RAS and BRAF in metastatic colorectal cancer management
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death in both men and women in the United States with an estimated 134,490 new cases and 49,190 deaths in 2016 (1).
Approximately 25% of patients present with metastatic disease at diagnosis, and about 50% of patients with CRC will eventually develop metastases (2). Since the 1950s and until the last two decades, 5-fluorouracil (5-FU) with leucovorin (LV) remained the standard therapy in advanced CRC (3,4). The current approach to treating metastatic CRC (mCRC) favors the use of combination cytotoxic therapy including 5-FU, LV, and irinotecan (FOLFIRI), 5-FU, LV, and oxaliplatin (FOLFOX), capecitabine and oxaliplatin (XELOX), or 5-FU, LV, oxaliplatin, and irinotecan (FOLFOXIRI) in the first-line setting (5-9). The addition of targeted agents against angiogenesis, in particular the vascular endothelial growth factor A (VEGF-A) inhibitor bevacizumab, to combination chemotherapy has afforded improved outcomes in mCRC in the first-line and second-line settings (10-12).
The treatment landscape in mCRC has been further refined with the advent of targeted therapy against the epidermal growth factor receptor (EGFR). Activation of the mitogen-activated protein kinase (MAPK) signaling pathway has long been recognized to promote colorectal carcinogenesis by affecting cell growth, proliferation, and survival (13). Activation of receptor tyrosine kinases including EGFR and related downstream signaling through RAS, RAF, MAPK kinase (MEK), and extracellular-signal-regulated kinase (ERK) comprise key steps that can be involved along the MAPK tumorigenic cascade. Notably, the presence of activating mutations in RAS and BRAF are associated with poorer prognosis and have been identified as predictors of resistance to anti-EGFR monoclonal antibodies, cetuximab and panitumumab, in mCRC (14-16). Findings from large, randomized clinical trials have recently confirmed the survival benefits afforded by the addition of anti-EGFR monoclonal antibodies to standard combination chemotherapy in RAS and BRAF wild-type metastatic colorectal tumors. Here, we review data from pivotal clinical trials that have redefined our treatment approach in mCRC with respect to RAS and BRAF mutation status.
RAS mutation status as a biomarker of response to anti-EGFR therapy
Oncogenic RAS mutations have historically been present in approximately 40–50% of CRC cases (17). In a recent pooled analysis, the prevalence of RAS mutations in mCRC has been shown to be as high as 55.9% with mutations in KRAS exon 2 being the most common (42.6%) followed by KRAS exon 3 (3.8%), KRAS exon 4 (6.2%), NRAS exon 2 (2.9%), NRAS exon3 (4.2%), and NRAS exon4 (0.3%) mutations (18). Mutations in codons G12D, G12V, and G12C were most common for KRAS exon 2, codons Q61H and Q61R for KRAS exon 3, codons A146T and A146V for KRAS exon 4, codon G12D for NRAS exon2, codons Q61K and Q61R for NRAS exon3, and codon A146T for NRAS exon4. In the initial RASCAL study, the presence of a KRAS mutation was associated with poorer overall survival (OS) and increased risk of relapse in mCRC (19). In addition, an analysis of the N0147 trial has shown an increased relapse rate for RAS-mutant CRC [codon 12 hazard ratio (HR) 1.52; 95% confidence interval (CI), 1.28–1.80; P<0.0001 and codon 13 HR 1.36; 95% CI, 1.04–1.77; P=0.0248] in comparison to RAS-wild-type patients (20). The prognostic value of KRAS mutation in the metastatic disease setting is more controversial, as many non-EGFR containing arms of treatment have failed to show a difference in outcome between KRAS-mutant and KRAS-wild-type CRC (21-23). Since then, seminal clinical trials have shown that RAS mutation status also predicts response to anti-EGFR therapy, in particular cetuximab and panitumumab, in first-line and beyond settings in the treatment of mCRC.
Chemotherapy refractory settings
Cetuximab first gained Food and Drug Administration (FDA) approval on the basis of the BOND trial. This multicenter, randomized control trial (RCT) investigated cetuximab given at initial dose of 400 mg/m2 followed by weekly infusions of 250 mg/m2 alone or in combination with irinotecan in 329 patients with EGFR-expressing mCRC who progressed on one or more lines of irinotecan-based chemotherapy (24). Cetuximab + irinotecan demonstrated a significantly improved overall response rate (ORR) and median progression-free survival (PFS) compared to cetuximab alone (Table 1). Grade 3 or 4 adverse events (AEs) were more frequent in the combination therapy arm including diarrhea (21.2%) and neutropenia (9.4%) than the monotherapy arm, but their incidence and severity were similar to those expected with irinotecan alone. The phase III CO.17 study randomized 572 patients with EGFR-expressing mCRC refractory to chemotherapy to single-agent cetuximab + best supportive care (BSC) vs. BSC alone (25). In contrast to the BOND trial, treatment with cetuximab demonstrated significantly improved OS (HR 0.77; 95% CI, 0.64–0.92; P=0.005) as well as PFS and ORR when compared to BSC alone (Table 1). The incidence of grade ≥3 AEs were higher with cetuximab treatment with respect to rash (11.8% vs. 0.4%), infection without neutropenia (12.8% vs. 5.5%), confusion (5.6% vs. 2.2%), pain (14.9% vs. 7.3%), and hypomagnesemia (5.8% vs. 0.0%). The presence of a grade ≥2 rash was associated with improved survival in the cetuximab arm (HR 0.33; 95% CI, 0.22–0.50; P<0.001).

Full table
Notably, these early studies evaluated the efficacy of anti-EGFR therapy in mCRC so long as tumors were EGFR-expressing; the effect of RAS mutation status and response to anti-EGFR therapy was not investigated. However, a post hoc analysis of the CO.17 trial involving KRAS mutation analysis in 394 tumor specimens collected at the time of diagnosis demonstrated median OS of 4.5 (cetuximab) vs. 4.6 months (BSC, HR 0.98; 95% CI, 0.70–1.37; P=0.89) among KRAS-mutant tumors and median OS of 9.5 (cetuximab) vs. 4.8 months (BSC, HR 0.55; 95% CI, 0.41–0.74; P<0.001) among KRAS-wild-type tumors (26). A similar benefit was seen in median PFS between cetuximab (3.7 months) vs. BSC (1.9 months; HR 0.40; 95% CI, 0.30–0.54; P<0.001) among KRAS-wild-type tumors whereas no benefit in PFS was observed between arms among KRAS- mutant tumors. Of note, KRAS mutation analysis was limited to codons 12 and 13 of exon 2.
KRAS mutation status has similarly been shown to predict benefit to the anti-EGFR monoclonal antibody, panitumumab, in chemotherapy-resistant mCRC. The phase III 408 study assigned 463 patients with EGFR-expressing mCRC who progressed on ≥2 lines of prior chemotherapy to panitumumab [60-minute intravenous (IV) infusion at 6 mg/kg once every 2 weeks] + BSC vs. BSC alone (27). Treatment with panitumumab conferred an advantage in PFS (HR 0.54; 95% CI, 0.44–0.66; P<0.0001) and ORR (10% vs. 0.0%, P<0.0001) over BSC alone. No significant difference in OS (HR 1.00; 95% CI, 0.82–1.22; P=0.81) was observed between arms though 76% of BSC patients were allowed to cross over to the panitumumab arm. Skin-related toxicities were more frequent in the panitumumab arm (90%) than the BSC group (9%). The presence of a grade ≥2 rash was associated with improved PFS (HR 0.62; 95% CI, 0.44–0.88) when compared to those treated with panitumumab having grade 1 skin toxicity. A subsequent exploratory analysis of this cohort involving KRAS mutation testing (codons 12 and 13) in 427 available tumors showed improved PFS in KRAS-wild-type tumors treated with panitumumab (median PFS 12.3 weeks) vs. BSC (median PFS 7.3 weeks; HR 0.45; 95% CI, 0.34–0.59) whereas no benefit was seen in the panitumumab arm (median PFS 7.4 weeks) vs. BSC (median PFS 7.3 weeks; HR 0.99; 95% CI, 0.73–1.36) among KRAS-mutant tumors (28). This treatment effect on PFS was statistically different between KRAS-wild-type tumors vs. KRAS-mutant tumors (P<0.0001). Response to panitumumab was higher in KRAS-wild-type tumors (17%) vs. KRAS-mutant tumors (0%), and patients with wild-type tumors experienced longer OS compared to mutant tumors after adjustment (HR 0.67; 95% CI, 0.55–0.82, both arms combined). Median OS was improved in those allowed to cross over to the panitumumab arm on progression for wild-type tumors (6.8 months) vs. mutant tumors (4.5 months; HR 0.65; 95% CI, 0.47–0.90).
Preliminary results from a recent phase III trial (20100007) involving panitumumab + BSC vs. BSC alone in 377 patients with chemorefractory KRAS-wild-type (exon 2) mCRC have confirmed advantages across all efficacy endpoints with panitumumab therapy over BSC (Table 1). Notably, cross over to panitumumab was not permitted and 270 patients with RAS-wild-type tumors (including exons 3 and 4 of KRAS and NRAS) were enrolled and similarly experienced benefits across all efficacy endpoints, including OS, when treated with panitumumab over BSC alone (29). The recent phase III ASPECCT trial pitted cetuximab vs. panitumumab in a head-to-head investigation in 999 patients with KRAS-wild-type (exon 2) mCRC refractory to prior chemotherapy (30). Panitumumab demonstrated non-inferiority to cetuximab in OS (HR 0.97; 95% CI, 0.84–1.11), PFS (HR 1.00; 95% CI, 0.88–1.14), and response rate [odds ratio (OR) 1.15; 95% CI, 0.83–1.58] in this population. The incidence of grade ≥3 AEs were similar across treatment arms though grade ≥3 skin toxicity was higher with panitumumab (13%) than cetuximab (10%) as well as grade ≥3 hypomagnesemia (7% panitumumab vs. 3% cetuximab). Grade ≥3 infusion reactions occurred less frequently with panitumumab (<0.5%) than cetuximab (2%). Final results have confirmed the non-inferiority of panitumumab to cetuximab for OS in chemotherapy-resistant wild-type KRAS (exon 2) mCRC (31).
In chemotherapy refractory settings, cetuximab or panitumumab offers survival advantages in mCRC that are dependent on KRAS mutation status. The addition of cetuximab to irinotecan can overcome irinotecan resistance in mCRC previously treated with irinotecan-based chemotherapy. Panitumumab is non-inferior in survival to cetuximab in chemotherapy-resistant wild-type KRAS mCRC. The choice of anti-EGFR agent should take into consideration patient factors (e.g., history of infusion reaction) and toxicity profiles of either drug.
First-line settings
The COIN trial randomized 1,630 patients with chemotherapy-naive mCRC to a control arm [choice of capecitabine 850 mg/m2 orally twice daily for 2 weeks + oxaliplatin 130 mg/m2 2-hour infusion (XELOX) every 3 weeks or 5-FU 400 mg/m2 bolus followed by 5-FU 2,400 mg/m2 infusion over 46 hours + LV 175 mg 2-hour infusion + oxaliplatin 85 mg/m2 2-hour infusion (FOLFOX) every 2 weeks] vs. treatment arm (same combination + cetuximab). Tumor samples from 1,316 patients were available for KRAS (codons 12, 13, and 61), NRAS (codons 12 and 61), and BRAF (codons 594 and 600) mutation analysis (32). Among wild-type KRAS tumors, the addition of cetuximab to oxaliplatin-based chemotherapy did not significantly improve OS and PFS compared to oxaliplatin-based chemotherapy alone (Table 2). On subgroup analysis, the addition of cetuximab to FOLFOX demonstrated improved PFS (HR 0.72, 95% CI, 0.53–0.98, P=0.037) but not in combination with XELOX. Notably, significantly more grade ≥3 toxicities involving skin rash, diarrhea, and hand-foot syndrome were observed with the addition of cetuximab to XELOX, which likely contributed to the lack of benefit in this cohort. OS was 8.8 months for BRAF mutants, 13.8 months for NRAS mutants, 14.4 months for KRAS mutants, and 20.1 months for all wild-type tumors.
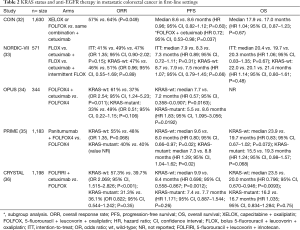
Full table
Further uncertainty on the benefits of adding cetuximab to oxaliplatin-based chemotherapy in previously untreated mCRC arose from the NORDIC-VII study (33). This phase III trial randomized 571 patients to oxaliplatin 85 mg/m2 30–90 minute infusion on day 1 + 5-FU 500 mg/m2 bolus followed by LV 60 mg/m2 bolus on days 1–2 (FLOX) every 2 weeks vs. cetuximab + FLOX vs. cetuximab + intermittent FLOX (discontinued after 16 weeks of treatment). KRAS (codons 12 and 13) and BRAF (codon 600) mutation analysis was performed on 498 and 457 metastatic colorectal tumors, respectively. Response rates, PFS, and OS did not differ between treatment arms among patients with KRAS-wild-type and KRAS-mutant tumors (Table 2). Notably, grade ≥3 neutropenia was seen in 46–49% of patients across all three treatment arms, and as expected, grade ≥3 skin rash was more frequently seen in cetuximab + FLOX (22%) and cetuximab + intermittent FLOX (29%) arms vs. FLOX alone (1%, P<0.01).
The OPUS study randomized 344 patients with mCRC to first-line oxaliplatin 85 mg/m2 2-hour infusion on day 1 + LV 200 mg/m2 2-hour infusion + 5-FU 400 mg/m2 bolus followed by 5-FU 600 mg/m2 22-hour infusion on days 1–2 (FOLFOX4) every 2 weeks vs. FOLFOX4 + cetuximab (34). In contrast to the NORDIC-VII study, this open-label, multicenter phase II trial demonstrated a significantly improved ORR and PFS with the addition of cetuximab to FOLFOX4 among wild-type KRAS tumors but a worse ORR and PFS with FOLFOX4 + cetuximab, when compared to FOLFOX4 alone, among KRAS-mutant tumors (Table 2). The most frequent grade ≥3 AEs were neutropenia (30%), rash (11%), and diarrhea (8%) in the cetuximab + FOLFOX4 arm and neutropenia (34%), rash (0.6%), and diarrhea (7%) in the FOLFOX4 alone arm.
The PRIME study provided convincing evidence for improved PFS and OS with the addition of anti-EGFR therapy to chemotherapy in previously untreated patients with wild-type KRAS (exon 2) metastatic colorectal tumors (35). In this phase III study, panitumumab was combined with FOLFOX4 and compared to FOLFOX4 alone in 1,183 patients in the first-line setting. Notably, a detriment to PFS and OS was observed with the addition of panitumumab to FOLFOX4 when compared to FOLFOX4 alone among those with KRAS-mutant tumors (Table 2). Toxicities were generally comparable across treatment arms except for those known to be associated with anti-EGFR therapy. A later analysis from the PRIME study cohort of 620 tumors absent for KRAS mutations in exon 2 identified the presence of extended RAS mutations [KRAS exon 3 (codon 61) and exon 4 (codons 117 and 146) and NRAS exon 2 (codons 12 and 13), exon 3 (codon 61), and exon 4 (codons 117 and 146)] in 17% of patients (37). The presence of extended RAS mutations was also associated with a worse outcome in PFS and OS when treated with panitumumab + FOLFOX4, while significant improvement in PFS and OS was seen with panitumumab + FOLFOX4, when compared to FOLFOX4 alone, in those without RAS mutations. Furthermore, a significant improvement in OS was seen with panitumumab + FOLFOX4 (28.3 months) over FOLFOX4 alone (20.9 months) in the wild-type RAS and BRAF group.
The phase III CRYSTAL study investigated the efficacy of first-line irinotecan 180 mg/m2 30–90 minute infusion on day 1 + LV 200 mg/m2 L-form or 400 mg/m2 racemic 2-hour infusion followed by 5-FU 400 mg/m2 bolus on day 1 followed by 5-FU 2,400 mg/m2 46-hour infusion (FOLFIRI) on days 1–2 every 2 weeks vs. FOLFIRI + cetuximab in 1,198 patients with mCRC (36). Among wild-type KRAS tumors (limited to codons 12 and 13), the addition of cetuximab to FOLFIRI conferred advantages to ORR, PFS, and OS when compared to FOLFIRI alone (Table 2). These benefits were not observed with the addition of cetuximab to FOLFIRI in KRAS-mutant tumors. Furthermore, early development of an acne-like rash in patients with wild-type KRAS tumors treated with FOLFIRI + cetuximab was associated with improved median OS (26.4 months) vs. those without an early rash (19.1 months). The presence of KRAS mutations predicted worse OS in both treatment groups compared to KRAS-wild-type tumors. The most common grade ≥3 AEs included neutropenia (28.2% FOLFIRI + cetuximab vs. 24.9% FOLFIRI), diarrhea (15.7% vs. 10.5%), and acne-form rash (16.2% vs. 0%). A subsequent post hoc analysis of 430 tumors previously typed as KRAS exon 2 wild-type focused on reevaluating for other RAS mutations [KRAS exons 3 (codons 59 and 61) and 4 (codons 117 and 146) and NRAS exons 2 (codons 12 and 13), 3 (codons 59 and 61), and 4 (codons 117 and 146)] from the CRYSTAL study cohort (38). Other RAS mutations were identified in 14.7% of this cohort (KRAS exon 4 being most common site) and were similarly associated with a lack of benefit across all efficacy endpoints with the addition of cetuximab to FOLFIRI vs. FOLFIRI alone. Treatment with cetuximab offered significant benefit in those with wild-type RAS tumors (28.4 vs. 20.2 months with FOLFIRI alone).
In sum, these findings support a benefit from the addition of cetuximab or panitumumab to combination chemotherapy in the first-line treatment of patients with wild-type KRAS metastatic colorectal tumors. In contrast, the addition of anti-EGFR therapy in metastatic KRAS-mutant tumors (including extended RAS mutations) offers no benefit in this setting and has even been shown to be detrimental. Cetuximab or panitumumab + FOLFOX and cetuximab + FOLFIRI are combinations of proven benefit in previously untreated wild-type KRAS mCRC. Nevertheless, a preferred backbone of chemotherapy has yet to be definitively proven in this setting.
Second-line settings
The EPIC study was an early phase III trial comparing irinotecan (350 mg/m2 90-minute infusion every 3 weeks) to cetuximab + irinotecan in 1,298 patients with EGFR-expressing mCRC who progressed on first-line fluoropyrimidine and oxaliplatin therapy (39). Although conducted prior to the establishment of KRAS as a predictive biomarker, the addition of cetuximab to irinotecan significantly improved PFS and ORR vs. irinotecan alone (Table 3). Median OS was comparable in both arms and the lack in difference may have been contributed by the 46.9% of patients randomized to irinotecan alone who eventually received cetuximab. Grade ≥3 AEs of diarrhea and acne-form rash were more frequent in the cetuximab + irinotecan group (28.4% and 8.2%, respectively) vs. the irinotecan alone group (15.7% and 0.2%, respectively).

Full table
The 20050181 trial, however, randomized 1,186 patients with mCRC to second-line FOLFIRI + panitumumab vs. FOLFIRI and prospectively analyzed efficacy by KRAS mutation status (40). Results from this phase III trial showed an improvement in PFS and ORR with FOLFIRI + panitumumab over FOLFIRI among wild-type KRAS tumors, but no benefits were observed in PFS and ORR with the addition of panitumumab among mutant KRAS tumors (Table 3). In the KRAS-wild-type population, improved median OS was observed in favor of FOLFIRI + panitumumab (14.5 months) over FOLFIRI (12.5 months, P=0.12) though not statistically significant likely secondary to the 31% of patients in the FOLFIRI arm who subsequently received anti-EGFR therapy. Grade ≥3 skin toxicities, neutropenia, and diarrhea were the most frequent AEs in the FOLFIRI + panitumumab arms in both mutant and wild-type cohorts. In an updated analysis, no benefits were observed again with FOLFIRI + panitumumab compared to FOLFIRI in those with extended RAS mutations (42). Significant improvement in PFS was seen with FOLFIRI + panitumumab over FOLFIRI alone in those with wild-type RAS tumors (KRAS and NRAS exons 2, 3, and 4, HR 0.70, 95% CI, 0.54–0.91, P=0.007) and wild-type RAS and BRAF tumors (HR 0.68, 95% CI, 0.51–0.90, P=0.006).
The PICCOLO trial prospectively assigned 460 patients with wild-type KRAS mCRC (codons 12, 13, and 61) who progressed on first-line fluoropyrimidine-based chemotherapy to irinotecan + panitumumab (9 mg/kg IV every 3 weeks) or irinotecan alone (41). The addition of panitumumab to irinotecan offered advantages in PFS and ORR compared to irinotecan alone, but no significant difference was observed in OS between arms (Table 3). The lack of significant difference in OS cannot be attributed to cross over to anti-EGFR therapy in the irinotecan alone group as only 6% subsequently received anti-EGFR therapy. Instead, more rapid tumor growth upon stopping anti-EGFR therapy may have contributed to inferior survival in the irinotecan + panitumumab arm. Notably, interaction tests identified no benefit on PFS or ORR and a detrimental effect on OS with the addition of panitumumab in patients with any mutation present [KRAS (codons 12, 13, 61, and 146), BRAF (codon 600), NRAS (codons 12, 13, and 61), PIK3CA (exons 9 and 20)]. Toxicities were consistent to those seen in prior trials involving anti-EGFR monoclonal antibodies in combination with irinotecan.
The addition of an anti-EGFR agent to the second-line treatment of mCRC offers improvements in PFS and ORR that are limited to those with wild-type KRAS tumors. The lack of OS benefit observed may be attributed to the relatively high degree of cross over to anti-EGFR therapy in control arms and/or worse outcomes seen with anti-EGFR therapy withdrawal in the setting of high dose every-3-week irinotecan. Treatment with anti-EGFR therapy confers a lack of benefit to even harm in those who are KRAS mutant in this setting. As evident from the above, determination of RAS mutation status is crucial in predicting benefit (or harm) from the addition of anti-EGFR therapy across all treatment settings in mCRC.
Anti-EGFR therapy versus bevacizumab in the first-line treatment of RAS-wild-type metastatic CRC
Several large RCTs have attempted to compare the benefits of adding anti-EGFR monoclonal antibodies or bevacizumab to the first-line treatment in patients with wild-type RAS mCRC (Table 4). The phase III FIRE-3 trial randomized 592 patients with wild-type KRAS (exon 2) mCRC to FOLFIRI + bevacizumab (5 mg/kg 90-minute infusion initially then over 60 minutes 2 weeks later followed by over 30 minutes every 2 weeks thereafter) vs. FOLFIRI + cetuximab (43). Significant differences in ORR and PFS were not observed between arms though OS was significantly improved with FOLFIRI + cetuximab over FOLFIRI + bevacizumab. Similar results were seen in those with wild-type tumors at other RAS loci though a marked advantage was observed in median OS with FOLFIRI + cetuximab (33.1 months) over FOLFIRI + bevacizumab (25.6 months; HR 0.70; 95% CI, 0.53–0.92; P=0.011). Hematologic toxicity and diarrhea were among the most common grade ≥3 AEs in both arms; skin reactions were more common with FOLFIRI + cetuximab. A subsequent independent radiological review of FIRE-3 did identify improved ORR with FOLFIRI + cetuximab (71.4%) over FOLFIRI + bevacizumab (56.4%, P=0.015), greater early tumor shrinkage with FOLFIRI + cetuximab (67.5% vs. 47.9%, P=0.0013), and greater depth of response with FOLFIRI + cetuximab (48.2% vs. 33.0%, P=0.0005) that may have all contributed to the discrepancy between OS and PFS across arms (47). However, the limited number of patients who crossed over to anti-EGFR therapy on progression in the bevacizumab-containing arm (41%) may also be contributing.

Full table
The PEAK trial was a phase II study investigating modified FOLFOX6 (mFOLFOX6) + panitumumab vs. mFOLFOX6 + bevacizumab in 285 treatment-naïve patients with wild-type KRAS (exon 2) metastatic colorectal tumors (44). ORR and PFS were similar between arms but OS was significantly improved with mFOLFOX6 + panitumumab over mFOLFOX6 + bevacizumab in the intent-to-treat wild-type KRAS exon 2 cohort (Table 4). In the extended wild-type RAS population, median PFS was significantly improved in favor of mFOLFOX6 + panitumumab (13.0 vs. 9.5 months; HR 0.65; 95% CI, 0.44–0.96; P=0.029) and an improvement in median OS approached significance in favor of mFOLFOX6 + panitumumab [41.3 vs. 28.9 months (HR 0.63, 95% CI, 0.39–1.02, P=0.058)]. Skin toxicity and hypertension were the most common grade ≥3 AEs in the panitumumab- and bevacizumab-containing arms, respectively. Notably, the PEAK trial was limited by relatively small sample size and low cross over rate (38%) onto salvage anti-EGFR therapy on progression in the bevacizumab-containing arm.
The phase III CALGB/SWOG 80405 study randomized 1,137 patients with wild-type KRAS (codons 12 and 13) metastatic colorectal tumors to first-line standard chemotherapy (choice of FOLFIRI or mFOLFOX6) + cetuximab or bevacizumab (45). The majority of patients (73.4%) received mFOLFOX6, and no differences were observed in PFS and OS between arms (Table 4). In an updated analysis, chemotherapy + cetuximab demonstrated an improved ORR in the wild-type KRAS (exon 2) group (65.6% vs. 57.2%) and extended wild-type RAS group (68.6% vs. 53.6%) over chemotherapy + bevacizumab, but again there were no differences in PFS and OS (46). Of 132 patients who were able to undergo curative surgery following chemotherapy, 82 were in the cetuximab arm and 50 were in the bevacizumab arm (48). In this group who achieved curative resection, median OS was more than 5 years but was not significantly different between cetuximab- or bevacizumab-treated patients.
To sum, the FIRE-3, PEAK, and CALGB 80405 studies failed to meet their respective primary endpoints but mature data for depth of response, early tumor shrinkage, and impact of post-progression therapy are still awaited for CALGB 80405. There is preliminary evidence to support the use of anti-EGFR therapy in the first-line treatment of wild-type RAS mCRC over bevacizumab-containing chemotherapy for patients with goals of tumor shrinkage for conversion to surgical resection or symptomatic disease. Nevertheless, an optimal treatment sequence in wild-type RAS mCRC cannot be definitively made with the current evidence, and the choice of first-line anti-EGFR- or bevacizumab-containing therapy should be made based on toxicity, cost, availability, and other parameters tailored to the patient.
KRAS mutant subtypes and impact on outcome
The RASCAL II study further expanded and explored the impact of various KRAS mutations on CRC outcome from the RASCAL study and identified that only those with a glycine to valine substitution on codon 12 (G12V) demonstrated significantly inferior disease-free survival (DFS) and OS in stage III but not stage IV CRC, when compared to wild-type controls, suggesting that KRASG12V mutations may predispose to a more aggressive phenotype (49). In mCRC, pooled analyses have shown that patients with KRASG13D mutated tumors may derive statistically significant benefits from the addition of cetuximab to chemotherapy when compared to other KRAS mutant subgroups in first-line and beyond settings (50,51).
However, in a recent pooled analysis of the 408, PRIME, and 20050181 studies, no KRAS mutant allele [out of the seven most common mutations evaluated in KRAS codons 12 and 13 (G12A, G12C, G12D, G12R, G12S, G12V, and G13D)] was consistently shown to be a prognostic biomarker for PFS or OS in patients with mCRC receiving panitumumab therapy (52). The phase II ICECREAM trial assessed the efficacy of cetuximab vs. cetuximab + irinotecan in mCRC patients stratified to KRASG13D mutation or no mutation (53). Of 51 patients so far enrolled with KRASG13D mutant tumors refractory to irinotecan- or oxaliplatin-based chemotherapy, median time to progression (TTP) was comparable between arms but stable disease (SD) was achieved in 58% of patients treated with cetuximab and 70% with cetuximab + irinotecan. In short, there remains a lack of convincing evidence to argue that anti-EGFR therapy should not be limited to patients with wild-type KRAS mCRC.
BRAF mutation status as a biomarker of response to anti-EGFR therapy
In a pooled analysis of RCTs, the prevalence of BRAF mutations in patients with mCRC has been shown to be 8.1% (18). Oncogenic BRAF mutations, of which the majority are codon V600E, have been associated with microsatellite instability (MSI), multiple sites of metastases, colon (in particular right-sided) rather than rectal tumors, high-grade tumors, mucinous histology, adverse histologic features, older age, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥2, female gender, and poor survival [especially those with microsatellite stable (MSS) tumors] in mCRC (18,54-58). In several mutational analyses, BRAF mutations have been identified as independent factors significantly associated with reduced survival in mCRC patients (57,58). Similar to RAS mutations, BRAF mutation status appears to be a predictor of benefit (or lack of) from anti-EGFR monoclonal antibodies, cetuximab or panitumumab, in first-line and beyond treatment settings in mCRC.
First-line and beyond settings
Early retrospective studies were among the first to identify the lack of benefit to anti-EGFR in those with BRAF-mutated metastatic colorectal tumors. In one study, 6 of 48 patients (12.5%) with BRAF-mutant mCRC were treated with cetuximab or panitumumab in the first-line to fourth-line settings, and none demonstrated an objective response (14). Similarly, out of 79 patients who were KRAS-wild-type, there were no responses seen in the 11 patients who had BRAF mutations treated with cetuximab or panitumumab for their chemorefractory mCRC (15). The addition of anti-EGFR therapy to the first-line or second-line treatment of mCRC has consistently demonstrated a lack of benefit in those with BRAF-mutant tumors compared to their wild-type counterparts (Table 5). These findings have been observed across several phase III trials (22,32,33,36,37,41,42,59). Indeed, a recent meta-analysis of phase II and III trials confirmed that the addition of anti-EGFR therapy to standard chemotherapy or BSC did not significantly improve PFS, OS, or ORR in those with BRAF-mutant mCRC, when compared to control regimens (60). Notably, addition of cetuximab to FOLFIRI in the CRYSTAL study showed a trend towards benefit in PFS and OS (significance was not reached) compared to FOLFIRI alone in those with wild-type KRAS but BRAF-mutant tumors (36). A pooled analysis of the OPUS and CRYSTAL studies also showed a trend towards benefit in PFS and OS (significance not reached) with addition of cetuximab to chemotherapy compared to chemotherapy alone in wild-type KRAS but BRAF-mutant tumors (61). These data, in aggregate, suggest that there is limited activity for anti-EGFR therapy in metastatic BRAF-mutant colorectal tumors. Subsequent investigations have focused on circumventing resistance to EGFR inhibition associated with BRAF mutations in mCRC.
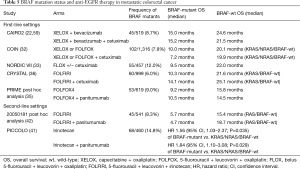
Full table
Targeting additional mediators of the RAS/RAF/MAPK pathway
Aside from targeting EGFR, an initial phase II study investigated vemurafenib, an oral BRAFV600E inhibitor, in 21 patients with predominantly chemorefractory and BRAFV600E-mutant mCRC at 960 mg twice daily (62). Single-agent vemurafenib did not show meaningful activity in this population (Table 6). The most common grade ≥3 AEs were squamous cell carcinoma (SCC) of the skin, hyperbilirubinemia, rash, and hyponatremia. Similarly, in a phase I dose-escalation study where the majority of patients were treated with a recommended phase II dose of the oral BRAF inhibitor dabrafenib (150 mg twice daily), only 1 of 9 evaluable patients (11.1%) with treatment-naïve or refractory BRAF-mutant mCRC experienced an objective response (63). The most frequent grade ≥2 AEs were cutaneous SCC or keratoacanthoma, fatigue, and pyrexia. Another phase I dose-escalation study investigating the oral MEK1/MEK2 inhibitor trametinib [majority at 2 mg daily for 21 days (every 28 days)] failed to identify an objective response in 28 patients with treatment-refractory KRAS- or BRAF-mutant advanced CRC (64). The most common AEs of the overall cohort were dermatitis acneiform (80%) and diarrhea (42%). The limited activity seen with single-agent BRAF and EGFR inhibitors in BRAF-mutant mCRC has been better elucidated with further understanding of feedback loops along the RAS/RAF/MAPK pathway.
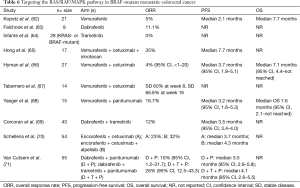
Full table
Constitutive activation of BRAFV600E promotes cell proliferation, growth, and survival through MEK/ERK signaling yet suppresses stimulation from related pathways including RAS-induced activation of RAF isoforms (CRAF and ARAF) through negative feedback (72). Inhibition of BRAFV600E results in loss of negative feedback and promotion of RAS/RAF signaling. Other mediators of BRAF-inhibitor resistance include EGFR activation, mutations in MEK itself, BRAFV600E amplifications, and MAP3K8/COT mutations. Furthermore, BRAF inhibitor-induced feedback activation of EGFR appears to be dependent, in part, by inhibition of the phosphatase CDC25C (73). MEK inhibition, like BRAF inhibition, has also been shown to activate EGFR through feedback regulation (73). In an attempt to overcome the various mechanisms of MAPK pathway-inhibitor resistance, preclinical studies have demonstrated improved antitumor efficacy with combination EGFR, BRAF, or MEK inhibition in BRAF-mutant CRC (73,74). Preclinical studies such as these have essentially established the framework for subsequent clinical trials in BRAF-mutant mCRC treatment.
One phase Ib study attempted to address the compensatory activation of EGFR with BRAF blockade by adding cetuximab to vemurafenib and irinotecan in 17 patients with BRAF-mutant mCRC (Table 6). An improvement in ORR was produced with a median duration of response of 12.5 cycles (65). The maximum tolerated dose (MTD) determined was vemurafenib 960 mg twice daily + cetuximab 250 mg/m2 weekly + irinotecan 180 mg/m2 every 14 days. Fatigue (94%), diarrhea (89%), nausea (83%), and rash (78%) were among the most common AEs. The phase II SWOG 1406 study will build on these findings and investigate irinotecan (180 mg/m2 IV every 2 weeks) + cetuximab (500 mg/m2 IV every 2 weeks) ± vemurafenib (960 mg oral twice daily) in patients with BRAFV600E-mutant mCRC who have progressed on first- or second-line therapies (75). Additionally, several other early phase clinical trials have explored the combination of vemurafenib + EGFR inhibitor and demonstrated modest activity in patients with chemorefractory metastatic BRAF-mutated colorectal tumors (66-68). Notably, data from these trials are preliminary with OS not reached thus rendering definitive conclusions difficult to make until maturation or evaluation in larger trials (Table 6). Interestingly, the incidence of grade ≥3 dermatologic toxicity was lower in combination BRAF + EGFR inhibitor arms than in single-agent BRAF or EGFR inhibitor arms, but abnormal liver function tests were more common in the combination arms (66,68).
Alternatively, combined BRAF and MEK inhibition with dabrafenib (150 mg twice daily) + trametinib (2 mg daily), respectively, demonstrated activity in 43 patients with predominantly pretreated mCRC bearing BRAFV600E mutations (Table 6). Anemia, pyrexia, fatigue, and vomiting were among the most frequent grade ≥3 AEs (69). A phase I study investigated the highly selective oral BRAFV600E inhibitor encorafenib + cetuximab ± the alpha-specific PI3K inhibitor alpelisib in 54 patients with mutated BRAF mCRC (70). The triple combination arm had an improved ORR and PFS than the encorafenib + cetuximab arm though statistical differences, if any, have yet to be reported (Table 6). The most common grade ≥3 AEs included fatigue, hypophosphatemia, and nausea and a MTD of encorafenib 200 mg daily + cetuximab 250 mg/m2 IV weekly ± alpelisib 300 mg daily was proposed. Combinations of dabrafenib (150 mg twice daily) + panitumumab (6 mg/kg IV every 2 weeks) or dabrafenib + trametinib (2 mg daily) + panitumumab were used in the treatment of 55 patients with BRAF-mutant mCRC (71). The triple combination arm demonstrated greater improvement in endpoints as well as more profound reduction in pERK levels on biopsies taken at day 15 compared to the dual combination arm (Table 6). The most common AEs were dermatitis acneiform and diarrhea (triple combination arm) and dermatitis acneiform and fatigue (dual combination arm).
In sum, there is growing evidence to suggest that there is a lack of clinically meaningful benefit with anti-EGFR therapy (single-agent or in combination with standard chemotherapy) in those with BRAF-mutant mCRC. Similarly, single-agent BRAF and MEK inhibitors appear to have limited activity in this group likely owing to mechanisms of resistance/feedback regulation intrinsic to the RAS/RAF/MAPK signaling pathway. Combined inhibition of targets such as EGFR, BRAF, and/or MEK is showing promising activity in BRAF-mutant mCRC compared to historical controls involving single targeting.
BRAF-mutant metastatic CRC and first-line therapy
In an initial phase II study investigating first-line irinotecan 165 mg/m2 IV on day 1 + oxaliplatin 85 mg/m2 IV on day 1 + LV 200 mg/m2 IV on day 1 + 5-FU 3,200 mg/m2 48-hour infusion over days 1–2 (FOLFOXIRI) + bevacizumab every 2 weeks, 214 eligible patients with mCRC were screened for BRAF mutational status (76). Fifteen patients with BRAF-mutant tumors were included in the validation cohort and demonstrated an ORR of 72%, median PFS of 9.2 (95% CI, 5.1–13.3), and median OS of 24.1 months (95% CI, 3.3–45.0). The subsequent phase III TRIBE study randomized 508 patients with mCRC to FOLFOXIRI + bevacizumab vs. FOLFIRI + bevacizumab (every 2 weeks up to 12 cycles then transitioned to maintenance 5-FU/LV + bevacizumab) in the first-line setting (77). FOLFOXIRI + bevacizumab demonstrated superior PFS and OS (median, 29.8 months) over FOLFIRI + bevacizumab [25.8 months (HR 0.80; 95% CI, 0.65–0.98; P=0.03)] in the overall intent-to-treat population. In the BRAF-mutant and extended RAS-mutant subgroups, FOLFOXIRI + bevacizumab also showed improved PFS and OS over FOLFIRI + bevacizumab. Grade ≥3 neutropenia, diarrhea, and asthenia were among the most common AEs in the FOLFOXIRI + bevacizumab arm and were more frequent than in the FOLFIRI + bevacizumab arm. Mutant BRAF status portends an aggressive phenotype and poor prognosis in mCRC. Given the benefits offered by FOLFOXIRI + bevacizumab irrespective of RAS or BRAF mutational status, this combination should be considered in the first-line treatment of BRAF-mutant mCRC.
Discussion and future directions
The management of mCRC with respect to RAS and BRAF mutational status has seen much change over the past decade or so. In 2006, the American Society of Clinical Oncology (ASCO) recommended against routine RAS mutational testing in mCRC due to insufficient and heterogeneous data at the time (78). In 2009, ASCO released a provisional clinical opinion based on available data and recommended routine testing for KRAS mutations (codons 12 and 13) for selection of candidates for treatment with cetuximab and panitumumab in mCRC (79). The National Comprehensive Cancer Network (NCCN) now mandates routine testing for KRAS exon 2 and extended RAS (KRAS non-exon 2 and NRAS) mutations at diagnosis of mCRC; patients with any of these mutations should not be treated with anti-EGFR therapy (80). Furthermore, the panel currently recommends for BRAFV600E mutation testing at diagnosis of stage IV CRC as this mutation has significant prognostic implications that may impact patient counseling and treatment.
The ability to maximize the benefits offered by anti-EGFR therapy in wild-type RAS and BRAF mCRC at present and in the future will likely center around several key issues. Firstly, optimal selection of patients with mCRC who can benefit from anti-EGFR monoclonal antibodies depends on the performance of available molecular analysis technologies in detecting mutations. In an early investigation, 16 KRAS mutations were identified out of 59 patients with mCRC (27%) with traditional direct sequencing analysis (81). The use of more sensitive polymerase chain reaction (PCR)-based techniques detected another 6 KRAS mutations missed by direct sequencing that ultimately led to the detection of 22 mutations (37%) in total. Sanger sequencing has been characterized by poor sensitivity that requires KRAS mutant alleles to represent ≥20% of the signal in order to reliably detect the presence of a mutation (82). In contrast, pyrosequencing or quantitative PCR (qPCR) has greater sensitivity in detecting lower frequency mutations ranging from 1–10% of KRAS mutant DNA in a background of wild-type DNA (83). The significance of low frequency mutations (defined as mutant:wild-type RAS DNA ratio ≤10%) has been shown in patients with mCRC carrying low frequency KRAS mutations that amounted to a significantly inferior ORR and PFS with anti-EGFR therapy compared to wild-type KRAS patients (82). Digital PCR has shown even greater sensitivity with the ability to detect mutant KRAS alleles representing as low as 0.01% of the signal (83). Notably, mCRC patients with mutant:wild-type KRAS DNA ratios less than 1% have similar PFS and OS compared to wild-type KRAS patients treated with anti-EGFR therapy (83). Future studies will need to focus on developing standardized and sensitive techniques to guide clinical decision making in selecting optimal candidates with mCRC for anti-EGFR therapy. The development of next-generation sequencing (NGS) with high-throughput functionality, which has shown comparable accuracy in detecting RAS mutations to real-time PCR, represents one increasingly popular strategy (84).
The optimal selection of mCRC patients who can benefit from anti-EGFR therapy is also dependent on identification of additional markers of resistance in those with wild-type RAS and BRAF tumors. Much progress has been made in understanding that extended RAS mutations including NRAS and non-exon 2 KRAS mutations are predictive of absence of benefit to EGFR blockade. RAS and BRAF mutations are now found in approximately 50–55% of cases of mCRC. Preclinical studies have identified that colorectal tumors harboring amplifications in KRAS, although infrequent (<1%), are not responsive to cetuximab or panitumumab (85). CpG island methylator phenotype (CIMP)-high methylation status (≥40%) has been significantly associated with reduced PFS in wild-type RAS and BRAF mCRC patients treated with first-line anti-EGFR therapy (86). ERRB2 (HER2/neu) and MET amplifications have also been shown to promote resistance to anti-EGFR monoclonal antibodies in colorectal tumors (87,88). Similarly mutations along the PTEN/PIK3CA/AKT signaling pathway have been associated with lack of response to anti-EGFR therapy in wild-type KRAS mCRC (89). PIK3CA mutations (exon 20) have also been associated with worse outcomes in wild-type KRAS mCRC treated with cetuximab (90). The Cancer Genome Atlas (TCGA) Network previously identified gene alterations in BRAF, ARAF, CRAF, and NRAS of very low frequency (<1% of cases) in their CRC dataset whose association with response to anti-EGFR therapy in wild-type RAS and BRAF metastatic colorectal tumors has yet to be elucidated (91). Recent genomic analyses have identified mutations in EGFR (including alterations in the ectodomain of EGFR), FGFR1, PDGFRA, and MAP2K1 as additional potential mediators of resistance to anti-EGFR therapy in wild-type KRAS mCRC (92). Interestingly, alterations in the tyrosine kinase receptor adaptor gene ISR2 may be associated with increased sensitivity to anti-EGFR agents (92). The list of potential markers of anti-EGFR resistance in wild-type RAS and BRAF mCRC continues to grow. Comprehensive genomic analyses via NGS, with its massive throughput capabilities, will likely continue to play a major role in facilitating the search for additional predictors of resistance that were previously undetectable due to feasibility and technical reasons.
A third issue that is important in maximizing the efficacy of anti-EGFR monoclonal antibodies in wild-type RAS and BRAF mCRC involves overcoming mechanisms of resistance. The mechanisms of acquired or secondary resistance to EGFR inhibition has been broadly characterized by mutations that disrupt binding of cetuximab or panitumumab to the EGFR, pathway mutations that bypass the site of inhibition (alterations in KRAS or BRAF), and mutations along parallel pathways (MET or HER2) (93). With the exception of the EGFR extracellular domain mutation S492R, mechanisms of primary resistance overlap with those of secondary resistance. More and more gene alterations are being identified as potential biomarkers of response to EGFR inhibition in wild-type tumors, yet it has been postulated that the majority of alterations will converge to activate the EGFR/RAS/MAPK pathway as evidenced by the frequency of mutations involving mediators of EGFR signaling (EGFR, RAS, BRAF, and PIK3CA). In order to improve the efficacy of anti-EGFR therapy in wild-type RAS and BRAF tumors, future studies will need to focus on the centrality of MAPK signaling in driving resistance.
Preclinical studies have recently demonstrated synergistic antitumor activity in BRAF-mutant CRC cells with addition of PI3K inhibitors or methyltransferase to vemurafenib (94). Similarly, greater tumor regression was seen in BRAF-mutant CRC cells treated with combined BRAF and dual PI3K/mTOR inhibition (95). In BRAF-mutant mCRC patients treated with RAF + EGFR inhibition or RAF + MEK inhibition, acquired resistance was characterized by development of a KRAS amplification, BRAF amplification, ARAF mutation, or MEK1 mutation (96). Addition of an ERK inhibitor was able to suppress MAPK activity and overcome resistance in this group. The growing number of clinical trials investigating combined EGFR, BRAF, and/or MEK inhibition that have shown promising activity in BRAF-mutant mCRC highlight the importance of inhibiting multiple steps along the EGFR signaling cascade to enhance activity. Future trials with combination therapy in both RAS- and BRAF-mutant mCRC are likely to increase in number with the goal to improve therapeutic efficacy through sustained MAPK pathway inhibition.
Lastly, recent analysis of the FIRE-3 trial demonstrated significant improvement in ORR, PFS, and OS with cetuximab therapy in patients with wild-type KRAS metastatic tumors arising from the left colon compared to right colon (97). Similarly, analysis of the CO.17 trial identified that wild-type KRAS mCRC patients with primary tumors located to the left colon experienced significantly improved PFS when treated with cetuximab vs. BSC (median 5.4 vs. 1.8 months; HR 0.28; 95% CI, 0.18–0.45; P<0.0001) while those with primaries located to the right colon did not (97). Median PFS in those with wild-type KRAS metastatic tumors of right colon primary treated with cetuximab was 1.9 vs. 1.9 months in those treated with BSC (HR 0.73; 95% CI, 0.42–1.27; P=0.26). In wild-type KRAS mCRC, there is growing evidence to suggest that tumor location is also predictive of benefit to anti-EGFR therapy. Further investigation is duly warranted to explore the interactions between primary right-sided and left-sided colorectal tumors and response to anti-EGFR therapy in the wild-type RAS and BRAF metastatic population.
Conclusions
All patients with mCRC should undergo RAS (KRAS and NRAS) and BRAF mutational analysis at the time of diagnosis. Treatment with anti-EGFR monoclonal antibodies (cetuximab or panitumumab), either as single-agent or combination therapy, offers improved outcomes in wild-type RAS mCRC. The optimal sequencing of EGFR inhibitors and bevacizumab has yet to be defined. The incorporation of anti-EGFR treatment in the continuum of treatment of metastatic RAS wild-type colon cancer is acceptable in the first, second, or subsequent lines of treatment. There is a lack of benefit and even harm with anti-EGFR therapy in RAS-mutant mCRC. Similarly, BRAF mutations have been shown to predict resistance to anti-EGFR therapy in mCRC. Clinical trials involving combinations of BRAF, EGFR, and/or MEK inhibitors have shown promising activity in BRAF-mutant mCRC. Comprehensive genomic profiling via NGS can facilitate the identification of additional markers of resistance to EGFR inhibition in wild-type RAS and BRAF metastatic colorectal tumors. Future studies will likely focus on combined inhibition of multiple targets along the MAPK signaling pathway to overcome resistance to anti-EGFR therapy in this population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1-9. [Crossref] [PubMed]
- de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997;15:808-15. [PubMed]
- Poon MA, O'Connell MJ, Moertel CG, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 1989;7:1407-18. [PubMed]
- Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12. [Crossref] [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47. [PubMed]
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-6. [Crossref] [PubMed]
- Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779-86. [Crossref] [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [Crossref] [PubMed]
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29-37. [Crossref] [PubMed]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol 2005;6:322-7. [Crossref] [PubMed]
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643-8. [Crossref] [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [Crossref] [PubMed]
- Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992-5. [Crossref] [PubMed]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989;49:4682-9. [PubMed]
- Peeters M, Kafatos G, Taylor A, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: A pooled analysis of randomised controlled trials. Eur J Cancer 2015;51:1704-13. [Crossref] [PubMed]
- Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst 1998;90:675-84. [Crossref] [PubMed]
- Yoon HH, Tougeron D, Shi Q, et al. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin Cancer Res 2014;20:3033-43. [Crossref] [PubMed]
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672-80. [Crossref] [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [Crossref] [PubMed]
- Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J Clin Oncol 2011;29:2675-82. [Crossref] [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [Crossref] [PubMed]
- Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [Crossref] [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [Crossref] [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [Crossref] [PubMed]
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34. [Crossref] [PubMed]
- Kim TW, Elme A, Kusic Z, et al. An open label, randomized phase III trial evaluating the treatment (tx) effects of panitumumab (pmab)+ best supportive care (BSC) versus BSC in chemorefractory wild-type (WT) KRAS exon 2 metastatic colorectal cancer (mCRC) and in WT RAS mCRC. J Clin Oncol 2016;34:abstr 642.
- Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569-79. [Crossref] [PubMed]
- Price TJ, Peeters M, Kim TW, et al. Final results from ASPECCT: Randomized phase 3 non-inferiority study of panitumumab (pmab) vs cetuximab (cmab) in chemorefractory wild-type (WT) KRAS exon 2 metastatic colorectal cancer (mCRC). J Clin Oncol 2015;33:abstr 3586.
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [Crossref] [PubMed]
- Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755-62. [Crossref] [PubMed]
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [Crossref] [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [Crossref] [PubMed]
- Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692-700. [Crossref] [PubMed]
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-9. [Crossref] [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [Crossref] [PubMed]
- Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol 2013;14:749-59. [Crossref] [PubMed]
- Peeters M, Oliner KS, Price TJ, et al. Updated analysis of KRAS/NRAS and BRAF mutations in study 20050181 of panitumumab (pmab) plus FOLFIRI for second-line treatment (tx) of metastatic colorectal cancer (mCRC). J Clin Oncol 2014;32:abstr 3568.
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. [Crossref] [PubMed]
- Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240-7. [Crossref] [PubMed]
- Venook AP, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32:abstr LBA3.
- Lenz H, Niedzwiecki D, Innocenti F, et al. 501ocalgb/Swog 80405: phase III trial of irinotecan/5-Fu/leucovorin (folfiri) or oxaliplatin/5-Fu/leucovorin (mfolfox6) with bevacizumab (bv) or cetuximab (cet) for patients (pts) with expanded Ras analyses untreated metastatic adenocarcinoma of the colon or rectum (mcrc). Ann Oncol 2014;25:mdu438.
- Heinemann V, Modest DP, von Weikersthal LF, et al. Independent radiological evaluation of objective response, early tumor shrinkage, and depth of response in FIRE-3 (AIO KRK-0306). Ann Oncol 2014;25:ii117. [Crossref]
- Venook A, Niedzwiecki D, Lenz H, et al. CALGB/SWOG 80405: Analysis of patients undergoing surgery as part of treatment strategy. Ann Oncol 2014;25:mdu438.
- Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer 2001;85:692-6. [Crossref] [PubMed]
- De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 2010;304:1812-20. [Crossref] [PubMed]
- Tejpar S, Celik I, Schlichting M, et al. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol 2012;30:3570-7. [Crossref] [PubMed]
- Peeters M, Douillard JY, Van Cutsem E, et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol 2013;31:759-65. [Crossref] [PubMed]
- Segelov E, Thavaneswaran S, Waring P. The AGITG ICECREAM Study: The irinotecan cetuximab evaluation and cetuximab response evaluation amongst patients with a G13D mutation—analysis of outcomes in patients with refractory metastatic colorectal cancer harbouring the KRAS G13D mutation. Eur J Cancer 2015;51:S726. [Crossref]
- Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res 2005;65:6063-9. [Crossref] [PubMed]
- Zlobec I, Bihl MP, Schwarb H, et al. Clinicopathological and protein characterization of BRAF- and K-RAS-mutated colorectal cancer and implications for prognosis. Int J Cancer 2010;127:367-80. [PubMed]
- Pai RK, Jayachandran P, Koong AC, et al. BRAF-mutated, microsatellite-stable adenocarcinoma of the proximal colon: an aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am J Surg Pathol 2012;36:744-52. [Crossref] [PubMed]
- Saridaki Z, Tzardi M, Sfakianaki M, et al. BRAFV600E mutation analysis in patients with metastatic colorectal cancer (mCRC) in daily clinical practice: correlations with clinical characteristics, and its impact on patients' outcome. PLoS One 2013;8:e84604. [Crossref] [PubMed]
- Russo AL, Borger DR, Szymonifka J, et al. Mutational analysis and clinical correlation of metastatic colorectal cancer. Cancer 2014;120:1482-90. [Crossref] [PubMed]
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98-9. [Crossref] [PubMed]
- Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 2015;51:587-94. [Crossref] [PubMed]
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75. [Crossref] [PubMed]
- Kopetz S, Desai J, Chan E, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol 2015;33:4032-8. [Crossref] [PubMed]
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901. [Crossref] [PubMed]
- Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:773-81. [Crossref] [PubMed]
- Hong DS, Morris VK, El Osta BE, et al. Phase Ib study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated metastatic colorectal cancer and advanced cancers. J Clin Oncol 2015;33:abstr 3511.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Tabernero J, Chan E, Baselga J, et al. VE-BASKET, a Simon 2-stage adaptive design, phase II, histology-independent study in nonmelanoma solid tumors harboring BRAF V600 mutations (V600m): Activity of vemurafenib (VEM) with or without cetuximab (CTX) in colorectal cancer (CRC). J Clin Oncol 2014;32:abstr 3518.
- Yaeger R, Cercek A, O'Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res 2015;21:1313-20. [Crossref] [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol 2015;33:4023-31. [Crossref] [PubMed]
- Schellens JH, van Geel R, Bendell JC, et al. Abstract CT136: Final biomarker analysis of the phase I study of the selective BRAF V600 inhibitor encorafenib (LGX818) combined with cetuximab with or without the α-specific PI3K inhibitor alpelisib (BYL719) in patients with advanced BRAF-mutant colorectal cancer. Cancer Res 2015;75:CT136. [Crossref]
- Atreya CE, Van Cutsem E, Bendell JC, et al. Updated efficacy of the MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal cancer (mCRC). Ann Oncol 2015;33:103.
- Das Thakur M, Stuart DD. Molecular pathways: response and resistance to BRAF and MEK inhibitors in BRAF(V600E) tumors. Clin Cancer Res 2014;20:1074-80. [Crossref] [PubMed]
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483:100-3. [Crossref] [PubMed]
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227-35. [Crossref] [PubMed]
- Kopetz S, McDonough S, Morris VK, et al. S1406: Randomized phase II study of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (mCRC). J Clin Oncol 2015;33:abstr TPS790.
- Loupakis F, Cremolini C, Salvatore L, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer 2014;50:57-63. [Crossref] [PubMed]
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306-15. [Crossref] [PubMed]
- Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313-27. [Crossref] [PubMed]
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines: Colon Cancer, Version 2. 2016. Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 2007;96:1166-9. [Crossref] [PubMed]
- Tougeron D, Lecomte T, Pagès JC, et al. Effect of low-frequency KRAS mutations on the response to anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol 2013;24:1267-73. [Crossref] [PubMed]
- Laurent-Puig P, Pekin D, Normand C, et al. Clinical relevance of KRAS-mutated subclones detected with picodroplet digital PCR in advanced colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res 2015;21:1087-97. [Crossref] [PubMed]
- Marques JM, Repetto L, Guggeri L, et al. Clinical validation of real-time PCR and SNaPshot methods in comparison to NGS for KRAS and NRAS Mutation Detection in colorectal cancer at Genia Laboratories (Uruguay). J Clin Oncol 2015;33:e22121.
- Valtorta E, Misale S, Sartore-Bianchi A, et al. KRAS gene amplification in colorectal cancer and impact on response to EGFR-targeted therapy. Int J Cancer 2013;133:1259-65. [Crossref] [PubMed]
- Lee MS, Overman MJ, Maru DM, et al. Association of CpG island methylator phenotype (CIMP) with inferior progression-free survival with anti-EGFR monoclonal antibody therapy in metastatic colorectal cancer. J Clin Oncol 2014;32:abstr 3633.
- Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3:99ra86. [Crossref] [PubMed]
- Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov 2013;3:658-73. [Crossref] [PubMed]
- De Roock W, De Vriendt V, Normanno N, et al. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 2011;12:594-603. [Crossref] [PubMed]
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010;11:753-62. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Bertotti A, Papp E, Jones S, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 2015;526:263-7. [Crossref] [PubMed]
- Misale S, Di Nicolantonio F, Sartore-Bianchi A, et al. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov 2014;4:1269-80. [Crossref] [PubMed]
- Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013;19:657-67. [Crossref] [PubMed]
- Coffee EM, Faber AC, Roper J, et al. Concomitant BRAF and PI3K/mTOR blockade is required for effective treatment of BRAF(V600E) colorectal cancer. Clin Cancer Res 2013;19:2688-98. [Crossref] [PubMed]
- Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov 2015;5:358-67. [Crossref] [PubMed]
- Heinemann V, Modest DP, Fischer von Weikersthal L, et al. Gender and tumor location as predictors for efficacy: Influence on endpoints in first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol 2014;32:abstr 3600.


