The genomics and therapeutics of HER2-positive gastric cancer—from trastuzumab and beyond
Introduction
Globally, gastric cancer is the fifth most common cancer and the third most common cause of cancer related death (1). It is estimated that 43,280 new cases of gastro-esophageal cancer will be diagnosed (2) and 26,420 deaths related to gastro-esophageal cancer will occur in the United States in 2016 (2).
The majority of patients diagnosed with gastric cancer present with advanced, incurable disease. Chemotherapy remains the standard of care treatment approach for patients with advanced stage disease however, response rates are relatively low and the prognosis is poor with a median survival of only 8–10 months. This underlines the importance of identifying potential therapeutic targets and developing targeted therapies to improve the outcomes of systemic treatment beyond those currently achieved with conventional chemotherapy.
As our understanding of cancer biology, cancer genomics and molecular oncology evolves we now recognize that gastric cancer is not a single disease entity. Gastric cancer represents a conglomerate of histologically and biologically heterogeneous diseases, which are characterized by various genomic alterations that result in activation of molecular pathways. We know more about the biological behavior of gastric cancer and its intrinsic subtypes, particularly the human epidermal growth factor receptor-2 (HER2) amplified gastric cancer subtype. HER2 is the first validated therapeutic target in esophagogastric cancer.
The aim of this article is to review HER2-positive gastric cancer, the importance of cancer genomics in classifying this disease entity, the standard therapeutic options available and the future directions of clinical investigation.
Pathogenesis
One of the most important targets in human malignancy is the epidermal growth factor receptor (EGFR) family. This family includes EGFR/HER1, HER2/neu, HER3 and HER4 (3). Each receptor consists of an extracellular ligand-binding domain, an intracellular domain with kinase activity and a short, lipophilic, transmembrane domain. These receptors are activated by ligand binding however, the specific ligand to HER2 has not been identified yet (4). The HER2-receptor is considered an orphan receptor that is ligand independent. It acts as a co-receptor that modulates signals after ligand binding to other receptors in the EGFR family. Homodimerization independently of a ligand and heterodimerization with other members of the EGFR family produces signals that activates downstream pathways including the Ras/Raf/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase/protein kinase-B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathways (5,6). Stimulation of these pathways influences many aspects of tumor cell biology including proliferation, differentiation, migration and apoptosis. The HER2/neu gene located on chromosome 17q21 encodes the HER2-protein. HER2/neu oncogene amplification results in HER2-receptor overexpression. This can enhance and prolong signals that produce advantageous properties for a malignant phenotype transformation by facilitating uncontrolled cell growth and tumorigenesis (7).
Incidence
The phase III Trastuzumab for Gastric Cancer (ToGA) trial reported the incidence of HER2-positive gastric cancer to be 22% (8). HER2-overexpression in gastric cancer is dependent of the location of the primary tumor and ranges between 6–30% (8,9). The reported rates of HER2-positivity are highest in gastro-esophageal junction (GEJ) or stomach cardia tumors compared to tumors arising more distally in the stomach (10). Gastric cancer HER2-overexpression is also influenced by histological subtype. HER2-positivity is predominantly seen in Lauren’s intestinal type, well to moderately differentiated gastric cancers with a low prevalence in diffuse type gastric cancer (32% vs. 6%) (9).
HER2 as a prognostic and predictive biomarker
Although, HER2-positivity selects the patients most likely to obtain benefit from HER2-targeted therapy some patients with HER2-positive gastric cancer experience primary resistance to HER2-directed therapy. Therefore, HER2-positivity alone is not an adequate predictive biomarker of response to HER2-targeted agents (11). The role of HER2-overexpression as a prognostic biomarker in gastroesophageal cancer is controversial. Retrospective studies have demonstrated HER2-positivity as a poor prognostic marker associated with increased risk of invasion, metastasis and worse survival (12,13). Surgical series have also determined HER2-status to be the second poorest prognostic marker after nodal status (14,15), while other studies have found no association between HER2 and prognosis in both early and advanced stage disease (16,17).
Detection of HER2-positivity
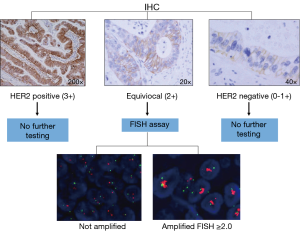
HER2-positivity is determined by quantification of the HER2 cell surface receptors via immunohistochemistry (IHC) and/or by measuring the number of HER2 gene copy numbers using fluorescent in-situ hybridization (FISH) techniques (Figure 1).
In gastric cancer testing for HER2 is recommended in patients with inoperable, locally advanced, recurrent or metastatic disease in whom trastuzumab therapy is being considered (18). Determination of HER2-status via IHC is distinct for gastric cancer. The main difference in scoring HER2 IHC staining between gastric and breast cancer is that an incomplete basolateral or lateral staining alone in gastric cancer is considered positive in addition to complete membrane staining. This results in tumor heterogeneity and the potential for inaccuracy in the detection of HER2-positive tumors.
In gastric cancer HER2-positivity is determined by 3+ scoring on IHC or FISH positivity (HER2: CEP 17 ratio ≥2.0). Due to the higher rate of tumor heterogeneity and incomplete membranous reactivity seen in gastric cancer compared to breast cancer an IHC scoring criteria specific for HER2-overexpression in gastric cancer was devised (19). The IHC staining pattern that determines the highest level of HER2 expression by IHC (IHC 3+) is different depending on whether a surgical specimen or biopsy is tested. Basolateral or lateral membranous reactivity in ≥10% of tumor cells represents an IHC 3+ staining pattern in the setting of a surgical specimen. An IHC 3+ staining pattern on a tumor biopsy is determined by tumor cell clusters with a strong complete, basolateral or lateral membranous reactivity irrespective of percentage of tumor cells stained (8).
Tumors with equivocal IHC scores (2+) should be tested further using FISH or other in situ methods. Research efforts are endeavoring to improve the quality and precision with which HER2 status is determined in gastric cancer. A recent study suggested that the proteomics-based collaborative enzyme enhanced reactive (CEER) immunoassay is a useful technique to investigate HER2 expression in gastric cancer. CEER-based assays showed higher sensitivity and specificity as compared to IHC-based assays (20). Another technique to consider is to use a quantitative variable, as used in breast cancer, that could be objectively measured, such as HER2 gene copy number or the HER2 amplification ratio, compared to the subjective IHC scoring assessment (21).
Pathologic concordance
High concordance between HER2 status measured on the primary tumor and corresponding sites of metastatic disease has been reported in gastric cancer (17,22). This suggests that the evaluation of HER2 status in the primary tumor alone is sufficient to determine appropriateness for treatment with anti-HER2-therapy in gastro-esophageal cancer.
Management of HER2-positive gastro-esophageal cancers
Inoperable locally advanced/metastatic disease
Trastuzumab is a monoclonal anti-body that binds to the extracellular domain of the HER2-receptor blocking its downstream signaling pathway. It promotes an antibody-dependent cell-mediated cytotoxicity by activating apoptotic signals in tumor cells (23).
The ToGA trial was the first phase III study to investigate the role of trastuzumab in the setting of HER2-positive gastric or GEJ cancer. This open-label, international trial randomized 594 patients to receive a combination of fluoropyrimidine and cisplatin administered every 3 weeks for 6 cycles with or without trastuzumab. This study met its primary end point by demonstrating a significant improvement in overall survival favoring the trastuzumab arm (13.8 vs. 11.1 months; P=0.0046). Overall response rate was also significantly better in the trastuzumab arm (47% vs. 35%, P=0.0017). However, this response rate highlights that the considerable proportion of individuals that are primary refractory to HER2-inhibition even in the presence of the drug target. An exploratory analysis identified that the patients with the highest level of HER2 expression (IHC2+ & FISH+ or IHC3+) compared to patients with FISH positivity and low level of HER2 expression via IHC (IHC 0 or 1 & FISH positive) derived the greatest benefit from trastuzumab in combination with chemotherapy (16.0 vs. 11.8 months; HR 0.68; 95% CI, 0.5–0.83). This was not a preplanned exploratory analysis (8). However, as a consequence of this post-hoc exploratory analysis different requirements for the approval of trastuzumab in this indication have emerged in the US and in Europe.
The US Food and Drug Administration (FDA) granted trastuzumab approval for use in patients with advanced gastric cancer with positive FISH results or an IHC score 3+ (24). The European Medicines Agency (EMA) limited the approval to patients with gastric cancers with HER2 overexpression as defined by HER2 IHC score 2+, confirmed by a positive FISH result, or by HER2 IHC score of 3+ (25).
The ToGA trial also demonstrated safety for the combination of trastuzumab with chemotherapy in gastric cancer. The rates of grade 3 or 4 adverse events were similar between the treatment groups. Notably, the proportion of patients that experienced cardiac dysfunction (defined as a ≥10% drop in LVEF to an absolute value <50%) was low in both treatment arms [trastuzumab plus chemotherapy, 11 (5%) of 237 vs. chemotherapy alone, two (1%) of 187] (8).
First-line therapy
Following publication of the results of the ToGA trial, trastuzumab in combination it cisplatin/fluoropyrimidine became a standard first-line treatment for patients the HER2-positive advanced gastric cancer. Due to its more favorable toxicity profile flouropyrimidine in combination with oxaliplatin has become a preferred first-line treatment option for the management of advanced gastro-esophageal cancers. To date two phase II studies have explored the combination trastuzumab with capecitabine and oxaliplatin inpatients with HER2-positive advanced gastric cancer. Both studies demonstrated objective response rates of 67% and safety with this combination therapy (26,27).
Second-line setting and beyond
Patients with advanced HER2-positive gastric cancer have limited options in the second-line setting and beyond. Management approaches include irinotecan or paclitaxel/ramucirumab, enrollment in a clinical trial or best supportive care. Response rates to these treatment options are invariably low and outcomes are poor. In trastuzumab pre-treated patients with HER2-positive gastric cancer there is no data to support the continuation of trastuzumab in combination with chemotherapy beyond progression. In trastuzumab naive patients with HER2-positive gastric cancer progressing on first-line chemotherapy the benefit of adding trastuzumab to second-line chemotherapy also remains unknown.
HER2-targeted therapy in operable HER2-positive gastric cancer
Incorporation of HER2-directed therapy in combination with neoadjuvant chemotherapy improves recurrence free and overall survival and increases the rate of pathologic complete response (pCR) achieved in breast cancer (28-30).
Following the path of investigation paved by HER2-positive breast cancer clinical researchers, the role of trastuzumab in early stage HER2-positive gastric cancer is being explored. The NeoHx study, examined perioperative trastuzumab in combination with capecitabine-oxaliplatin in 36 patients with resectable, HER2-positive gastro-esophageal cancer. A partial response was observed in 14 patients (39%). An R0 resection was achieved in 78%. The pathological complete response rate was 19% (n=7). The primary endpoint of the study, disease free survival (DFS) at 18 months, has not yet been reported (31). A number of studies are currently exploring the role of trastuzumab in the adjuvant and neoadjuvant setting of HER2-positive gastro-esophageal cancer. The TOXAG study (clinical trial number NCT01748773), is a single arm trial actively recruiting patients with resected HER2-positive esophagogastric cancer to receive 3 cycles of adjuvant capecitabine/oxaliplatin and trastuzumab followed by 5 weeks of chemoradiotherapy. Adjuvant trastuzumab will be given for a duration of one year in this study. The HerFLOT study intends to evaluate pCR rate with trastuzumab in combination with a triplet chemotherapy regimen incorporating 5-fluorouracil, docetaxel and oxaliplatin in patients with locally advanced, resectable HER2-positive gastric adenocarcinoma (clinical trial number NCT01472029). Finally, the RTOG 1010 study (clinical trial number NCT01196390) is a large phase III study that aims to investigate the role of adding trastuzumab to tri-modality therapy (carboplatin, paclitaxel and radiotherapy) in patients with HER2 overexpressing esophageal adenocarcinomas.
Alternative HER2-directed therapies
Lapatinib, pertuzumab and T-DM1 are all HER2-directed agents approved for HER2-positive breast cancer that were also explored in the HER2-positive gastric cancer arena.
Lapatinib
Lapatinib is a small molecule, reversible tyrosine kinase, dual inhibitor of HER2 and EGFR. It binds to the intracellular domain of these targets and prevents activation of PI3K and Ras pathways. This results in downregulation of receptor tyrosine kinase phosphorylation in tumor cells. Preclinical data identified single-agent activity for lapatinib in a HER2-positive NCI-N87 gastric cancer model (32). However, lapatinib failed to live up to its potential promise in the clinical setting. Lapatinib demonstrated only modest single agent activity in the first line setting of an unselected group of advanced gastric cancer patients. It yielded a response rate of 9% (33). Two large phase III studies incorporating lapatinib in combination with chemotherapy in advanced HER2-positive gastric cancer failed to reach their primary endpoints. The TyTAN trial assessed the efficacy of paclitaxel with or without lapatinib in the second-line setting of an Asian population with HER2-FISH amplified advanced gastric cancer (34). The LOGiC study combined lapatinib with capecitabine/oxaliplatin in treatment naive patients with advanced HER2-FISH amplified esophagogastric cancers (35). Lapatinib prolonged median overall survival by almost two months in both studies. However, this improvement in OS did not reach statistical significance in either study. Both trials were ultimately classified as negative studies. In the LOGiC trial, a preplanned exploratory subgroup analysis demonstrated a significant improvement in overall survival in Asian patients [16.5 vs. 10.9 months; hazard ratio (HR), 0.68; 95% CI, 0.48–0.96; P=0.0261] and in younger patients (12.9 vs. 9.0 months; HR, 0.69; 95% CI, 0.51–0.94; P=0.0141). However, HER2 status is not known to vary based on these patient characteristics, which are unreliable predictors of outcome in this setting. In the TyTAN study, a preplanned subgroup analysis demonstrated that patients with HER2-IHC 3+ tumors that received the lapatinib containing regimen had improved median OS compared to those that received paclitaxel alone (14.0 vs. 7.6 months; HR, 0.59; P=0.0176). Notably, no correlation was observed in the LOGiC trial between HER2 IHC status and survival.
Pertuzumab
Pertuzumab is a recombinant, humanized, monoclonal antibody that targets HER2. It binds to the dimerization domain (extracellular domain II) of HER2 and disrupts ligand induced HER2 heterodimerization with other activated HER receptors and prevents HER2 pathway signaling. The HER2/HER3 dimer is the most potent signaling dimer (36). Up regulation of HER3 has been identified as a mechanism of resistance to HER2-targeted therapy in HER2-driven tumors. Pertuzumab has the potential to overcome this mechanism of resistance. Preclinical studies incorporating HER2-positive gastric cancer xenografts models have demonstrated more potent anti-tumor activity with dual HER2-blockade (pertuzumab and trastuzumab) compared to pertuzumab monotherapy (37). There are a number of clinical trials investigating the role of trastuzumab and pertuzumab in combination with chemotherapy in the first line and neoadjuvant setting of HER2-positive, gastro-esophageal adenocarcinoma (clinical trial numbers NCT01461057, NCT01774786, NCT02205047 and NCT02581462).
T-DM1
T-DM1 is an antibody-drug conjugate of trastuzumab and emtansine (DM1) 1, a microtubule inhibitor. Antibody-drug conjugates deliver cytotoxic drugs directly to cancer cells. In the advanced breast cancer setting T-DM1 has been associated with significant efficacy and minimal toxicity even in heavily pretreated with patients (38). This makes it an attractive, potential therapeutic option for patients with advanced gastric cancers. In the preclinical setting, T-DM1 demonstrated greater antitumor activity than trastuzumab in gastric cancer models (39). The efficacy of T-DM1 compared to a taxane, in the second line setting, in patients with advanced, trastuzumab refractory, HER2-positive gastric cancer was evaluated in the GATSBY, phase II/III, trial. The results of this study were recently reported. The study randomized 415 patients: 228 to T-DM1 weekly and 117 to a taxane (weekly paclitaxel or docetaxel 3 weekly). An independent data monitoring committee selected T-DM1 once weekly for further study and the majority of patients were randomized to this or a taxane, however 70 patients had previously been randomized to T-DM1 every three weeks but were not included in the results. This study failed to meet its primary endpoint and conveyed no significant difference in median overall survival between the taxane-monotherapy and T-DM1 arms [8.6 vs. 7.9; HR 1.15 (0.87–1.51); P=0.86]. The objective response rate was similar between both arms while T-DM1 was slightly better tolerated (40).
The HER2 biology in gastric versus breast tumors
HER2-overexpression/amplification is an established therapeutic target in breast and gastro-esophageal cancer. Can we think of all HER2-positive tumors as being alike irrespective of their site of origin?
The body of preclinical and clinical experience of these diseases suggests that the answer is—No. Similarities do exist between HER2-positive breast and gastric cancer, in terms of the incidence of HER2-positivity, pathologic concordance rates and until recently their management in the first-line metastatic setting. However, differences are also apparent including how we detect and determine HER2-positivity and the prognostic capabilities of the biomarker in each tumor type. Notably, lapatinib identified as a standard of care therapeutic for the management of advanced HER2-positive breast cancer was not shown to provide clinically meaningful benefit in HER2-positive gastro-esophageal cancer.
HER2-overexpression was identified as an important therapeutic target in breast cancer 15 years ago. Research has continued to investigate potential mechanisms of resistance to HER2-targeted therapy and this has led to the development of alternative HER2-directed therapies. In parallel, significant improvement in patients outcomes are now commonly observed. For example, the median overall survival of treatment naïve, women presenting with advanced HER2-positive breast cancer treated with dual HER2-blockade and chemotherapy is 3.5 times longer than that seen in women treated with chemotherapy and trastuzumab alone (41).
However, although HER2-positive esophagogastric cancer cannot be viewed as the breast cancer of gastrointestinal tumors it does not mean that the information generated from breast cancer research cannot provide meaningful information relevant to esophagogastric cancers.
Ultimately, we have learned from developments in breast cancer to examine each patient’s tumor individually using comprehensive molecular and genomic analyses. Each patient’s tumor carries a unique genetic signature. The key is to understand how the molecular and signaling effects of the gene signature relate to and predict for response and resistance to HER2- and other targeted therapies.
Future directions of clinical investigation in HER2-positive gastric cancer
Genomic sequencing
The majority of patients with HER2-positive esophagogastric cancer treated with trastuzumab develop resistance. The exact mechanisms underlying this resistance are unknown. Tumor heterogeneity and interactions between HER2 and other signaling pathways including receptor tyrosine kinase signaling provide potential means for signal diversification and tumor escape from HER2-blockade.
Significant research efforts have been made to define the complexity and heterogeneity of gastric cancer through the development of various classification systems using tumor characteristics reflecting histology (42), anatomic site (42), gene expression (43), gene amplification (43), DNA methylation or oncogenic pathways (44). Recently, emphasis has focused upon the categorization of gastric cancer into distinct molecular entities. As part of The Cancer Genome Atlas (TCGA) project, comprehensive molecular evaluation of 295-treatment naive, primary gastric adenocarcinomas were performed. Four molecularly distinct gastric cancer subgroups were identified and categorized as follows: EBV-positive (9%); microsatellite instability (MSI)-high (22%); genomically stable [absence of extensive somatic copy number alterations (SCNAs)] (20%); and those exhibiting chromosomal instability (CIN; 50%). Integrative pathway analysis was performed in an attempt to characterize the potentially actionable genomic alterations frequently identified within each molecular subgroup. EBV-enriched tumors showed evidence of hypermethylation and harbored PIK3CA mutations (80%), PD-L1/2 amplification, recurrent JAK2 and HER2-amplifications. MSI-high tumors generally lacked targetable amplifications but were associated with DNA hypermethylation and elevated somatic mutation rates including mutations of PIK3CA (42%) and ERBB3 (26%). Genomically stable tumors lacked aneuploidy, hypermethylation and hypermutation. A third of this group exhibited recurrent RHOA mutations and CLDN18 events. Finally, CIN tumors were shown to have frequent oncogenic amplifications of receptor tyrosine kinases including HER2 (24%), VEGFR2 and cell cycle mediators (CCNE1, CCND1 and CDK6) (44,45).
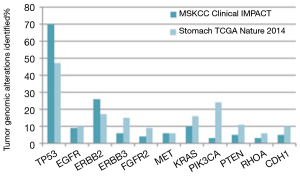
At Memorial Sloan Kettering Cancer Center, molecular profiling of tumor from patients with advanced gastric cancer is routinely performed using the Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACTTM) assay. This next generation sequencing assay can identify point mutations, small insertion or deletion events and gene-copy-number aberrations in 410 cancer-associated genes. MSKCC gastric cancer specific IMPACT data was recently compared with the TCGA gastric cancer data. An over-representation of the CIN subtype was seen in the MSKCC cohort (65% versus 50% in TCGA). The remaining tumors in the MSKCC dataset were classified as chromosomally stable (29%), EBV-enriched (<3%) and MSI (<3%) (46) (Figure 2). Notably, the MSKCC data reflects a metastatic esophagogastric cancer population whereas the TCGA data included treatment naïve patients only and used the primary tumor tissue for analysis. This suggests that the inherent molecular profile of a gastric cancer changes from the primary treatment naïve state to the metastatic setting with higher proportions of p53 mutations evident in the pretreated population.
As the genomic landscape of gastric cancer continues to evolve efforts are centered upon establishing the clinical relevance of these distinct molecular subtypes. The Asian Cancer Research group utilizing gene expression data from 251 patients with gastric cancer identified four molecular subtypes: the mesenchymal-like type; MSI-high type, TP53-active and TP53-inactive types (47). They correlated each molecular subtype with survival outcome and patterns of recurrence. The MSI subtype has the best prognosis followed by MSS/TP53+ and MSS/TP53−, with the MSS/EMT subtype displaying the worst prognosis (log-rank, P=0.0004). In terms of recurrence patterns the MSS/EMT group displayed a higher chance of recurrence compared to the MSI group (63% versus 23%). The ACRG validated their molecular sub-classification system using the TCGA (44) and the Gastric Cancer Project ’08 Singapore datasets (48). Comparison of the ACRG subtypes with the TCGA genomic subtypes when applied to both ACRG and TCGA cohorts showed significant overlap and similarities between the respective molecular subtypes identified by each classification system. Both systems identified MSI-high tumors as a molecularly distinct subgroup. The TCGA genomically stable, EBV positive and CIN subtypes were enriched in ACRG microsatellite stable (MSS)/EMT, MSS/TP53+ve and MSS/TP53-ve subtypes, respectively. The ACRG explored copy number profiles in terms of focal amplifications in known cancer genes and chromosome-wise copy number variation highlighted that chromosomal instability was significantly associated with TP53 mutations (Fisher’s test, P=0.01) and the MSS/TP53− subtype (Fisher’s test, P=8e−6). Recurrent focal amplifications in HER2 and other genes such as EGFR, CCNE1 and CCND1 were most prevalent in the MSS/TP53-ve subgroup. These gene amplifications also tended towards mutual exclusivity in the MSS/TP53− subtype (P=0.05). HER2-positive tumors are molecularly characterized by dependency on tyrosine kinase signaling. It is possible that the development of recurrent amplifications in key receptor tyrosine kinases including EGFR, HER3, HER4 and MET contribute to resistance to HER2-targeted therapy.
Routine incorporation of next generation sequencing analysis of HER2-positive gastric tumors of clinical trial participants may help to identify gene signatures that are predictive of resistance to HER2-targeted therapy (49).
Molecular classification of gastric tumors provides the opportunity to investigate new-targeted therapies in a molecularly refined; select patient population most likely to gain benefit from the therapeutic agent under evaluation. For example, recurrent focal gene amplifications in HER2 and EGFR most frequently occur in the MSS/TP53-ve gastric subgroup. Given the mutual exclusivity of these alterations and the uniform molecular background of these tumors this identifies a patient population in which to explore novel HER2-directed or EGFR targeted therapies. Molecular categorization and definition of gastric cancers also highlights new potential therapeutic targets. Preclinical models that reflect the four molecularly distinct subtypes can be developed to facilitate the necessary preclinical work to identify promising new-targeted treatments for gastric cancer.
Patient-derived xenografts (PDXs)
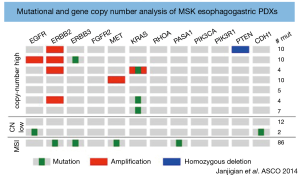
Preclinical models including cell culture lines and mouse xenografts often fail to accurately incorporate the tumor microenvironment and replicate the complex interactions that occur between tumor cells and the immune system which are key players in tumor proliferation and metastasis (50). Consequently, these preclinical models have variable predictive power in the translation of new therapies in to the clinical arena (51). PDXs represent a novel method to reproduce the complexities of the gastric cancer microenvironment in a preclinical setting. PDXs involve the transfer of tumor tissue directly from the patient to an immunodeficient mouse. One study has established PDX models, which reflect patients with esophagogastric cancers with HER2-positive, MET-positive, CDH1 germ-line mutant and diffuse/signet ring pathological features (Figure 3). This study highlighted the challenges associated with developing PDXs including relatively low rates of tumor engraftment. The tumor engraftment rate was 46% for PDXs created in an orthotopic (direct implantation of tumor into a specific mouse organ) fashion and 26% for those with heterotopic (direct implantation of tumor into the subcutaneous tissue of the mouse) implants (52). Other factors limiting their use include the higher cost and specialized maintenance associated with PDXs compared to cultured cell lines. However, despite these limitations PDXs are an exciting preclinical development that provides the platform to validate differences in tumor biology and inform the rational design of future clinical trials.
Novel alternative HER2-targeted therapies
Innovations in molecular oncology and developmental therapeutics that may overcome potential resistance pathways in HER2-positive gastric cancers include pan-HER TKIs, mTOR and MET inhibitors.
Pan-HER TKI inhibition: afatinib, neratinib, dacomitinib
Afatinib is an irreversible inhibitor of EGFR, HER2 and HER4. Preclinical data demonstrated potent anti-tumor activity of afatinib in an NCI-N87 HER2-positive esophagogastric cancer xenograft. The addition of afatinib to trastuzumab produced greater anti-tumor efficacy than either drug alone (53). Safety and efficacy for afatinib has been demonstrated in a phase II study involving trastuzumab refractory patients with advanced HER2-positive (IHC 3+ or FISH amplified) esophagogastric adenocarcinomas. In 19 out of 20 evaluable patients a disease stabilization rate (partial response and stable disease) of 42% at four months was reported (54). An expansion cohort looking at the safety and efficacy of afatinib in combination with trastuzumab is actively recruiting (clinical trial number NCT01522768).
Neratinib is a pan-HER TKI that has shown promising results in advanced, trastuzumab pre-treated, HER2-positive breast cancer (55). The efficacy of neratinib is being explored in other solid tumors with HER2, HER3 or EGFR mutations in a multicenter, open-label, phase II study (clinical trial number NCT 01953926).
Dacomitinib is an irreversible, pan-HER inhibitor that binds to the adenosine triphosphate domain of each of the three kinase-active members of the HER family: HER1, HER2 and HER4. In preclinical studies dacomitinib produced strong anti-tumor activity in HER2-positive gastric cancer cell lines. Dacomitinib was shown to be synergistic with chemotherapy and molecularly targeted agents including trastuzumab (56). A small, phase II study has recently demonstrated single agent activity for dacomitinib in previously treated patients with advanced HER2-positive gastric cancer. The disease control rate was 40.7% (n=11/27; 95% CI, 21.9–59.6%) with 7.4% (n=2/27; (95% CI, 0–17.5%) experiencing a partial response. A tolerable safety profile was also documented (57). These results suggest that simultaneous targeting of EGFR/pan HER may be a potential therapeutic avenue in patients with metastatic, trastuzumab resistant, HER2-positive esophagogastric cancer via potent signaling inhibition.
mTOR inhibition
Dysregulation of the HER2 downstream signal substrate, including PI3K/Akt/mTOR pathway is associated with trastuzumab resistance. PI3K mutations and phosphate and tensin homolog (PTEN) inactivation result in activation of downstream signals, which may affect the effectiveness of HER2-directed therapy. Everolimus is an mTOR inhibitor. The combination of everolimus with anti-HER2-therapy represents a potential combination strategy to consider in HER2-positive esophagogastric cancer. The BOLERO3 study explored this strategy in combination with vinorelbine in an advanced trastuzumab refractory breast cancer setting. The study was positive yielding a significant, albeit modest improvement in median progression free survival. However the toxicity observed with this combination was considerable (58).
MET inhibition
MET signaling has also been implicated as a mechanism for resistance to HER2-targeted therapy. The combination of a MET inhibitor with anti-HER2 therapy may facilitate efficacy and help to overcome resistance to HER2-directed therapy (6).
Pharmacokinetic and pharmacodynamic factors may contribute to resistance to HER2-directed therapies
We know that trastuzumab pharmacokinetics differs in the setting of breast and gastric cancer. Pharmacokinetics data from the ToGA trial demonstrated a rate of trastuzumab clearance of 0.378 L/d based on the standard dosing schedule. This represents a 70% higher rate of trastuzumab clearance in patients with gastro-esophageal cancer than the rates observed in trastuzumab-treated patients with metastatic breast cancer (59,60). The clinical relevance of the pharmacokinetic differences of trastuzumab observed in patients with gastro-esophageal cancer compared to breast cancer has not been defined. The HELOISE study aims to address this question. It is a randomized phase IIIb study that aims to compare two different trastuzumab dosing regimens in combination with cisplatin/capecitabine in treatment naïve, metastatic, HER2-positive esophagogastric adenocarcinomas. The trastuzumab dosing schedules being investigated in this trial includes the standard dose versus a higher dosing schedule of 8 mg/kg loading dose followed by 10 mg/kg every 3 week.
Poor drug absorption may also contribute to drug resistance and may be especially important in orally administered drugs. In general, early phase studies focus on toxicity assessments in order to establish the maximum tolerated dose and radiological assessments in order to determine a drug’s therapeutic activity. However, molecularly targeted agents necessitate target engagement in order to exert their effect. To date this fundamental specification unique to targeted therapeutics has been relatively ignored during the clinical therapeutic developmental process. In an attempt to address this issue early phase studies are increasingly incorporating tumor tissue correlative analyses using biopsies from baseline and shortly after trial initiation to characterize the effects of targeted drugs at a molecular level. However, analyses of small biopsy specimens fail to provide the whole picture and disregard the heterogeneity that exists within the remaining tumor tissue. In the future, it may be possible to capture this information using liquid biopsies utilizing whole-exome sequencing of circulating tumor DNA however further research is required to determine this.
89Zr-trastuzumab PET represents a useful non-invasive pharmacodynamic biomarker that can serially monitor therapeutic target engagement and functional activity in the entire tumor over a prolonged period of time. Trastuzumab labeled with 89zirconium (Zr) (t1/2) used as a positron emission tomography (PET) imaging tracer has been shown to successfully delineate HER2-positive gastric cancer and monitor the pharmacodynamics effects of afatinib (53) and other HER2-directed therapies (clinical trial number NCT02023996). This form of functional imaging is superior to [18F] fluorodeoxyglucose PET in evaluating HER2-directed therapy in HER2-positive gastric tumors because it is HER2-specific.
Conclusions
Significant progress has been made to stratify gastric tumors into molecular subtypes linked to distinct patterns of molecular alterations, disease progression and prognosis (44,47). Gastric cancer was once seen as a single disease entity and is now known to encompass a molecularly heterogeneous group of cancers. HER2-overexpression/amplification is an established therapeutic target in advanced gastric cancer. Trastuzumab in combination with chemotherapy was the first molecularly targeted agent to demonstrate improvement in response rates and overall survival in the setting of advanced HER2-positive gastric cancer. Trastuzumab is now being explored in the adjuvant and neoadjuvant setting. Alternative HER2-directed therapies, such as pertuzumab and TDM-1, with known clinical benefit in HER2-positive breast cancer have also been explored in the setting of HER2-positive gastric cancer. However, similar to HER2-positive breast cancer, patients with HER2-positive gastric cancer are primary refractory or acquire resistance to trastuzumab therapy. Novel HER2-directed therapies including pan-HER TKIs, MET and mTOR inhibitors and dual HER2-blockade offer promise as potential therapeutic strategies to overcome mechanisms of resistance to trastuzumab. The molecular classification system identified through next generation sequencing of gastric tumors provides the platform to identify new therapeutic targets and develop rational therapeutics for molecularly distinct subtypes of gastric cancer. The landscape of future clinical investigation in gastric cancer will be very different from the traditional clinical trial design. The success of future molecular specific studies will depend on the incorporation of next generation sequencing to refine patient selection and the use of tissue correlative and functional imaging to enhance our understanding of the biology of esophagogastric cancer. The hope is that this will lead to clinically meaningful progress for gastric cancer patients similar to that witnessed amongst other molecularly sophisticated tumors, such as breast cancer, lung cancer and melanoma over the last decade.
Acknowledgements
None.
Footnote
Conflicts of Interest: YY Janjigian has received, or has pending grants or patents from Boehringer Ingelheim, Bayer, Genentech, Eli Lilly, Pfizer, Merck. The other author has no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644-6. [Crossref] [PubMed]
- Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159-67. [Crossref] [PubMed]
- Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 2011;8:492-503. [Crossref] [PubMed]
- Matsuoka T, Yashiro M. Recent advances in the HER2 targeted therapy of gastric cancer. World J Clin Cases 2015;3:42-51. [Crossref] [PubMed]
- Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol 2001;12 Suppl 1:S3-8. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [Crossref] [PubMed]
- Won E, Janjigian YJ, Ilson DH. HER2 directed therapy for gastric/esophageal cancers. Curr Treat Options Oncol 2014;15:395-404. [Crossref] [PubMed]
- Ock CY, Lee KW, Kim JW, et al. Optimal Patient Selection for Trastuzumab Treatment in HER2-Positive Advanced Gastric Cancer. Clin Cancer Res 2015;21:2520-9. [Crossref] [PubMed]
- Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 2011;29:3030-6. [Crossref] [PubMed]
- Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol 2011;18:2833-40. [Crossref] [PubMed]
- Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 1999;85:1894-902. [Crossref] [PubMed]
- Park DI, Yun JW, Park JH, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 2006;51:1371-9. [Crossref] [PubMed]
- Gómez-Martin C, Garralda E, Echarri MJ, et al. HER2/neu testing for anti-HER2-based therapies in patients with unresectable and/or metastatic gastric cancer. J Clin Pathol 2012;65:751-7. [Crossref] [PubMed]
- Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol 2012;23:2656-62. [Crossref] [PubMed]
- NCCN. NCCN Guidelines Version 3. 2015 Gastric Cancer. 2015 [02.17.2016]. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [Crossref] [PubMed]
- Lee J, Kim S, Kim P, et al. A novel proteomics-based clinical diagnostics technology identifies heterogeneity in activated signaling pathways in gastric cancers. PLoS One 2013;8:e54644. [Crossref] [PubMed]
- Gomez-Martin C, Plaza JC, Pazo-Cid R, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol 2013;31:4445-52. [Crossref] [PubMed]
- Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 2011;104:1372-6. [Crossref] [PubMed]
- Fendly BM, Winget M, Hudziak RM, et al. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res 1990;50:1550-8. [PubMed]
- FDA. Full Prescribing Information for Trastuzumab. 2010 [2016]; Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- EMA. Summary of Positive Opinion for Herceptin. 2009 [cited 2016]; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000278/WC500059913.pdf
- Gong J, Liu T, Fan Q, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer 2016;16:68. [Crossref] [PubMed]
- Ryu MH, Ryoo BY, Park YS, et al. Phase II study of trastuzumab in combination with capecitabine and oxaliplatin in patients with advanced gastric cancer. J Clin Onco 2014;32:abstr 83.
- Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005;23:3676-85. [Crossref] [PubMed]
- Perez EA, Romond EH, Suman VJ Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366-73. [Crossref] [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [Crossref] [PubMed]
- Rivera F, Jimenez P, Garcia Alfonso P, et al. NeoHx study: Perioperative treatment with trastuzumab in combination with capecitabine and oxaliplatin (XELOX-T) in patients with HER2 resectable stomach or esophagogastric junction (EGJ) adenocarcinoma--R0 resection, pCR, and toxicity analysis. J Clin Oncol 2013;31:abstr 4098.
- Wainberg ZA, Anghel A, Desai AJ, et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res 2010;16:1509-19. [Crossref] [PubMed]
- Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol 2011;22:2610-5. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC-A Randomized Phase III Trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996;16:5276-87. [Crossref] [PubMed]
- Yamashita-Kashima Y, Iijima S, Yorozu K, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res 2011;17:5060-70. [Crossref] [PubMed]
- Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Barok M, Tanner M, Koninki K, et al. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett 2011;306:171-9. [Crossref] [PubMed]
- Kang YK, Shah MA, Ohtsu A, et al. A randomized, open-label, multicenter, adaptive phase 2/3 study of trastuzumab emtansine (T-DM1) versus a taxane (TAX) in patients (pts) with previously treated HER2-positive locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma (LA/MGC/GEJC). J Clin Oncol 2016;34:abstr 5.
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 2011;17:2693-701. [Crossref] [PubMed]
- Tay ST, Leong SH, Yu K, et al. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res 2003;63:3309-16. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Lordick F, Janjigian YY. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol 2016;13:348-60. [Crossref] [PubMed]
- Riches JC, Schultz N, Ku GY, et al. Genomic profiling of esophagogastric (EG) tumors in clinical practice. J Clin Oncol 2015;33:abstr 57.
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Ooi CH, Ivanova T, Wu J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet 2009;5:e1000676. [Crossref] [PubMed]
- Janjigian YY. Lapatinib in Gastric Cancer: What Is the LOGiCal Next Step? J Clin Oncol 2016;34:401-3. [Crossref] [PubMed]
- Daniel VC, Marchionni L, Hierman JS, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res 2009;69:3364-73. [Crossref] [PubMed]
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res 2006;66:3351-4, discussion 4. [Crossref] [PubMed]
- Janjigian YY, Vakiani E, Imtiaz T, et al. Patient-derived xenografts as models for the identification of predictive biomarkers in esophagogastric cancer. J Clin Oncol 2014;32:abstr 4059.
- Janjigian YY, Viola-Villegas N, Holland JP, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med 2013;54:936-43. [Crossref] [PubMed]
- Janjigian YY, Ku GY, Ilson DH, et al. A phase II study of afatinib in patients (pts) with metastatic human epidermal growth factor receptor (HER2)-positive trastuzumab refractory esophagogastric (EG) cancer. J Clin Oncol 2015;33:abstr 59.
- Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 2010;28:1301-7. [Crossref] [PubMed]
- Nam HJ, Ching KA, Kan J, et al. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther 2012;11:439-51. [Crossref] [PubMed]
- Oh DY, Lee KW, Cho JY, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer 2016;19:1095-103. [Crossref] [PubMed]
- André F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [Crossref] [PubMed]
- Bruno R, Washington CB, Lu JF, et al. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 2005;56:361-9. [Crossref] [PubMed]
- Roche Inc: Herceptin package insert. Available online: http://www.medsafe.govt.nz/profs/datasheet/h/Herceptininf.pdf. Access date: 3rd February 2016. Cited 2016 3rd February 2016.