolute numbers, and intergroup comparisons are conducted using an χ2-test. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Prognostic variables were analyzed by univariate analysis, and single factors with statistical significance were included in the Cox regression model for multivariate analysis, and patients’ independent risk factors affecting overall survival (OS) were analyzed. Statistical significance was considered when P<0.05.
Results
Baseline data of enrolled patients
A total of 59 patients with pelvic malignancies were included in the institution, all of whom successfully completed PE surgery without intraoperative deaths, 1 patient died within 30 days after surgery. Their median age was 54 years (range, 40–77 years), 29 were male, and 30 were female. The operative time was 1.3–11.9 hours (mean 3.3 hours). The operative bleeding volume was 200–3,500 mL (mean 931 mL). The mean total hospital stay was 27 days (range, 12–60 days). A total of 37 patients received preoperative treatment (Tables 1,2), of which 18 received neoadjuvant radiotherapy and chemotherapy, 17 received adjuvant chemotherapy, and 2 received adjuvant radiotherapy [median dose 45 Gy (range, 40–50 Gy)].
Table 1
| Variable | Rectal cancer group | Cervical cancer group | Statistical value | P value |
|---|---|---|---|---|
| Median age (years) | 55.5 [51.0, 65.7] | 54.0 [49.0, 58.0] | Z=−1.097 | 0.272 |
| Gender | K2=27.091 | <0.001 | ||
| Male | 29 (72.5) | 0 | ||
| Female | 11 (27.5) | 19 (100.0) | ||
| TNM stage | Z=0.152 | 0.697 | ||
| I | 0 | 0 | ||
| II | 3 (7.5) | 2 (10.5) | ||
| III | 37 (92.5) | 17 (89.5) | ||
| Neoadjuvant therapy | Z=7.315 | 0.026 | ||
| Yes | 21 (52.5) | 16 (84.2) | ||
| No | 15 (37.5) | 3 (15.8) | ||
| Unknown | 4 (10.0) | 0 | ||
| Types of neoadjuvant therapy | Z=12.761 | 0.013 | ||
| Chemoradiotherapy | 10 (25.0) | 11 (57.9) | ||
| Chemotherapy alone | 11 (27.5) | 3 (15.8) | ||
| Radiotherapy alone | 0 | 2 (10.5) | ||
| Not | 15 (37.5) | 3 (15.8) | ||
| Unknown | 4 (10.0) | 0 | ||
| Hospital stay (days) | 26.0 [18.3, 32.6] | 25.0 [22.0, 30.0] | Z=−0.642 | 0.521 |
| Tumor primary/recurrence | K2=1.259 | 0.262 | ||
| Primary | 29 (72.5) | 11 (57.9) | ||
| Recurrence | 11 (27.5) | 8 (42.1) |
Data are presented as median [interquartile range] or N (%). PE, pelvic exenteration; TNM, tumor-node-metastasis.
Table 2
| Variable | Rectal cancer group | Cervical cancer group | Statistical value | P value |
|---|---|---|---|---|
| Surgical procedure | Z=−0.750 | 0.941 | ||
| TPE | 24 (60.0) | 11 (57.9) | ||
| APE | 3 (7.5) | 2 (10.5) | ||
| PPE | 13 (32.5) | 6 (31.6) | ||
| Operation time (min) | 214.7±108.0 | 227.8±79.6 | t=−0.472 | 0.881 |
| Blood loss (mL) | 600 [425, 1,200] | 1,100 [600, 1,500] | Z=−1.881 | 0.060 |
| R0 resection of tumor | K2=4.358 | 0.037 | ||
| R0 | 40 (100.0) | 17 (89.5) | ||
| R1 | 0 | 2 (10.5) | ||
| Maximum diameter of tumor (cm) | 8.8±4.2 | 6.2±2.9 | t=2.450 | 0.077 |
Data are presented as mean ± standard deviation, median [interquartile range] or N (%). PE, pelvic exenteration; TPE, total pelvic exenteration; APE, anterior pelvic exenteration; PPE, posterior pelvic exenteration.
In terms of preoperative comorbidities, 2 patients in the rectal cancer group had rectovaginal fistula, there was 1 case of anemia complicated by urinary tract infection, 1 case of severe anemia complicated by intussusception, 1 case of pelvic infection, 7 cases of intestinal obstruction, 1 case of sigmoid colon perforation, and 1 case of lacunar cerebral infarction; 4 patients in the cervical cancer group had rectovaginal fistula, 1 of which was combined with presacral abscess, and 3 cases of vesicovaginal fistula. After surgery, 38 patients underwent non-anal sphincter preserving surgery, of which 34 underwent sigmoidostomy, 3 underwent transverse colostomy, and 1 underwent ileostomy, all of which were permanent stomas; 22 patients underwent anal sphincter preservation surgery, of which 12 underwent functional ileostomy and 10 underwent rectosigmoid anastomosis. Urinary tract reconstruction was performed by ileal substitution of the bladder in 11 patients, cystostomy in 6 patients, and ureteral skin fistula in 24 patients.
PE postoperative complications
The incidence of postoperative complications was 33.9% in all patients, and some patients had more than 1 combination of postoperative complications. The incidence of postoperative complications in the rectal cancer group was 27.5%, with perineal wound infection (63.6%), intestinal obstruction (54.5%), and pneumonia (36.3%) being the most common. The incidence of complications was similar in patients with locally advanced colorectal cancer (LACRC) and locally recurrent colorectal cancer (LRCRC); in the cervical cancer group it was 47.3%, with urinary tract infection (55.5%), pelvic abscess (33.3%), pneumonia (22.2%), and enterocutaneous fistula (22.2%) being the most common (Table 3). There was 1 patient who died within 30 days after surgery in the above patients, who had postoperative complications of perineal wound infection, intestinal fistula, and sepsis.
Table 3
| Complication | Rectal cancer group, N (%) | Cervical cancer group, N (%) |
|---|---|---|
| Perineal wound infection | 7 (63.6) | 1 (11.1) |
| Pelvic abscess | 2 (18.1) | 3 (33.3) |
| Intestinal obstruction | 6 (54.5) | 1 (11.1) |
| Intestinal fistula | 2 (18.1) | 2 (22.2) |
| Ureteral obstruction | 0 | 1 (11.1) |
| Pneumonia | 4 (36.3) | 2 (22.2) |
| Urinary tract infection | 3 (27.7) | 5 (55.5) |
| Stomal necrosis | 1 (9.0) | 0 |
| Anastomotic fistula | 0 | 0 |
| Postoperative bleeding | 2 (18.1) | 1 (11.1) |
| Sepsis | 1 (9.0) | 0 |
| Thrombus | 3 (27.7) | 0 |
| 30-day readmissions | 6 (15.0) | 4 (21.1) |
PE, pelvic exenteration.
The Clavien-Dindo complication grading system was used to grade postoperative complications. The postoperative complication grades I, II, III, and IV of patients in the rectal cancer group were 2, 6, 2, and 1, respectively, and those in the cervical cancer group were 1, 3, 3, and 2, respectively (Table 4). The comparison between the two groups of patients showed no statistically significant results (P=0.730).
Table 4
| Clavien-Dindo grading of complications | Rectal cancer group, N (%) | Cervical cancer group, N (%) | Statistical value | P value |
|---|---|---|---|---|
| I | 2 (18.2) | 1 (11.1) | K2=1.867 | 0.730 |
| II | 6 (54.5) | 3 (33.3) | ||
| III | 2 (18.2) | 3 (33.3) | ||
| IV | 1 (9.1) | 2 (22.2) |
The Clavien-Dindo complication grading system was used to grade them and the results were not statistically significant when comparing the two groups of patients (P=0.730). PE, pelvic exenteration.
Survival analysis of patients after PE operation
Survival curves of the rectal cancer group and cervical cancer group
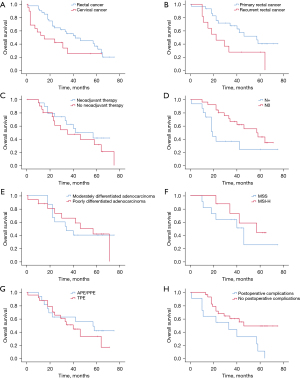
The median survival times for the rectal and cervical cancer groups were 42 and 15 months, respectively, and the 5-year survival rates were 36.6% and 25.3% (Figure 3A), respectively, with the rectal cancer group tending to have a better survival prognosis compared to the cervical cancer group (P=0.031).
Subgroup analysis of rectal cancer
Patients in the LACRC and LRCRC groups had median survival times of 57 and 22 months and 5-year survival rates of 40.1% and 27.3%, respectively (Figure 3B). Patients in the LACRC group had a better prognosis for postoperative survival (P=0.031). Patients in the neoadjuvant group and no neoadjuvant group had median survival times of 42 and 35 months, respectively. The 5-year survival rates were 41.6% and 31.5%, respectively (Figure 3C). The median survival times for patients in the postoperative pathological lymph node metastasis and lymph node non-metastasis groups were 19 and 57 months, respectively. The 5-year survival rates were 24.4% and 43.2%, respectively (Figure 3D), and patients in the lymph node non-metastasis group had a better prognosis for postoperative survival (P=0.035); the 5-year survival rates for patients in the postoperative pathological moderately differentiated adenocarcinoma and poorly differentiated adenocarcinoma groups were 44.0% and 40.0%, respectively (Figure 3E).
As genetic testing has gradually gained popularity in recent years, some patients were screened with genetic testing. A total of 19 patients underwent microsatellite stability (MSS) genetic testing, which revealed that 11 patients had MSS and 8 patients had MSI-high (MSI-H), with 5-year survival rates of 43.8% and 25.5%, respectively (Figure 3F), and patients in the MSI-H group tended to have a better survival prognosis, but the difference was not statistically significant (P=0.362).
For patients in the APE/PPE and TPE groups, the median survival times were 42 and 40 months, and the 5-year survival rates were 29.4% and 42.4%, respectively (Figure 3G). The median survival times for patients in the group with and without postoperative complications were 32 and 50 months, respectively, and the 5-year survival rates were 10.9% and 49.0%, respectively (Figure 3H), with a better prognosis for patients in the group without postoperative complications (P=0.015).
Univariate and multivariate analysis of prognosis in patients with rectal cancer
Univariate analysis confirmed that recurrent rectal cancer, lymph node metastasis, presence of postoperative complications, and MSS status were significant prognostic factors for poor survival prognosis (Table 5). The above-mentioned factors with statistical significance were included in the multifactorial analysis, and the results suggested that postoperative lymph node metastasis [hazard ratio (HR) =4.380; 95% confidence interval (CI): 1.493–12.850; P=0.007] and postoperative complications (HR =0.217; 95% CI: 0.074–0.636; P=0.005) affected the prognostic factors of patient survival (P<0.05).
Table 5
| Variable | N (%) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| 5-year survival (%) | P value | HR | 95% CI | P value | |||
| Age, years | 0.499 | ||||||
| ≤60 | 27 (67.5) | 34.3 | |||||
| >60 | 13 (32.5) | 40.3 | |||||
| Gender | 0.300 | ||||||
| Male | 29 (72.5) | 40.9 | |||||
| Female | 11 (27.5) | 25.5 | |||||
| Primary site, n | 0.804 | ||||||
| Rectum | 26 (65.0) | 35.9 | |||||
| Sigmoid colon | 14 (35.0) | 37.4 | |||||
| Preoperative CEA (ng/mL) | 0.876 | ||||||
| ≤5 | 23 (57.5) | 39.5 | |||||
| >5 | 17 (42.5) | 32.4 | |||||
| Preoperative CA199 (U/mL) | 0.428 | ||||||
| ≤37 | 26 (65.0) | 42.5 | |||||
| >37 | 14 (35.0) | 18.2 | |||||
| Operative time (min) | 0.626 | ||||||
| >200 | 18 (45.0) | 33.3 | |||||
| ≤200 | 22 (55.0) | 38.0 | |||||
| Blood loss (mL) | 0.250 | ||||||
| >500 | 23 (57.5) | 27.2 | |||||
| ≤500 | 17 (42.5) | 51.5 | |||||
| Primary/recurrent tumor | 0.031 | 0.674 | 0.277–1.644 | 0.386 | |||
| LACRC | 29 (72.5) | 40.1 | |||||
| LRCRC | 11 (27.5) | 27.3 | |||||
| Surgery type | 0.452 | ||||||
| TPE | 24 (60.0) | 33.2 | |||||
| PE | 16 (40.0) | 41.7 | |||||
| Lymph node metastasis | 0.035 | 4.380 | 1.493–12.850 | 0.007 | |||
| N0 | 25 (62.5) | 43.2 | |||||
| N+ | 15 (37.5) | 24.4 | |||||
| Postoperative complication | 0.015 | 0.217 | 0.074–0.636 | 0.005 | |||
| No | 29 (72.5) | 49.0 | |||||
| Yes | 11 (27.5) | 10.9 | |||||
| MSI state | 0.362 | ||||||
| MSI-H | 8 (20.0) | 43.8 | |||||
| MSS | 11 (27.5) | 25.5 | |||||
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CA119, carbohydrate antigen 199; LACRC, locally advanced colorectal cancer; LRCRC, locally recurrent colorectal cancer; TPE, total pelvic exenteration; PE, pelvic exenteration; MSI, microsatellite instability; MSI-H, MSI-high; MSS, microsatellite stable.
Discussion
PE is used with curative intent for advanced primary and recurrent pelvic malignancies and is now the mainstay of treatment for pelvic malignancies. With the rise of immunotherapy, the application of radiotherapy, the constant updating of chemotherapy regimens, and the continuous improvement of surgical techniques, the mortality rate of patients undergoing PE has decreased from the initial 33% to less than 10% (7-9). However, the postoperative complications remain high (13–75%) (10,11), the 5-year survival rate of patients with primary pelvic malignancies undergoing PE is 32–66%, and the 5-year survival rate of patients with recurrence is 0–23% (12,13). The safety and feasibility of PE has been demonstrated based on the high R0 resection rate and manageable postoperative complications as well as the good prognosis.
A large collaborative study including data from 1,184 patients from 27 international treatment centers from 2004 to 2014 showed that 55.4% of patients achieved R0 resection (14), and their OS was closely related to the margin status, with 5-year OS rates of 28.2% and 17.3% for R0 and R1 resected patients, respectively, and 3% for R2 resected patients.
As for gynecologic tumors, radiotherapy is preferred for the treatment of locally advanced cervical cancer, followed by surgery. Approximately one-third of primary patients have local residual tumor or recurrent changes after surgery, and PE (APE, PPE or TPE) resection is feasible to achieve radical tumor resection in patients with locally advanced disease who are selected appropriately and do not have distant metastases (15). The overall 5-year postoperative survival rate of the 19 cervical cancer patients included in the study was 25.3%, which was low due to the small number of cervical cancer patients and the presence of 2 cases with R1 resection and poor long-term prognosis.
In addition, the extensive resection and trauma associated with PE surgery resulted in a high rate of postoperative complications. Some 47.3% of cervical cancer patients at our institution had postoperative complications, with urinary complications being more common, which were reported in the literature to be related to urinary reconstruction, Houvenaeghel et al. also reported a rate of similar complications associated with ureteral skin fistula of 42% (16). For patients with rectal cancer invading only the male reproductive system, we tend to protect the bladder when R0 can be guaranteed, and there is a study (17) that confirm that the difference in OS between patients with rectal cancer plus prostatectomy and those with the addition of cystectomy was not statistically significant. Ileal neobladder surgery is frequently used in our department to reconstruct the urinary system. This technique has a lower infection rate compared to the colon-in-lieu bladder procedure, and there was no statistically significant difference between the two in terms of complications (18). Nguyen et al. (19) figure that double-barrelled uro-colostomy (DBUC) had a lower incidence of postoperative urological complications than the ileal conduit (IC) group, but there was no statistical difference between the two groups. The DBUC technique has the advantage of having only one stoma, but we did not use the DBUC technique due to the fact that postoperative urine and feces are discharged together, which may make nursing care more difficult and reduce the quality of life of the patient.
The long-term prognosis of patients with rectal cancer was better than that of those in the cervical cancer group, with a 5-year survival rate of 36.7% on the basis of R0 radical resection achieved in all 40 patients, the main cause of postoperative death in rectal cancer patients is distant metastasis (lung, liver, brain), followed by severe complications such as sepsis, intestinal fistula, and abdominal bleeding. Corresponding R0 radical resection was a determining factor in the survival prognosis of patients. In addition, the incidence of postoperative complications in patients with rectal cancer was lower than that of those with cervical cancer, at 33.9%. Perineal infections were predominant, and other complications mainly included gastrointestinal manifestations.
In the rectal cancer group, this study reported long-term postoperative prognostic outcomes for LACRC/LRCRC undergoing PE, with a 5-year postoperative survival rate of 40.1% for LACRC patients compared to 27.3% for LRCRC patients. Nielsen et al. (20) also derived a 5-year survival rate of 46% for LACRC and 17% for LRCRC, and this institution’s LACRC/LRCRC patients had a slightly higher survival rate than the mean, which may be related to the fact that all 40 patients in the rectal cancer group achieved R0 resection, providing further evidence that R0 resection is still an important factor affecting patient survival.
In this study, no significant difference in postoperative long-term survival was observed between patients treated with neoadjuvant therapy and those not treated with neoadjuvant therapy. It has been suggested that neoadjuvant radiotherapy combined with TPE for primary T4b rectal cancer did not show an advantage in reducing the local recurrence rate, nor did it prolong the recurrence-free survival and OS of patients, and it also increased the incidence of postoperative complications (21). In addition, Duldulao et al. investigated the distribution of residual cancer cells in the various layers of the intestinal wall in patients treated with neoadjuvant radiotherapy and found that most of the tumor cells did not appear in the mucosal and submucosal layers of the intestinal wall after standard neoadjuvant therapy, but were mostly distributed in the lamina propria and subserous layer. In particular, patients with ypT4 stage were more common (22). In addition, neoadjuvant radiotherapy increases the cost of hospitalization and the complications associated with it can be painful for patients.
The 5-year survival rate of patients with MSI-H status in this study was 43.8%, which was higher than that of patients with MSS status (25.5%). A large number of lymphocytes were clustered around MSI-H tumor cells and most of them showed Crohn’s-like inflammatory response. This altered tumor cell microenvironment may be related to patient prognosis (23); MSI-H has a better prognosis for long-term survival in patients with stage II and III colorectal cancer (6), and immune checkpoint inhibitors are expected to be effective agents in the treatment of patients with locally advanced primary and recurrent rectal cancer of MSI-H type in future comprehensive tumor therapy.
Lymph node metastasis, as the main metastatic route of rectal cancer, is an important indicator for evaluating the long-term prognosis of patients. A study has shown that lymph node ratio (LNR) can be an important predictor of overall postoperative survival and disease-free survival in patients with locally progressive rectal cancer (24). In this study, the results showed that the 5-year survival rate was 24.4% for patients with postoperative pathologically positive lymph nodes and 43.2% for patients with negative lymph nodes, with statistically significant differences. In fact, the detection of positive postoperative lymph nodes is influenced by multiple factors, such as the complete clearance of the surgical lymph node extent area, the technique of the pathologist in freeing and sorting the lymph nodes, and the influence of preoperative neoadjuvant radiotherapy (25).
The surgical indications, areas of dissection and principles of management of lateral lymph node dissection for rectal cancer have been highly controversial in Eastern and Western countries (26,27). Western countries prefer total mesorectal excision (TME) for rectal cancer after neoadjuvant therapy. Eastern countries prefer prophylactic lateral lymph node dissection (28). According to the results of a meta-study (29), lateral lymph node dissection after neoadjuvant therapy does not increase long-term patient survival and only reduces local recurrence compared with TME surgery after neoadjuvant therapy. However, a single-centre study (30) from a Chinese authority reported significant differences in both overall and disease-free survival in patients with positive lateral lymph nodes in studies spanning a decade. For patients undergoing PE, if preoperative imaging suggests the presence of suspected malignant lymph nodes, MRI and PET-CT should be performed to determine this and to avoid unnecessary extended dissection. If lymph node positivity cannot be determined preoperatively, prophylactic dissection is considered necessary by us because it is related to the long-term survival of the patient.
Postoperative complications in the rectal cancer group were an independent prognostic factor affecting patient survival. In terms of postoperative complications (31) after PE, more clinical attention should be paid to perineal wound infection and intestinal obstruction, and some patients will be complicated by sepsis, renal failure, intestinal obstruction, and urinary tract obstruction in the distant postoperative period, and more rare complications include pulmonary embolism, skin flap necrosis, infectious shock, and enterostomy necrosis, which will seriously threaten patients’ lives if not promptly treated. The management of perineal wounds is individualised and we routinely perform direct suturing and negative pressure drainage. For patients with unsatisfactory intraoperative haemostasis, intraoperative contamination or large perineal wounds, we choose to tamponade the perineum with iodophor gauze. For difficult-to-heal perineal incisions, we choose to perform flap grafting at a later stage.
There are some limitations of this study: first, the study was conducted retrospective and the sample size was small, so a large multicenter study is needed. Second, it is difficult to perform long-term studies with the same regimen because of the differences in patients’ radiotherapy regimens and the influence of patients’ physical condition. Third, the cases included in this study were not from the same treatment group, and there were some differences in the procedure performed, which may have had some influence on the results.
Conclusions
Patients with rectal cancer undergoing PE have a better survival prognosis compared to those with cervical cancer. For patients in the rectal cancer group, patients with primary tumor, R0 resection of tumor, negative postoperative lymph nodes, genetic testing for MSI-H and fewer postoperative complications had a better survival prognosis.
PE is a viable option for pelvic malignancies, and aggressive radical resection of the lesion and reduction of postoperative complications can effectively improve the prognosis of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-973/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-973/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-973/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-973/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committees of the Affiliated Cancer Hospital of Zhengzhou University (No. 2016ct083). Due to the retrospective nature of the study and because no patient specimens were used, the requirement for individual consent for this retrospective analysis was waived by the ethics committees.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rottoli M, Vallicelli C, Boschi L, et al. Outcomes of pelvic exenteration for recurrent and primary locally advanced rectal cancer. Int J Surg 2017;48:69-73. [Crossref] [PubMed]
- Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 2013;100:E1-33. [Crossref] [PubMed]
- Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948;1:177-83. [Crossref] [PubMed]
- Brintnall ES, Flocks RH. En masse "pelvic viscerectomy" with ureterointestinal anastomosis. AMA Arch Surg 1950;61:851-68. [Crossref] [PubMed]
- Yan XL, Wang K, Bao Q, et al. En bloc right hemicolectomy with pancreatoduodenectomy for right-sided colon cancer invading duodenum. BMC Surg 2021;21:302. [Crossref] [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [Crossref] [PubMed]
- Khan O, Patsouras D, Ravindraanandan M, et al. Total Pelvic Exenteration for Locally Advanced and Recurrent Rectal Cancer: Urological Outcomes and Adverse Events. Eur Urol Focus 2021;7:638-43. [Crossref] [PubMed]
- Law WL, Chu KW, Choi HK. Total pelvic exenteration for locally advanced rectal cancer. J Am Coll Surg 2000;190:78-83. [Crossref] [PubMed]
- Yamada K, Ishizawa T, Niwa K, et al. Pelvic exenteration and sacral resection for locally advanced primary and recurrent rectal cancer. Dis Colon Rectum 2002;45:1078-84. [Crossref] [PubMed]
- Falk RE, Moffat FL, Makowka L, et al. Pelvic exenteration for advanced primary and recurrent adenocarcinoma. Can J Surg 1985;28:539-41. [PubMed]
- Boey J, Wong J, Ong GB. Pelvic exenteration for locally advanced colorectal carcinoma. Ann Surg 1982;195:513-8. [Crossref] [PubMed]
- Liu SY, Wang YN, Zhu WQ, et al. Total pelvic exenteration for locally advanced rectal carcinoma. Dis Colon Rectum 1994;37:172-4. [Crossref] [PubMed]
- Shirouzu K, Isomoto H, Morodomi T, et al. Total pelvic exenteration for locally advanced colorectal carcinoma--postoperative complications. Kurume Med J 1995;42:33-7. [Crossref] [PubMed]
- Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. Br J Surg 2018;105:650-7. [Crossref] [PubMed]
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33-64, 1.
- Houvenaeghel G, Moutardier V, Karsenty G, et al. Major complications of urinary diversion after pelvic exenteration for gynecologic malignancies: a 23-year mono-institutional experience in 124 patients. Gynecol Oncol 2004;92:680-3. [Crossref] [PubMed]
- Platt E, Dovell G, Smolarek S. Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Tech Coloproctol 2018;22:835-45. [Crossref] [PubMed]
- Hagemans JAW, Voogt ELK, Rothbarth J, et al. Outcomes of urinary diversion after surgery for locally advanced or locally recurrent rectal cancer with complete cystectomy; ileal and colon conduit. Eur J Surg Oncol 2020;46:1160-6. [Crossref] [PubMed]
- Nguyen TM, Traeger L, Vather R, et al. Double barrelled uro-colostomy versus Ileal conduit for urinary diversion following pelvic exenteration: a single centre experience. ANZ J Surg 2023;93:2450-6. [Crossref] [PubMed]
- Nielsen MB, Rasmussen PC, Lindegaard JC, et al. A 10-year experience of total pelvic exenteration for primary advanced and locally recurrent rectal cancer based on a prospective database. Colorectal Dis 2012;14:1076-83. [Crossref] [PubMed]
- Wu T, Wen L, Zhang J, et al. Efficacy analysis of neoadjuvant chemoradiotherapy combined with total pelvic exenteration in the treatment of primary T4b rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2019;22:59-65. [PubMed]
- Duldulao MP, Lee W, Streja L, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum 2013;56:142-9. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Dekker JW, Peeters KC, Putter H, et al. Metastatic lymph node ratio in stage III rectal cancer; prognostic significance in addition to the 7th edition of the TNM classification. Eur J Surg Oncol 2010;36:1180-6.
- Wang J, Hassett JM, Dayton MT, et al. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg 2008;12:1790-6. [Crossref] [PubMed]
- Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol 2018;25:1454-5.
- Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42. [Crossref] [PubMed]
- Kusters M, Uehara K, Velde CJHV, et al. Is There Any Reason to Still Consider Lateral Lymph Node Dissection in Rectal Cancer? Rationale and Technique. Clin Colon Rectal Surg 2017;30:346-56. [Crossref] [PubMed]
- Kroon HM, Hoogervorst LA, Hanna-Rivero N, et al. Systematic review and meta-analysis of long-term oncological outcomes of lateral lymph node dissection for metastatic nodes after neoadjuvant chemoradiotherapy in rectal cancer. Eur J Surg Oncol 2022;48:1475-82. [Crossref] [PubMed]
- Tang JQ, Li HY, Liu T, et al. Thirty years' changes of the strategy of lateral lymph node dissection in low rectal cancer: treatment experience and prognostic analysis of 289 cases in one single center. Zhonghua Wei Chang Wai Ke Za Zhi 2021;24:889-96. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]