
Prognostic nomogram in patients with gastrointestinal stromal tumors: a SEER-based study
Highlight box
Key findings
• The nomogram for the prognosis of gastrointestinal stromal tumor (GIST) has been introduced.
What is known and what is new?
• GISTs are rare tumors of the digestive system, which have poor prognosis. At present, there is no standard to evaluate the prognosis of patients with GIST. tumor-node-metastasis stage is a well-known prognostic factor.
• We found new prognostic factors, such as age, sex, pathological differentiation level, surgical intervention, radiotherapy, and marital status.
What is the implication, and what should change now?
• Leveraging this nomogram offers clinicians a reliable tool, enhancing the precision of their judgments. Nevertheless, performing broader external validations remains a crucial next step.
Introduction
Gastrointestinal stromal tumors (GISTs) are rare tumors of the digestive system and include gastrointestinal stromal sarcoma (GISS) (1). Stemming from pluripotent mesenchymal cells, GISTs can evolve into the interstitial cells of Cajal (ICCs), pivotal pacemaker entities nestled between the circular and longitudinal muscular layers of the gastrointestinal tract’s muscular propria (2). For patients with non-metastatic GISTs, laparoscopic surgical removal remains the primary therapeutic approach (3). In advanced GIST cases, clinicians typically resort to tyrosine kinase inhibitors (TKIs), such as imatinib, sunitinib, and regorafenib. These pharmaceutical agents demonstrate efficacy against certain GIST subtypes, yet their challenges persist, necessitating innovative therapeutic strategies (4). The recurrence rate remains high for GIST due to its rarity, even after intensive treatment (5). The literature regarding this malignancy primarily encompasses case studies and limited-scope retrospective analyses. Typically, prognostic assessments for GIST rely heavily on clinical expertise. Hence, the creation of a visual nomogram for survival prediction can offer clinicians an enhanced tool for individual prognosis assessments. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-27/rc).
Methods
Data source
In this investigation, we utilized a retrospective approach with data sourced from the Surveillance Epidemiology, and End Results (SEER) database. Utilizing SEER*Stat software, information based on histological and pathological assessments from 2010–2019 was extracted. The dataset incorporated factors such as age, sex, primary location of the tumor, pathological grading, and staging (T, N, M, and overall), along with treatment methods, tumor dimensions, marital status, and ethnicity. Eligibility for inclusion encompassed (I) diagnoses between 2010 and 2019; (II) tumors primarily situated in the stomach; (III) confirmation of GIST via ICD-O-3 pathology; and (IV) availability of comprehensive follow-up details. On the other hand, exclusion criteria included (I) the presence of multiple primary tumors; (II) incomplete or unverified histological/pathological data; (III) ambiguous surgical details; (IV) lack of clarity regarding tumor categorization, differentiation level, and staging; (V) uncertain survival duration; and (VI) mortality linked to other malignancies or undetermined origins. After screening, we identified a cohort of 1,213 patients. These individuals were subsequently distributed into modeling and validation cohorts at a 2:1 ratio, resulting in 809 participants in the modeling subset, with the residual 404 assigned to the validation subset. Within the scope of inclusion criteria, we randomly selected a part of people as the validation cohort and the training. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Dataset fundamentals were articulated as percentages. Chi-square tests were used to compare rates, with significance acknowledged when P<0.05. Lasso regression was applied for formative steps on the modeling group’s data. This allowed for the identification of substantial prognostic elements, which then entered the multifaceted analysis via the Cox proportional hazard regression model, presenting their respective hazard ratios (HRs) and 95% confidence intervals (95% CIs). Survival rates at distinct intervals—1, 3, and 5 years—were illustrated graphically. The discriminatory ability of the model was gauged through the area under the receiver operating characteristic (ROC) curve (AUC). Calibration curves were used to further this analysis by juxtaposing predictions against tangible outcomes. Concurrently, a clinical decision curve analysis (DCA) illuminated the clinical utility of the nomogram. Based on inherent prognostic components, risk stratifications for GIST patients emerged. Employing R language, we generated survival state scatter plots. The Kaplan-Meier approach was applied to distinguish disparities between risk tiers, high and low. R language software, version 4.1.3, was used to craft all relevant visualizations and tables.
Results
Patient baseline characteristics
In our investigation, we sourced eligible patients diagnosed with GIST from 2010 to 2019 using the SEER database. The distribution between the modeling and validation groups was set at a 2:1 ratio as detailed in Table 1. A comparative analysis between these two groups revealed no significant variances in their clinical characteristics (P>0.05), ensuring the robustness and reliability of our model.
Table 1
| Characteristic | Training cohort (n=809) | Validation cohort (n=404) | P |
|---|---|---|---|
| Age (years) | 0.531 | ||
| <60 | 252 (20.8) | 118 (9.7) | |
| ≥60 | 557 (45.9) | 286 (23.6) | |
| Sex | 0.902 | ||
| Female | 387 (31.9) | 191 (15.7) | |
| Male | 422 (34.8) | 213 (17.6) | |
| Primary site | 0.033 | ||
| Body | 86 (7.1) | 30 (2.5) | |
| Cardia | 65 (5.4) | 31 (2.6) | |
| Fundus | 132 (10.9) | 79 (6.5) | |
| Greater curvature | 100 (8.2) | 60 (4.9) | |
| Lesser curvature | 84 (6.9) | 59 (4.9) | |
| Overlapping lesion | 34 (2.8) | 22 (1.8) | |
| Pylorus | 61 (5.0) | 25 (2.1) | |
| Stomach | 247 (20.4) | 98 (8.1) | |
| Grade | 0.171 | ||
| I | 174 (14.3) | 69 (5.7) | |
| II | 83 (6.8) | 45 (3.7) | |
| III | 20 (1.6) | 18 (1.5) | |
| IV | 38 (3.1) | 18 (1.5) | |
| Unknown | 494 (40.7) | 254 (20.9) | |
| T | 0.053 | ||
| T1 | 134 (11.0) | 54 (4.5) | |
| T2 | 244 (20.1) | 136 (11.2) | |
| T3 | 204 (16.8) | 85 (7.0) | |
| T4 | 143 (11.8) | 93 (7.7) | |
| TX | 84 (6.9) | 36 (3.0) | |
| N | 0.208 | ||
| N0 | 776 (64.0) | 394 (32.5) | |
| N1 | 33 (2.7) | 10 (0.8) | |
| M | 0.983 | ||
| M0 | 695 (57.3) | 348 (28.7) | |
| M1 | 114 (9.4) | 56 (4.6) | |
| Stage | 0.173 | ||
| I | 354 (29.2) | 165 (13.6) | |
| II | 79 (6.5) | 57 (4.7) | |
| III | 55 (4.5) | 33 (2.7) | |
| IV | 131 (10.8) | 59 (4.9) | |
| Unknown | 190 (15.7) | 90 (7.4) | |
| Surgery | 0.637 | ||
| No | 196 (16.2) | 108 (8.9) | |
| Local surgery | 80 (6.6) | 39 (3.2) | |
| Gastrectomy | 533 (43.9) | 257 (21.2) | |
| Radiation | >0.99 | ||
| None/unknown | 806 (66.4) | 402 (33.1) | |
| Yes | 3 (0.2) | 2 (0.2) | |
| Chemotherapy | 0.899 | ||
| No/unknown | 464 (38.3) | 234 (19.3) | |
| Yes | 345 (28.4) | 170 (14.0) | |
| Systemic treatment | 0.673 | ||
| No | 578 (47.7) | 291 (24.0) | |
| Before surgery | 44 (3.6) | 28 (2.3) | |
| After surgery | 147 (12.1) | 67 (5.5) | |
| Both before and after surgery | 40 (3.3) | 18 (1.5) | |
| Months from diagnosis to treatment | 0.414 | ||
| <1 | 382 (31.5) | 179 (14.8) | |
| ≥1 | 345 (28.4) | 175 (14.4) | |
| Unknown | 82 (6.8) | 50 (4.1) | |
| Tumor size (cm) | 0.275 | ||
| <5 | 125 (10.3) | 64 (5.3) | |
| ≥5 | 150 (12.4) | 60 (4.9) | |
| Unknown | 534 (44.0) | 280 (23.1) | |
| Marital status | 0.644 | ||
| Married | 450 (37.1) | 214 (17.6) | |
| Single | 7 (0.6) | 6 (0.5) | |
| Divorced/widowed/separated | 310 (25.6) | 163 (13.4) | |
| Unknown | 42 (3.5) | 21 (1.7) | |
| Race recode | 0.699 | ||
| White | 484 (39.9) | 229 (18.9) | |
| Black | 166 (13.7) | 93 (7.7) | |
| Other | 154 (12.7) | 80 (6.6) | |
| Unknown | 5 (0.4) | 2 (0.2) |
Univariate and multivariate Cox regression analyses
Single-factor analysis indicated that variables such as age, pathological differentiation, stages T, N, M, and tumor-node-metastasis (TNM) as well as surgical intervention radiation treatment, systemic treatments, post-diagnosis therapy duration, and marital status played a pivotal role in influencing the overall survival (OS) of patients. From an examination of both single-factor and multifactor analysis for the modeling group, it became evident that age, gender, pathological grading, TNM staging, surgical procedures, radiation exposure, and marital status stood out as autonomous determinants of an unfavorable prognosis for those diagnosed with GIST. This is elaborated upon in Table 2.
Table 2
| Characteristics | Total | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| Age (years) | <0.001 | <0.001 | ||||
| <60 | 252 | Reference | ||||
| ≥60 | 557 | 2.111 (1.502–2.968) | <0.001 | 2.414 (1.678–3.472) | <0.001 | |
| Sex | 0.046 | 0.029 | ||||
| Female | 387 | Reference | ||||
| Male | 422 | 1.267 (1.059–1.673) | 0.046 | 1.413 (1.039–1.921) | 0.028 | |
| Primary site | 0.097 | 0.475 | ||||
| Fundus | 132 | Reference | ||||
| Body | 86 | 0.864 (0.513–1.453) | 0.580 | 1.256 (0.731–2.158) | 0.408 | |
| Lesser curvature | 84 | 0.849 (0.513–1.404) | 0.524 | 1.043 (0.610–1.784) | 0.878 | |
| Stomach | 247 | 1.372 (0.956–1.969) | 0.086 | 1.239 (0.843–1.822) | 0.276 | |
| Cardia | 68 | 1.090 (0.628–1.892) | 0.760 | 0.773 (0.430–1.387) | 0.387 | |
| Overlapping lesion | 34 | 1.260 (0.873–1.485) | 0.760 | 0.837 (0.574–1.175) | 0.387 | |
| Greater curvature | 100 | 1.294 (0.673–2.488) | 0.440 | 1.214 (0.614–2.400) | 0.577 | |
| Pylorus | 61 | 0.599 (0.294–1.221) | 0.159 | 0.684 (0.329–1.420) | 0.308 | |
| Grade | 0.037 | 0.044 | ||||
| I | 174 | Reference | ||||
| II | 83 | 1.277 (0.719–2.270) | 0.404 | 1.437 (0.788–2.622) | 0.237 | |
| III | 20 | 1.880 (0.782–4.518) | 0.158 | 2.167 (0.855–5.494) | 0.103 | |
| IV | 38 | 2.281 (1.227–4.242) | 0.009 | 1.660 (0.787–3.504) | 0.183 | |
| Unknown | 494 | 1.673 (1.125–2.488) | 0.011 | 0.832 (0.521–1.330) | 0.443 | |
| T | <0.001 | 0.305 | ||||
| T1 | 134 | Reference | ||||
| T2 | 244 | 0.941 (0.580–1.526) | 0.806 | 0.925 (0.558–1.534) | 0.764 | |
| T3 | 204 | 1.139 (0.763–1.699) | 0.524 | 1.124 (0.727–1.738) | 0.599 | |
| T4 | 143 | 1.558 (1.024–2.371) | 0.038 | 1.700 (0.987–2.928) | 0.056 | |
| TX | 84 | 3.253 (2.143–4.936) | <0.001 | 1.339 (0.790–2.268) | 0.278 | |
| N | 0.013 | 0.232 | ||||
| N0 | 776 | Reference | ||||
| N1 | 33 | 1.970 (1.144–3.392) | 0.014 | 0.598 (0.260–1.375) | 0.226 | |
| M | <0.001 | 0.370 | ||||
| M0 | 695 | Reference | ||||
| M1 | 114 | 2.692 (1.967–3.686) | <0.001 | 0.599 (0.199–1.797) | 0.360 | |
| Stage | <0.001 | 0.040 | ||||
| I | 354 | Reference | ||||
| II | 79 | 0.499 (0.239–1.039) | 0.063 | 0.458 (0.201–1.041) | 0.062 | |
| III | 55 | 1.765 (1.021–3.051) | 0.042 | 1.345 (0.660–2.738) | 0.414 | |
| IV | 131 | 3.328 (2.342–4.730) | <0.001 | 2.896 (0.892–9.396) | 0.077 | |
| Unknown | 190 | 2.081 (1.446–2.995) | <0.001 | 1.080 (0.673–1.734) | 0.749 | |
| Surgery | <0.001 | 0.031 | ||||
| No | 196 | Reference | ||||
| Local surgery | 80 | 0.343 (0.215–0.549) | <0.001 | 0.632 (0.221–1.803) | 0.391 | |
| Gastrectomy | 533 | 0.231 (0.172–0.310) | <0.001 | 0.407 (0.154–1.077) | 0.070 | |
| Radiation | 0.002 | 0.008 | ||||
| None/unknown | 806 | Reference | ||||
| Yes | 3 | 6.988 (1.721–28.382) | 0.007 | 8.783 (1.796–42.940) | 0.007 | |
| Chemotherapy | 0.069 | 0.487 | ||||
| No/unknown | 464 | Reference | ||||
| Yes | 345 | 1.292 (0.980–1.704) | 0.069 | 1.410 (0.542–3.666) | 0.481 | |
| Systemic treatment | 0.025 | 0.349 | ||||
| No | 578 | Reference | ||||
| Before surgery | 44 | 0.986 (0.520–1.870) | 0.966 | 1.014 (0.308–3.335) | 0.982 | |
| After surgery | 147 | 0.557 (0.373–0.833) | 0.004 | 0.554 (0.209–1.467) | 0.235 | |
| Both before and after surgery | 40 | 0.614 (0.272–1.389) | 0.242 | 0.693 (0.192–2.506) | 0.576 | |
| Months from diagnosis to treatment | <0.001 | 0.248 | ||||
| <1 | 345 | Reference | ||||
| ≥1 | 82 | 2.949 (1.999–4.352) | <0.001 | 2.024 (0.795–5.152) | 0.139 | |
| Unknown | 382 | 0.877 (0.645–1.192) | 0.402 | 1.197 (0.849–1.688) | 0.306 | |
| Tumor size (cm) | 0.540 | 0.637 | ||||
| <5 | 125 | Reference | ||||
| ≥5 | 150 | 1.388 (0.674–2.859) | 0.374 | 0.889 (0.413–1.917) | 0.764 | |
| Unknown | 534 | 1.401 (0.768–2.556) | 0.272 | 1.137 (0.607–2.129) | 0.688 | |
| Marital status | 0.004 | 0.007 | ||||
| Married | 450 | Reference | ||||
| Single | 7 | 0.739 (0.103–5.301) | 0.763 | 1.156 (0.156–8.544) | 0.887 | |
| Divorced/widowed/separated | 310 | 1.670 (1.257–2.219) | <0.001 | 1.765 (1.281–2.431) | <0.001 | |
| Unknown | 42 | 1.399 (0.728–2.687) | 0.313 | 1.166 (0.581–2.338) | 0.666 | |
| Race | 0.081 | 0.508 | ||||
| White | 484 | Reference | ||||
| Black | 166 | 1.405 (1.010–1.957) | 0.044 | 1.313 (0.926–1.862) | 0.127 | |
| Other | 154 | 0.887 (0.605–1.298) | 0.536 | 1.085 (0.728–1.617) | 0.687 | |
| Unknown | 5 | 0.000 (0.000–Inf) | 0.993 | 0.000 (0.000–Inf) | 0.993 | |
CI, confidence interval.
Construction and validation of the prognostic nomogram
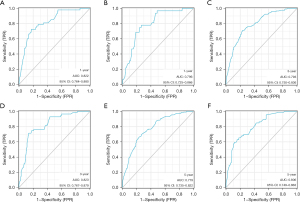
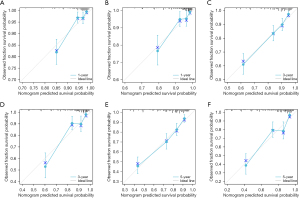
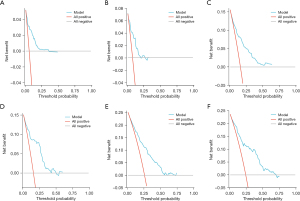
Based on the significance of various parameters from the multifactorial assessment, we allocated distinct scores to each. By summing these scores, we generated a comprehensive prognosis evaluation for patients. In our modeling cohort, the multivariate investigation identified age, sex, pathological grade, TNM classification, surgical intervention, radiation therapy, and marital status as pivotal factors determining the outcome in GIST cases. Using the aforementioned seven criteria, we devised a nomogram to project survival outcomes, particularly focusing on the 1-, 3-, and 5-year OS probabilities; details can be observed in Figure 1. For the ROC analysis, the AUC metrics for the 1-, 3-, and 5-year survival probabilities in the modeling cohort were 0.822, 0.793, and 0.779, respectively, while those for the validation cohort were respectively 0.796, 0.823, and 0.806, suggesting robust model precision (refer to Figure 2). Furthermore, a calibration curve was formulated to reaffirm the predictive efficiency of our model. In this curve, the X-axis illustrates the survival probabilities as per the nomogram, while the Y-axis shows the actual patient outcomes. The congruence between the dotted and solid trajectories within the diagram denotes the nomogram’s precision. Intriguingly, the calibration curve’s observed versus projected values for the 1-, 3-, and 5-year survival probabilities in both the modeling and validation cohorts were notably aligned (see Figure 3). Additionally, our clinical DCA reaffirmed the efficacy of our model in both groups, indicating its potential clinical utility and patient benefit (displayed in Figure 4).

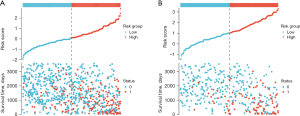
Risk stratification analysis
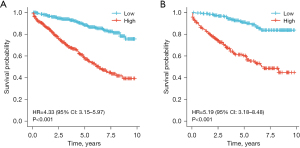
Utilizing the predictive indicators present in the nomogram, we categorized the individuals from both the modeling and validation cohorts into high-risk and low-risk segments. With R language, we graphically represented the survival patterns of these subgroups (refer to Figure 5). Evidently, the scatterplot elucidated a higher mortality rate among high-risk participants than among their low-risk counterparts. A subsequent Kaplan-Meier survival evaluation revealed that the low-risk segment demonstrated a substantially elevated survival trajectory in both cohorts relative to the high-risk segment. The marked discrepancy in the OS trajectories between these risk divisions was underpinned by a significant difference (P<0.001), reinforcing the adept predictive caliber of our constructed nomogram (see Figure 6).
Discussion
Over the decade from 2010 to 2019, our investigation encompassed 1,213 cases diagnosed with GIST. This expansive dataset allowed for a robust assessment of the survival metrics, specifically at the 1-, 3-, and 5-year marks. From our evaluations, several variables emerged as key independent prognostic indicators: age, sex, pathological differentiation, TNM stage, surgical intervention, radiotherapy, and marital status.
The literature suggests that individuals around the age of 60 are predominantly diagnosed with this condition (6,7). Aligning with these findings, we utilized 60 years as a critical age threshold, deducing age’s profound impact on prognosis. In a unique revelation of this study, gender was identified as an influential prognostic factor, with females in China exhibiting a heightened predisposition toward soft tissue sarcomas (8). Furthermore, marital status’s role in cancer prognosis has been underscored in various retrospective evaluations (9,10). Our multivariate analysis echoes these sentiments, reinforcing marital status as a pivotal prognostic element, likely attributed to the emotional and psychological support offered through marital bonds. Beyrouti and colleagues elucidated the rarity of stomach sarcoma and the dependence of its prognosis on varied parameters (11). Consistent with prior research, this study accentuates the inverse relationship between the AJCC staging post-GIST resection and the 5-year disease-free survival (DFS) rate (12). The prognosis of GIST is significantly influenced by TNM stage. Surgical interventions remain paramount in managing GIST. Shannon et al. pinpointed augmented OS in older patients undergoing resection, albeit with notable mortality risks (13). Peiper et al. highlighted the favorable outcomes following an extensive initial resection (14). Meanwhile, a study by Gronchi et al. underscored the disparities in OS among patients with varied resection extents (15). Our data included some ambiguities regarding surgical methodologies. However, we broadly categorized surgical intervention as a binary factor, underscoring its profound positive influence on prognosis. Traditionally, GIST treatments seldom involve radiotherapy. However, scholars in certain studies propose its potential benefits (16,17). Zhang et al. conducted a comprehensive review, suggesting radiotherapy’s efficacy in selecting advanced GIST cases, albeit without discernible survival enhancements (18). Our research posits radiotherapy as an influential prognostic factor, albeit indicative of an adverse prognosis. Further prospective evaluations are warranted to delve deeper into this association.
Our investigations demonstrate that the nomogram model holds promise in accurately forecasting the survival outcomes of individuals with GIST, thereby enhancing the precision of clinical judgments. Nevertheless, the limitations of this study also need to be noted. First, the SEER database, a prominent clinical cancer repository in the U.S., predominantly represents Black and White populations, leaving the Asian demographic underrepresented. Second, inherent to its retrospective nature, this work may encompass unforeseen selection biases. Last, our validation was internally confined; expanded validation through alternate databases or prospective evaluations remains paramount. For those diagnosed with GIST, early surgical intervention combined with reinforced psychological support is pivotal. However, large-sample, multicenter retrospective and prospective studies are still needed to provide better guidance on whether postoperative adjuvant radiotherapy should be performed and what kind of surgery should be followed by adjuvant radiotherapy. The original data in SEER database did not contain these clinical characteristics (e.g., mitotic rate, depth of invasion), so statistics could not be made. The study does not show any effect of tumor size, which may be related to the insufficient number of cases and the lack of external validation, and further research is needed. Factors such as type of driver mutations, germline vs. sporadic, and variant mutations within specific exons, allow selecting the type of targeted therapy that would benefit the most patients with advanced disease or patients at increased risk for recurrence. The proposed nomogram ignores the most important advances in the prognostication and therapy of GISTs. This is a limitation of this study, and further research is needed.
Conclusions
Our research is pioneering in devising a survival prognosis tool for individuals diagnosed with GIST. Leveraging this nomogram offers clinicians a reliable tool, enhancing the precision of their judgments. Nevertheless, performing broader external validations remains a crucial next step.
Acknowledgments
Funding: The work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-27/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ueyama T, Guo KJ, Hashimoto H, et al. A clinicopathologic and immunohistochemical study of gastrointestinal stromal tumors. Cancer 1992;69:947-55. [Crossref] [PubMed]
- Khan J, Ullah A, Waheed A, et al. Gastrointestinal Stromal Tumors (GIST): A Population-Based Study Using the SEER Database, including Management and Recent Advances in Targeted Therapy. Cancers (Basel) 2022;14:3689. [Crossref] [PubMed]
- Mazer L, Worth P, Visser B. Minimally invasive options for gastrointestinal stromal tumors of the stomach. Surg Endosc 2021;35:1324-30. [Crossref] [PubMed]
- Klug LR, Khosroyani HM, Kent JD, et al. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol 2022;19:328-41. [Crossref] [PubMed]
- Beham AW, Schaefer IM, Schüler P, et al. Gastrointestinal stromal tumors. Int J Colorectal Dis 2012;27:689-700. [Crossref] [PubMed]
- Piso P, Schlitt HJ, Klempnauer J. Stromal sarcoma of the stomach: therapeutic considerations. Eur J Surg 2000;166:954-8. [Crossref] [PubMed]
- Irving JA, Lerwill MF, Young RH. Gastrointestinal stromal tumors metastatic to the ovary: a report of five cases. Am J Surg Pathol 2005;29:920-6. [Crossref] [PubMed]
- Yang Z, Zheng R, Zhang S, et al. Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol Med 2019;16:565-74. [Crossref] [PubMed]
- Krajc K, Miroševič Š, Sajovic J, et al. Marital status and survival in cancer patients: A systematic review and meta-analysis. Cancer Med 2023;12:1685-708. [Crossref] [PubMed]
- Zhou R, Yan S, Li J. Influence of marital status on the survival of patients with gastric cancer. J Gastroenterol Hepatol 2016;31:768-75. [Crossref] [PubMed]
- Beyrouti MI, Beyrouti R, Ben Amar M, et al. Gastric sarcoma. Presse Med 2008;37:e60-6. [Crossref] [PubMed]
- Rutkowski P, Wozniak A, Dębiec-Rychter M, et al. Clinical utility of the new American Joint Committee on Cancer staging system for gastrointestinal stromal tumors: current overall survival after primary tumor resection. Cancer 2011;117:4916-24. [Crossref] [PubMed]
- Shannon AB, Song Y, Fraker DL, et al. Surgical resection of gastric gastrointestinal stromal tumors (GIST) in octogenarians. Am J Surg 2022;223:325-30. [Crossref] [PubMed]
- Peiper M, Schröder S, Zornig C. Stromal sarcoma of the stomach--a report of 20 surgically treated patients. Langenbecks Arch Surg 1998;383:442-6. [Crossref] [PubMed]
- Gronchi A, Bonvalot S, Poveda Velasco A, et al. Quality of Surgery and Outcome in Localized Gastrointestinal Stromal Tumors Treated Within an International Intergroup Randomized Clinical Trial of Adjuvant Imatinib. JAMA Surg 2020;155:e200397. [Crossref] [PubMed]
- Ozkan EE. Radiotherapy for Gastrointestinal Stromal Tumors. Chin Med J (Engl) 2018;131:235-40. [Crossref] [PubMed]
- Corbin KS, Kindler HL, Liauw SL. Considering the role of radiation therapy for gastrointestinal stromal tumor. Onco Targets Ther 2014;7:713-8. [Crossref] [PubMed]
- Zhang H, Jiang T, Mu M, et al. Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers (Basel) 2022;14:3169. [Crossref] [PubMed]


