Regorafenib combined with a PD-1 inhibitor in the second-line setting for unresectable hepatocellular carcinoma in real-world practice
Highlight box
Key findings
• This study delineates the enhanced clinical benefit derived from combining regorafenib with a programmed cell death-1 (PD-1) inhibitor in the second-line setting for advanced hepatocellular carcinoma (HCC) cases.
What is known and what is new?
• The regorafenib and PD-1 inhibitor combination for treating advanced HCC has shown efficacy in previously reported studies.
• This research, including 46 HCC patients, contributes specific findings. Among cases treated with regorafenib plus PD-1 inhibitor, the objective response rate was 21.7%, and the DCR was 47.8%. Notably, median progression-free survival in this group was 11.5 months, significantly surpassing the 5.1 months observed in the regorafenib monotherapy group (P=0.049).
What are the implications, and what should change now?
• The current study underscores the potential of regorafenib plus a PD-1 inhibitor in enhancing outcomes in HCC patients post-first-line therapy. Nevertheless, to consolidate these findings, expansive real-world studies with larger cohorts are warranted.
Introduction
Hepatocellular carcinoma (HCC) represents a major malignancy and the third deadliest cancer globally (1). As a global disease, the epidemiological features and treatment modalities of HCC show regional differences (2). Unfortunately, most HCC patients are diagnosed at a relatively late stage, without options for curative therapy (3). Therefore, developing novel systemic therapeutic strategies for advanced HCC is crucial.
According to the SHARP trial, sorafenib increases in HCC cases (4). Since then, there has been a continual increase in studies investigating vascular endothelial growth factor (VEGF) inhibitors in HCC cases.
Regorafenib is a multi-kinase suppressor, which conferred survival benefit to HCC cases with progression following sorafenib administration in the first-line setting in the RESORCE study (5). However, the survival benefit following regorafenib administration remains limited. At present, there is an urgent need to explore promising therapeutics for the development of new combination therapy regimens involving regorafenib and other systemic agents.
Programmed cell death-1 (PD-1) suppressors have demonstrated durable responses and enhanced long-term survival both in first line treatment (6,7) and after sorafenib failure (8). Recently, encouraging data have provided increasing evidence to support combination regimens including regorafenib and PD-1 suppressors (9-13).
Although multiple second-line treatments for HCC have been developed, patient prognosis in HCC remains poor. However, to our knowledge, administration of regorafenib and a PD-1 inhibitor in combination for HCC has not been reported so far, and no clinical studies have compared the effectiveness of regorafenib and regorafenib plus PD-1 suppressors after first-line failure in HCC in China. Herein, we assessed the combination of regorafenib and a PD-1 inhibitor versus regorafenib alone as second-line treatment for individuals with advanced HCC in real-world practice. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-618/rc).
Methods
Patients
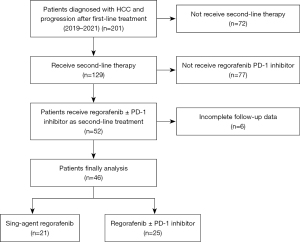
The present single-center, retrospective, real-world study was performed at the First Affiliated Hospital of the Third Military Medical University (Army Medical University) between January 2019 and July 2021. Inclusion criteria were: (I) >18 years old; (II) pathological or clinical diagnosis of HCC; (III) Barcelona Clinic Liver Cancer (BCLC) stage B or C disease and no eligibility for locoregional therapy; and (IV) progression following first-line therapy and administration of regorafenib plus a PD-1 inhibitor or regorafenib monotherapy in the second-line setting. The medical data of all patients were reviewed. This study was carried out in compliance with the Declaration of Helsinki (as revised in 2013). Ethical approval for the current research was granted by the Research Ethics Committee of the First Affiliated Hospital of Third Military Medical University (Army Medical University) with approval number KY2021012. Prior to inclusion in the study, all patients provided signed informed consent. Finally, 46 patients were included from 2019 to 2021. Figure 1 shows the patient selection flowchart.
Outcomes
Response assessment used the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, which uses dynamic computed tomography (CT) or dynamic magnetic resonance imaging (MRI). The disease control rate (DCR) was the total percentage of patients with best response as complete response (CR), partial response (PR), or stable disease (SD). The overall response rate (ORR) was the total percentage of patients with best response as CR or PR. Progression-free survival (PFS) was the time from treatment initiation to disease progression, death, or final recorded contact.
Statistical analyses
Groups were compared by the Chi-square test or Fisher’s exact test. PFS analysis utilized Kaplan-Meier curve analysis and the log-rank test. Subgroup analysis employed a univariable Cox model, and a forest plot was obtained with various hazard ratios (HRs) and 95% confidence intervals (CIs). SPSS 16.0 (SPSS, USA) was used for data analysis.
Results
Baseline characteristics
Between January 2019 and July 2021 , 46 advanced HCC cases administered regorafenib in the second-line setting were consecutively included in the current study. Among them, 21 patients (45.7%) received regorafenib monotherapy, and 25 (54.3%) received combination therapy. Both groups were comparable in sex, age (≤60 vs. >60 years), Child-Pugh grade, alpha-fetoprotein (AFP) (≤400 vs. >400 µg/L), BCLC stage, Eastern Cooperative Oncology Group (ECOG) score, distant metastases, previous systemic treatment, previous local treatment, previous target therapy, portal vein tumor thrombosis (PVTT) type and the starting and final daily doses of regorafenib. Table 1 summarizes all patient features. All included patients shared a common feature of hepatic reserve capacity, with Child-Pugh grade A (N=46, 100%). As for pre-treatment with tyrosine kinase inhibitors (TKIs), most patients had been treated with sorafenib as the primary TKI prior to receiving regorafenib (61.9% and 72% in the regorafenib and combination groups, respectively). Cheng’s classification was used to categorize vascular tumor thrombosis due to its prominence in assessing the condition, treatment selection, and prognosis of Chinese patients. The Chi-square test was conducted to analyze vascular tumor thrombosis, and the results indicated similar PVTT distributions in both groups (P>0.05), with 47.6% type I0, 28.6% type I, 5% type II, 14.3% type III and 5% type IV in the monotherapy group, and 80% type I0, 12% type I, 8% type II, 0% type III and 0% type IV in the combination group (Table 1).
Table 1
| Characteristic | Regorafenib (N=21), n (%) | Regorafenib + PD-1 inhibitor (N=25), n (%) | P value |
|---|---|---|---|
| Sex | 0.439 | ||
| Female | 5 (23.8) | 3 (12.0) | |
| Male | 16 (76.2) | 22 (88.0) | |
| Age >60 years | 0.514 | ||
| No | 17 (81.0) | 18 (72.0) | |
| Yes | 4 (19.0) | 7 (28.0) | |
| Hepatic reserve capacity | 1 | ||
| Child-Pugh grade A | 21 (100.0) | 25 (100.0) | |
| Child-Pugh grade B | 0 | 0 | |
| Child-Pugh grade C | 0 | 0 | |
| BCLC stage | 0.512 | ||
| A | 1 (4.8) | 0 | |
| B | 4 (19.0) | 4 (16.0) | |
| C | 16 (76.2) | 21 (84.0) | |
| ECOG | 0.259 | ||
| 0 | 19 (90.5) | 18 (72.0) | |
| 1 | 2 (9.5) | 7 (28.0) | |
| Distant metastases | 0.179 | ||
| No | 15 (71.4) | 13 (52.0) | |
| Yes | 6 (28.6) | 12 (48.0) | |
| Previous systemic treatment | 0.256 | ||
| Combination therapy | 9 (42.9) | 11 (44.0) | |
| Immunotherapy | 2 (9.5) | 0 | |
| Target therapy | 10 (47.6) | 14 (56.0) | |
| Previous local treatment | 0.163 | ||
| No | 4 (19.0) | 1 (4.0) | |
| Yes | 17 (81.0) | 24 (96.0) | |
| Previous target treatment | 0.102 | ||
| Sorafenib | 13 (61.9) | 18 (72.0) | |
| Renvastinib | 5 (23.8) | 1 (4.0) | |
| Apatinib | 1 (4.8) | 3 (12.0) | |
| Sorafenib and bevacizumab | 1 (4.8) | 0 | |
| Sorafenib and apatinib | 1 (4.8) | 3 (12.0) | |
| Portal vein tumor thrombosis type | 0.079 | ||
| I0 | 10 (47.6) | 20 (80.0) | |
| I | 6 (28.6) | 3 (12.0) | |
| II | 1 (4.8) | 2 (8.0) | |
| III | 3 (14.3) | 0 | |
| IV | 1 (4.8) | 0 | |
| Start dose daily | 0.329 | ||
| 80 mg | 2 (9.5) | 5 (20.0) | |
| 120 mg | 10 (47.6) | 14 (56.0) | |
| 160 mg | 9 (42.9) | 6 (24.0) | |
| Final dose daily | 0.629 | ||
| 80 mg | 5 (23.8) | 9 (36.0) | |
| 120 mg | 9 (42.9) | 8 (32.0) | |
| 160 mg | 7 (33.3) | 8 (32.0) | |
| AFP >400 μg/L | 0.301 | ||
| No | 13 (61.9) | 19 (76.0) | |
| Yes | 8 (38.1) | 6 (24.0) |
PD-1, programmed cell death-1; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; AFP, alpha-fetoprotein.
Main clinical outcomes
Four (8.70%) and 1 (2.17%) patient in the combination and monotherapy groups achieved a CR, respectively, whereas 6 (13.04%) and 3 (6.52%) patients, respectively, achieved a PR, as determined by RECIST 1.1 (Table 2). There were no significant differences in DCR (P=0.158) and ORR (P=0.124) between the two groups.
Table 2
| Response | Regorafenib + PD-1 inhibitor, n (%) | Regorafenib, n (%) | P value |
|---|---|---|---|
| CR | 4 (8.70) | 1 (2.17) | 0.357 |
| PR | 6 (13.04) | 3 (6.52) | 0.478 |
| SD | 12 (26.09) | 11 (23.91) | 0.767 |
| ORR | 10 (21.74) | 4 (8.70) | 0.124 |
| DCR | 22 (47.83) | 15 (32.61) | 0.158 |
Based on RECIST v1.1. PD-1, programmed cell death-1; CR, complete response; PR, partial response; SD, stable disease; ORR, objective response rate; DCR, disease control rate; RECIST, Response Evaluation Criteria in Solid Tumors.
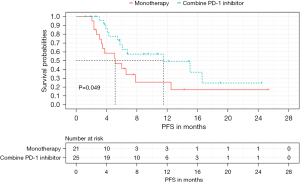
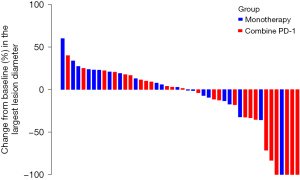
As for survival outcomes, 12 and 14 patients in the combination and monotherapy groups had disease progression, respectively. Median PFS for all patients was 6.57 months (95% CI: 4.20–8.94). PFS was 11.50 months (95% CI: 1.98–21.02) in the combination group, which was remarkably longer compared with that of the monotherapy group (5.13 months, 95% CI: 2.22–8.04; P=0.049) (Figure 2). A Waterfall map was constructed to illustrate long-diameter changes in the largest lesions in the 46 patients (Figure 3).
Subgroup analyses
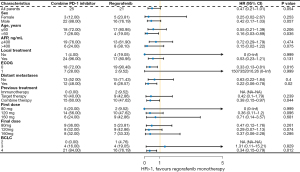
PFS was comparable in all subgroups of the combination and monotherapy groups (Figure 4). Subgroup analysis showed significantly prolonged PFS between groups among patients with age >60 years (P=0.036), ECOG =0 (P=0.016), and distant metastases (P=0.02).
Different regorafenib doses combined with the PD-1 inhibitor
In the combined PD-1 cohort, three different starting doses and a final daily dose of regorafenib were examined, including 80, 120, and 160 mg. However, there were no significant differences in PFS and response among groups with different starting or final regorafenib doses.
Discussion
This study tested the antitumor effects of regorafenib and combined regorafenib and PD-1 inhibitor in HCC cases with disease progression following first-line treatment. The results demonstrated regorafenib plus a PD-1 inhibitor provided PFS benefit in HCC patients as second-line treatment.
Regorafenib has shown survival benefits in HCC cases progressing on sorafenib treatment. In the RESOURCE study, median PFS in the regorafenib group was 3.1 months, which was shorter than observed for the regorafenib monotherapy cohort in this study (5). However, additional studies are still needed to develop new methods for improving treatment efficacy.
The emerging strategy of combining treatments is gaining prominence in HCC’s systemic therapy. The growing antitumor efficacy of this approach is rooted in the synergistic effects of some TKIs with immune checkpoint inhibitors (14). It is crucial to delve deeper into the mechanistic underpinnings that drive the impressive antitumor effects of the regorafenib plus PD-1 inhibitor combination therapy. A recent study has shed light on the intricate crosstalk between the immune system and regorafenib’s unique properties. Specifically, regorafenib displays anti-immunosuppressive properties, promoting antitumor immunity. It achieves this via modulation of macrophages and enhanced CD8+ T cell proliferation and activation (15). As we continue to explore therapeutic frontiers for HCC, integrating such potent combinations holds great promise in optimizing patient outcomes.
Meanwhile, mounting evidence shows immune checkpoint inhibitors can be used as second or above-line treatments (8,16,17). The effects of immune checkpoint inhibitors are dependent upon the tumor microenvironment (TME). Tumor vasculature normalization by anti-angiogenic agents results in increased infiltration of effector immune cells into tumors and the conversion of intrinsic immunosuppressive TME into an immune-supportive TME, which may lead to better results (18). A preclinical study reported that combined treatment with an anti-PD-1 antibody and regorafenib markedly enhances PD-1 blockade dose-dependently in experimental HCC. Moreover, in a phase III study of HCC (7), a strategy combining anti-VEGF (bevacizumab) and anti-PD-L1 (atezolizumab) antibodies also conferred a survival benefit. Therefore, we examined a combination regimen including regorafenib and a PD-1 inhibitor in HCC cases. As demonstrated above, regorafenib + PD-1 inhibitor achieved significantly better PFS as a second-line regimen compared with regorafenib administered alone. Additionally, the combination therapy yielded enhanced response.
As demonstrated above, patients aged >60 years were more prone to benefit from the combination PD-1 inhibitor therapy. Patient age is typically considered an unfavorable prognostic factor. However, some evidence demonstrates that older age might be favorable in terms of survival benefits in patients treated with immunotherapy (19,20). For instance, patients aged 70–80 years showed better PFS and overall survival (OS) after treatment with anti-PD-1 than younger patients (21). Furthermore, a clinical study demonstrated that younger patients have lower levels of CD8+ effector T-cells (22), which likely accounts for the improved efficacy in older patients. Moreover, the presence of extrahepatic metastases may also make it more feasible to benefit from the combination PD-1 inhibitor therapy (23), although the underpinning mechanism remains undefined and deserves further investigation.
Low doses of anti-VEGF TKIs may induce tumor vascular normalization (24). A previous study has reported that low-dose regorafenib and a PD-1 inhibitor exhibit synergistic antitumor effects (25). In the combined PD-1 cohort in the current work, PFS, ORR, and DCR were independent of the starting and final doses of regorafenib, which might be attributed to the limited sample size and short follow-up duration.
Conclusions
This study had several limitations. It had a small sample size (49 patients), and safety data were not assessed. In addition, a relatively short follow-up time was adopted.
Our preliminary data indicate a combination of regorafenib and a PD-1 inhibitor could provide clinical benefits in HCC cases progressing upon first-line treatment. Large-scale prospective studies should be conducted to further investigate the effectiveness of regorafenib in combination with a PD-1 inhibitor in real-world clinical practice.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-618/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-618/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-618/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-618/coif). G.A. receives consulting fees for Amgen, Astellas, Bayer, MSD, BMS, Servier and Lilly, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study complied with the Declaration of Helsinki (as revised in 2013). Ethical approval for the current research was granted by the Research Ethics Committee of the First Affiliated Hospital of Third Military Medical University (Army Medical University) with approval number KY2021012. Prior to inclusion, all patients provided signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Lin CH, Chang CY, Lee KR, et al. Cold-water extracts of Grifola frondosa and its purified active fraction inhibit hepatocellular carcinoma in vitro and in vivo. Exp Biol Med (Maywood) 2016;241:1374-85. [Crossref] [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid 2022;1:EVIDoa2100070.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open 2018;3:e000455. [Crossref] [PubMed]
- Liu JY, Chiang T, Liu CH, et al. Delivery of siRNA Using CXCR4-targeted Nanoparticles Modulates Tumor Microenvironment and Achieves a Potent Antitumor Response in Liver Cancer. Mol Ther 2015;23:1772-82. [Crossref] [PubMed]
- Li Z, Li L, Zhong J, et al. Regorafenib combined with PD-1 monoclonal antibody in the second-line setting for hepatocellular carcinoma (HCC): A retrospective, real-world study in China. J Clin Oncol 2022;40:e16145.
- Yan T, Peng C, Yu L, et al. A retrospective study on the efficacy and safety of regorafenib or regorafenib combined with immune-checkpoint-inhibitors (ICIs) after first-line therapy in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2022;40:e16115.
- Zhao J, Yang C, Han G, et al. Regorafenib joins immune checkpoint inhibitors (ICIs) for previously ICIs plus TKIs failed advanced hepatocellular carcinoma (HCC) patients: A retrospective study. J Clin Oncol 2022;40:e16118.
- Stefanini B, Ielasi L, Chen R, et al. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2023;23:279-91. [Crossref] [PubMed]
- Granito A, Forgione A, Marinelli S, et al. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol 2021;14:17562848211016959. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. [Crossref] [PubMed]
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. [Crossref] [PubMed]
- Hong H, Wang Q, Li J, et al. Aging, Cancer and Immunity. J Cancer 2019;10:3021-7. [Crossref] [PubMed]
- Elias R, Karantanos T, Sira E, et al. Immunotherapy comes of age: immune aging & checkpoint inhibitors. J Geriatr Oncol 2017;8:229-35. [Crossref] [PubMed]
- Bastholt L, Schmidt H, Bjerregaard JK, et al. Age favoured overall survival in a large population-based Danish patient cohort treated with anti-PD1 immune checkpoint inhibitor for metastatic melanoma. Eur J Cancer 2019;119:122-31. [Crossref] [PubMed]
- Kasanen H, Hernberg M, Mäkelä S, et al. Age-associated changes in the immune system may influence the response to anti-PD1 therapy in metastatic melanoma patients. Cancer Immunol Immunother 2020;69:717-30. [Crossref] [PubMed]
- Huang C, Zhu XD, Shen YH, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res 2021;9:19. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Li J, Cong L, Liu J, et al. The Efficacy and Safety of Regorafenib in Combination With Anti-PD-1 Antibody in Refractory Microsatellite Stable Metastatic Colorectal Cancer: A Retrospective Study. Front Oncol 2020;10:594125. [Crossref] [PubMed]