Clinicopathological features and prognoses in younger and older patients with mismatch repair defects colorectal cancer: a retrospective comparative cohort study
Highlight box
Key findings
• In this paper, we found that the composition of microsatellite instability (MSI) status varied among patients of different ages.
• MSI-high (MSI-H) mutations were more common in younger patients.
What is known, and what is new?
• Immunotherapy agents have had surprising efficacy in colorectal cancers (CRCs) with MSI-H mutations.
• MSI-H is presented in only about 10% of all CRCs, so testing for MSI is not widely available.
What is the implication, and what should change now?
• It could prevent physicians from not testing young CRC patients for MSI because the detection rate is too low.
Introduction
With 1.8 million new cases diagnosed every year and 860,000 deaths per year, colorectal cancer (CRC) is the third most common cancer and the second common cause of cancer-related death, exceeded only by lung cancer (1). CRC is a heterogeneous disease that can be classified into different subtypes with different underlying molecular alterations, such as microsatellite instability (MSI), chromosomal instability, and cytosine-phosphate-guanine (CpG)-islet methylation phenotype (2). MSI is a genomic instability in which mutations occur in nucleotide repeat sequences throughout the genome. These repeats are called microsatellites, and the differences among these sequences in tumors and germ cells are referred to as MSI. MSI arises from defects in the DNA mismatch repair system (3). In recent years, the use of immunotherapy-based drugs to treat CRCs with high MSI-high (MSI-H) or DNA deficient mismatch repair (dMMR) has been surprisingly successful (4,5). However, we found that the proportion of MSI-H patients in CRC patients with different ages was significantly different. In this study, we focused on a large cohort of younger patients (aged ≤40 years old) and older patients (aged 71–80 years old) with colorectal tumors to explore the incidence of colorectal MSI in patients of different ages and the relationship between colorectal MSI and prognosis. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-4/rc).
Methods
Patient characteristics and data
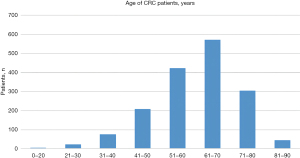
This study was conducted at the Affiliated Cancer Hospital of Zhengzhou University. A total of 1,901 patients who underwent CRC-related genetic testing from January 2017 to December 2019, of whom 1,654 had MSI test results, were included in the study. Of these 1,654 patients, 960 (58.1%) were men and 694 (41.9%) were women. The patients’ ages ranged from 17 to 90 years, with a median age of 61 years. We allocated 100 patients with CRC aged less than 41 years to the younger group, and 305 patients with CRC aged 71 and older but younger than 80 in the older group. The age distribution of these patients is shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Affiliated Cancer Hospital of Zhengzhou University (No. 2021-KY-0012-001), and the requirement of individual consent for this retrospective analysis was waived.
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have undergone preoperative colonoscopy and have a pathological diagnosis of CRC; (II) received perioperative adjuvant therapy; (III) underwent curative surgery for CRC. Each patient was followed up from the time of surgery until April 30, 2023 or death (every 3 months for the first 2 years after surgery, and every 6 months thereafter).
We recorded the patients’ age, sex, body mass index (BMI), tumor depth (T), number of metastatic lymph nodes (N), distant metastasis (M), the tumor, node, metastasis (TNM), the staging was based on the 8th edition of the TNM classification system of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC), radicality, tumor size, tumor site (we classified the tumors as proximal and distal, bounded by the splenic flexure of the colon), degree of differentiation, carcinoembryonic antigen (CEA) (ng/mL) level, and MSI status [fluorescence in situ hybridization (FISH) was used to determine MSI expression in CRC patients].
Statistical analysis
The data analysis was performed using SPSS 26.0 (IBM Corp, Armonk, NY, USA). A Chi-squared test was used to assess the significant differences in the clinicopathological features between the younger and older CRC patients. The survival curves were calculated by a Kaplan-Meier survival analysis, and the equivalences of the survival curves were analyzed using the log-rank test. Univariate and multivariate analyses were evaluated using the Cox proportional hazards model. A P value <0.05 was considered statistically significant.
Follow up
All patients were followed up postoperatively. Patients were followed up with postoperative imaging at an interval of 3 months for the first 3 years and at an interval of 6 months for the next 2 years, and annually thereafter. Each follow-up visit included not only a computed tomography scan of the entire body, but also quantitative testing of tumor markers, including CEA, cancer antigen 19-9, and carbohydrate antigen 72-4. The patients also underwent a colonoscopy once a year after surgery.
Results
Study cohort
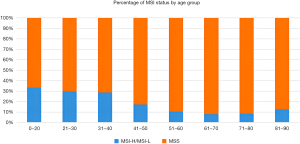
Of the 1,901 patients, 247 were excluded due to poor sample conditions or inadequate clinical information. Figure 1 shows the age distribution of the patients in the remaining sample of 1,654. In relation to age, the frequency of MSI-H was significantly higher in patients younger than or equal to 40 years old (33%) than those aged 51–60 years (10.3%, P<0.0001) (Figure 2). The staging was based on the 8th edition of the TNM classification system of the Union for International Cancer Control (UICC)/AJCC.
Clinicopathological features of the younger and older CRC patients
The clinicopathological characteristics of the 100 younger CRC patients and 305 older CRC patients are shown in Table 1. There were more women in the older CRC patient group than the younger CRC patient group (47.2% vs. 36.3%, P=0.108). The proportion of MSI-H was significantly higher in the younger CRC patients than the older CRC patients (33% vs. 10.16%, P<0.001). The proportion of undifferentiated adenocarcinoma was significantly higher in the younger CRC patients than the older CRC patients (53% vs. 31.15%, P<0.001). The younger patients had a lower BMI than the older patients (P=0.019). In terms of tumor size, tumor diameter was significantly larger in the younger CRC patients than the older CRC patients. Moreover, tumor differentiation was worse in the younger patients than the older patients (P<0.001). However, in relation to the other characteristics, such as surgical eradication, tumor location, vascular invasion, and nerve invasion, there were no significant differences between the younger and older CRC patients.
Table 1
| Characteristic | Younger group (n=100) | Older group (n=305) | P value |
|---|---|---|---|
| Sex | 0.108 | ||
| Male | 62 | 161 | |
| Female | 38 | 144 | |
| MSI status | <0.001 | ||
| MSS | 67 | 274 | |
| MSI-H or MSI-L | 33 | 31 | |
| Tumor location | 0.064 | ||
| Proximal colon | 17 | 84 | |
| Distal colon | 59 | 144 | |
| Rectum | 24 | 77 | |
| BMI | 0.019 | ||
| <24 kg/m2 | 81 | 210 | |
| ≥24 kg/m2 | 19 | 95 | |
| Differentiation | <0.001 | ||
| Poor | 53 | 95 | |
| Well and moderate | 47 | 210 | |
| Tumor size | 0.047 | ||
| <5 cm | 44 | 169 | |
| ≥5 cm | 56 | 136 | |
| TNM stage | 0.067 | ||
| I | 14 | 41 | |
| II | 38 | 155 | |
| III | 41 | 84 | |
| IV | 7 | 25 | |
| Vascular invasion | 0.594 | ||
| No | 58 | 173 | |
| Yes | 40 | 105 | |
| Perineural invasion | 0.535 | ||
| No | 74 | 216 | |
| Yes | 24 | 59 | |
| CEA (ng/mL) | 0.307 | ||
| Negative | 67 | 187 | |
| Positive | 33 | 118 |
Patients aged 40 or younger are defined as the young group, and patients aged 71 and older but younger than 80 are defined as the elderly group. MSI, microsatellite instability; MSS, microsatellite stability; MSI-H, MSI-high; MSI-L, MSI-low; BMI, body mass index; TNM, tumor, node, metastasis; CEA, carcinoembryonic antigen.
Univariate and multivariate analyses
In the Kaplan-Meier survival analyses, the overall survival (OS) time of the 100 younger CRC patients was much better than that of the 305 older CRC patients [Figure 2, P=0.111, hazard ratio (HR), 0.725, 95% confidence interval (CI): 0.488–1.076]. We conducted univariate and multivariate analyses to assess the risk factors for prognosis in the 405 CRC patients (Table 2). The univariate analysis of patient survival in both cohorts showed that the prognosis of CRC patients was associated with MSI status (P<0.001, HR, 0.297, 95% CI: 0.157–0.565), tumor site (proximal to distal and rectum; P=0.034, HR, 1.406 and 1.846, 95% CI: 0.92–2.147, 1.162–2.931), TNM stage (P<0.001, HR, 1.972, 95% CI: 1.432–2.714), vascular invasion (P=0.007, HR, 1.581, 95% CI: 1.135–2.204), and perineural invasion (P=0.003, HR, 1.751, 95% CI: 1.217–2.519). However, in the multivariate analysis, only MSI status (P<0.001, HR, 0.183, 95% CI: 0.088–0.381), tumor location (P<0.001, HR, 1.25, 2.604, 95% CI: 0.797–1.96, 1.599–4.241) and TNM stage (P=0.005, HR, 1.853, 95% CI: 1.208–2.84) were independent risk factors.
Table 2
| Characteristic | Univariate analysis | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 0.111 | ||||||
| Older | 1 | ||||||
| Younger | 0.725 | 0.488–1.076 | |||||
| Gender | 0.447 | ||||||
| Male | 1 | ||||||
| Female | 1.132 | 0.822–1.558 | |||||
| MSI status | <0.001 | <0.001 | |||||
| MSS | 1 | 1 | |||||
| MSI-H or MSI-L | 0.297 | 0.157–0.565 | 0.183 | 0.088–0.381 | |||
| Tumor location | 0.034 | <0.001 | |||||
| Proximal colon | 1 | 1 | |||||
| Distal colon | 1.406 | 0.920–2.147 | 1.250 | 0.797–1.960 | |||
| Rectum | 1.846 | 1.162–2.931 | 2.604 | 1.599–4.241 | |||
| BMI | 0.232 | ||||||
| <24 kg/m2 | 1 | ||||||
| ≥24 kg/m2 | 0.797 | 0.550–1.156 | |||||
| Differentiation | 0.495 | ||||||
| Poor | 1 | ||||||
| Well and moderate | 1.121 | 0.807–1.559 | |||||
| Tumor size | 0.293 | ||||||
| <5 cm | 1 | ||||||
| ≥5 cm | 1.187 | 0.862–1.633 | |||||
| TNM stage | <0.001 | 0.005 | |||||
| I and II | 1 | 1 | |||||
| III and IV | 1.972 | 1.432–2.714 | 1.853 | 1.208–2.840 | |||
| Vascular invasion | 0.007 | 0.905 | |||||
| No | 1 | 1 | |||||
| Yes | 1.581 | 1.135–2.204 | 0.973 | 0.617–1.532 | |||
| Perineural invasion | 0.003 | 0.091 | |||||
| No | 1 | 1 | |||||
| Yes | 1.751 | 1.217–2.519 | 1.407 | 0.947–2.091 | |||
| CEA (ng/mL) | 0.141 | ||||||
| Negative | 1 | ||||||
| Positive | 1.276 | 0.922–1.764 | |||||
Patients aged 40 or younger are defined as the young group, and patients aged 71 and older but younger than 80 are defined as the elderly group. CRC, colorectal cancer; HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; MSS, microsatellite stability; MSI-H, MSI-high; MSI-L, MSI-low; BMI, body mass index; TNM, tumor, node, metastasis; CEA, carcinoembryonic antigen.
Subgroup analysis
We then independently analyzed the risk factors for prognosis in the 100 younger patients and the 305 older patients. In the younger patients, MSI status, AJCC TNM stage, and vascular invasion were all associated with OS in the univariate analysis, but only MSI status and AJCC TNM stage were independent risk factors for OS in the multivariate analysis (Table 3). Among the 305 elderly CRC patients, MSI status, tumor site, tumor size, AJCC TNM stage, and neurological invasion were all associated with OS in the univariate analysis; however, only MSI status, tumor site, and AJCC TNM stage were independent risk factors for OS in the multivariate analysis (Table 4).
Table 3
| Characteristic | Univariate analysis | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender | 0.380 | ||||||
| Male | 1 | ||||||
| Female | 1.373 | 0.676–2.790 | |||||
| MSI status | 0.003 | 0.030 | |||||
| MSS | 1 | 1 | |||||
| MSI-H or MSI-L | 0.169 | 0.051–0.557 | 0.258 | 0.076–0.880 | |||
| Tumor location | 0.435 | ||||||
| Proximal colon | 1 | ||||||
| Distal colon | 1.912 | 0.654–5.594 | |||||
| Rectum | 1.363 | 0.384–4.837 | |||||
| BMI | 0.648 | ||||||
| <24 kg/m2 | 1 | ||||||
| ≥24 kg/m2 | 0.812 | 0.332–1.985 | |||||
| Differentiation | 0.539 | ||||||
| Poor | 1 | ||||||
| Well and moderate | 1.247 | 0.616–2.524 | |||||
| Tumor size | 0.169 | ||||||
| <5 cm | 1 | ||||||
| ≥5 cm | 0.609 | 0.301–1.234 | |||||
| TNM stage | <0.001 | 0.010 | |||||
| I and II | 1 | 1 | |||||
| III and IV | 5.656 | 2.317–13.810 | 3.837 | 1.375–10.707 | |||
| Vascular invasion | 0.030 | 0.935 | |||||
| No | 1 | 1 | |||||
| Yes | 2.191 | 1.077–4.458 | 1.034 | 0.465–2.295 | |||
| Perineural invasion | 0.072 | ||||||
| No | 1 | ||||||
| Yes | 1.970 | 0.941–4.124 | |||||
| CEA (ng/mL) | 0.913 | ||||||
| Negative | 1 | ||||||
| Positive | 0.959 | 0.451–2.038 | |||||
CRC, colorectal cancer; HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; MSS, microsatellite stability; MSI-H, MSI-high; MSI-L, MSI-low; BMI, body mass index; TNM, tumor, node, metastasis; CEA, carcinoembryonic antigen.
Table 4
| Characteristic | Univariate analysis | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender | 0.810 | ||||||
| Male | 1 | ||||||
| Female | 1.045 | 0.730–1.495 | |||||
| MSI status | 0.036 | <0.001 | |||||
| MSS | 1 | 1 | |||||
| MSI-H or MSI-L | 0.442 | 0.206–0.949 | 0.171 | 0.067–0.436 | |||
| Tumor location | 0.018 | <0.001 | |||||
| Proximal colon | 1 | 1 | |||||
| Distal colon | 1.343 | 0.842–2.143 | 1.136 | 0.687–1.877 | |||
| Rectum | 2.008 | 1.222–3.299 | 2.686 | 1.575–4.578 | |||
| BMI | 0.210 | ||||||
| <24 kg/m2 | 1 | ||||||
| ≥24 kg/m2 | 0.770 | 0.511–1.159 | |||||
| Differentiation | 0.447 | ||||||
| Poor | 1 | ||||||
| Well and moderate | 1.158 | 0.794–1.689 | |||||
| Tumor size | 0.033 | 0.115 | |||||
| <5 cm | 1 | 1 | |||||
| ≥5 cm | 1.477 | 1.032–2.113 | 1.375 | 0.926–2.043 | |||
| TNM stage | 0.008 | 0.023 | |||||
| I and II | 1 | 1 | |||||
| III and IV | 1.625 | 1.133–2.331 | 1.599 | 1.067–2.398 | |||
| Vascular invasion | 0.058 | ||||||
| No | 1 | ||||||
| Yes | 1.440 | 0.987–2.101 | |||||
| Perineural invasion | 0.013 | 0.212 | |||||
| No | 1 | 1 | |||||
| Yes | 1.698 | 1.117–2.581 | 1.321 | 0.853–2.046 | |||
| CEA (ng/mL) | 0.095 | ||||||
| Negative | 1 | ||||||
| Positive | 1.361 | 0.948–1.953 | |||||
CRC, colorectal cancer; HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; MSS, microsatellite stability; MSI-H, MSI-high; MSI-L, MSI-low; BMI, body mass index; TNM, tumor, node, metastasis; CEA, carcinoembryonic antigen.
Discussion
In the digestive system, MSI-H tumors are commonly found in CRC, pancreatic and gastric cancers (6). No previous studies had been conducted on the relationship between MSI-H tumors and age. A large sample study of unresectable or metastatic solid tumors in Japan examined the proportion of MSI-H in all tumors and found that the proportion was higher in patients aged less than 40 years than those aged over 80 years (7) The reason for the high proportion of MSI-H in patients aged over 80 years may be related to the occurrence of MLH1 promoter methylation (8). The high proportion of MSI-H in young patients may be related to genetic factors, and it is possible that these patients have Lynch syndrome or dMMR syndrome (9,10).
The proportion of MSI-H in the younger patients in this study was as high as 33%, while in the older group of patients, it was only 10.16% (P<0.001). The clinicopathological characteristics of the 100 younger and 305 older CRC patients are set out in Table 1. We found that more patients in the younger CRC group had a poorly differentiation of tumors than patients in the older CRC group (53% vs. 31.15%, P<0.001). However, in terms of the other characteristics, such as the tumor depth, CEA positive rate, distant metastasis, AJCC TNM stage, tumor size, and tumor depth, there were no significant differences between the younger and older CRC patients.
The 5-year OS rate obtained in our CRC patient cohort (59.3%) was similar to those reported in studies in Brazil (63.5%), Australia (63.0%), and Taiwan (68.7%) (11-13). In our study, the survival analysis showed that the patients in the younger group had a better prognosis than those in the older group. The multivariate analysis showed that MSI status was a significant prognostic factor for survival. The subgroup analysis revealed that MSI-H/MSI-low (MSI-L) status had an independent factor affecting on good prognosis both in the overall CRC cohort and in the younger and older patients, which may be related to the use of immunotherapy in patients (14). In terms of the relationship between tumor site and prognosis, this factor was not statistically significant in the younger group of patients (P=0.435), but was statistically significant in the older group, which may be related to the small sample size of the younger group. Further large-scale and multicenter studies need to be conducted to understand the potential reasons for the differences in outcomes, such as progression-free survival.
After analyzing the data from our unit, we believe that the detection of MSI status is essential for young CRC patients, as it can guide clinical treatment plans. In earlier studies, we found that MSI-H tumors may be insensitive to basic chemotherapy with 5-fluorouracil (10). However, with the introduction of immunotherapy and the advent of immunosuppressive agents [e.g., immune checkpoint inhibitors (ICIs)], ICIs have been shown to have surprising efficacy in the treatment of MSI-H tumors. In the KEYNOTE-164 study, researchers proposed that anti-programmed death-1 therapy has a controlled safety profile for MSI-H/dMMR CRC patients (15). A follow-up KEYNOTE-158 study concluded that ICIs have clinical benefits in patients with unresectable or metastatic MSI-H/dMMR non-CRC (16). The CheckMate 142 trial used a different type of ICIs, but similarly concluded that ICIs could provide a durable response and disease control in patients with dMMR/MSI-H metastatic CRC (17). Moreover, this is not limited to CRC, as similar results have been shown in gastric cancer, indicating that patients with MSI-H can benefit from immunotherapy (18). Thus MSI-H is an important biomarker for predicting the efficacy of ICIs on tumors. However, as CRCs exhibiting MSI-H represent only 10–15% of all CRCs (2), most clinicians do not test for MSI status in CRC patients, despite the availability of more convenient liquid biopsies for clinical implementation (19). Furthermore, high tumor mutational burden (high-TMB), which often co-occurs with MSI-H, can also serve as a prognostic factor for evaluating patient overall survival (20). As a result, patients may not receive the most appropriate treatments, which in turn may affect their prognosis. To our knowledge, this is the first time that MSI data from younger patients in Asia have been compared to the data of older patients. The data and results obtained from this study are informative given the testing protocols and treatment options.
This study had some limitations. First, it was a retrospective study conducted at a single hospital, which may have led to some errors. Second, and perhaps most importantly, while we advised all of the patients who needed adjuvant chemotherapy to attend follow-up appointments at our hospital or other hospitals, not all of them received the follow-up standard treatment. We intend to further validate our findings in a multicenter, large sample study conducted in conjunction with additional units.
Conclusions
MSI-H mutations occur more frequently in younger patients than older patients. This finding provides an important reference basis for clinicians to more accurately conduct targeted testing for patients and to select the most appropriate treatment regimen. By understanding the status of MSI-H mutations, doctors can better evaluate each patient’s condition and develop a more personalized treatment plan to improve the treatment effects and patient survival. Physicians should consider MSI-H mutations in young CRC patients. However, in elderly patients, physicians should pay more attention to their physical condition and drug tolerance due to their decreased physical function. When formulating a treatment plan, physicians should fully consider each patient’s age, physical condition, and the severity of the disease to select the most appropriate treatment plan. In addition, doctors should pay close attention to the nutritional status and psychological state of elderly patients and provide them with necessary support and nursing. By understanding the occurrence of MSI-H mutations in young CRC patients, physicians can better develop personalized treatment plans for patients, improve treatment efficacy, and patient survival. Doctors should also pay attention to the ages of patients and provide targeted diagnosis and treatment plans for patients of different ages.
Acknowledgments
Funding: This study was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-4/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-4/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-4/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Affiliated Cancer Hospital of Zhengzhou University (No. 2021-KY-0012-001), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-2087.e3. [Crossref] [PubMed]
- Muc R, Naidoo R. Microsatellite instability in diagnostic pathology. Current Diagnostic Pathology 2002;8:318-27.
- Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566-76. [Crossref] [PubMed]
- Diaz LA Jr, Shiu KK, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2022;23:659-70. [Crossref] [PubMed]
- Chang L, Chang M, Chang HM, et al. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl Immunohistochem Mol Morphol 2018;26:e15-21. [Crossref] [PubMed]
- Akagi K, Oki E, Taniguchi H, et al. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci 2021;112:1105-13. [Crossref] [PubMed]
- Moreno-Ortiz JM, Jiménez-García J, Gutiérrez-Angulo M, et al. High frequency of MLH1 promoter methylation mediated by gender and age in colorectal tumors from Mexican patients. Gac Med Mex 2021;157:618-23. [Crossref] [PubMed]
- Lynch HT, Lynch PM, Lanspa SJ, et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009;76:1-18. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Aguiar S Junior, Oliveira MM, Silva DRME, et al. Survival of patients with colorectal cancer in a cancer center. Arq Gastroenterol 2020;57:172-7. [Crossref] [PubMed]
- Roder D, Karapetis CS, Wattchow D, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev 2015;16:2431-40. [Crossref] [PubMed]
- Lee CH, Cheng SC, Tung HY, et al. The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran J Public Health 2018;47:519-30.
- Zhang X, Wu T, Cai X, et al. Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities. Front Immunol 2022;13:795972. [Crossref] [PubMed]
- Le DT, Kim TW, Van Cutsem E, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol 2020;38:11-9. [Crossref] [PubMed]
- Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol 2022;33:929-38. [Crossref] [PubMed]
- Lenz HJ, Van Cutsem E, Luisa Limon M, et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol 2022;40:161-70. [Crossref] [PubMed]
- Duan Y, Xu D. Microsatellite instability and immunotherapy in gastric cancer: a narrative review. Precis Cancer Med 2023;6:14.
- Fu Y, Ye Y, Liu X, et al. Analyzing microsatellite instability and gene mutation in circulating cell-free DNA to monitor colorectal cancer progression. Transl Cancer Res 2021;10:2812-21. [Crossref] [PubMed]
- Zhou Z, Li K, Wei Q, et al. Tumor mutation burden determined by a 645-cancer gene panel and compared with microsatellite instability and mismatch repair genes in colorectal cancer. J Gastrointest Oncol 2021;12:2775-87. [Crossref] [PubMed]