
Efficacy and safety of gemcitabine plus raltitrexed or S-1 versus standard third-line therapies in metastatic colorectal cancer: a retrospective cohort study
Highlight box
Key findings
• This study found that the gemcitabine plus raltitrexed or S-1 regimen is a novel, efficient, and economical regimen with tolerable adverse reactions for patients with metastatic colorectal cancer (mCRC).
What is known and what is new?
• Till now, FOLFIRI, FOLFOX, or CAPOX with targeted drugs such as cetuximab, bevacizumab, and panitumumab are the first- or second-line treatments of mCRC. In China, TAS-102, regorafenib, and fruquintinib have been approved as third-line regimens for mCRC. However, these regimens have the drawbacks of mediocre efficacy, substantive side effects, and high cost without superior survival.
• The efficacy and safety of gemcitabine plus raltitrexed or S-1 was evaluated in comparison to the standard third-line regimens in mCRC patients.
What is the implication, and what should change now?
• The gemcitabine plus raltitrexed or S-1 regimen achieved a similar therapeutic effect as did the currently practiced standard third-line treatments. With tolerable adverse reactions, this regimen represents a potentially effective and economical therapeutic option for patients with mCRC.
Introduction
Colorectal cancer (CRC), including colon and rectal cancer, is the third leading cause of cancer death worldwide (1) and one of the most common malignant tumors in China. Thus far, surgical resection is the main treatment for non-metastatic colorectal cancer (non-mCRC), while for unresectable mCRC, chemotherapy combined with targeted therapies is the standard palliative treatment (2) and can include the combination of FOLFIRI (folinic acid + fluorouracil + irinotecan), FOLFOX (folinic acid + fluorouracil + oxaliplatin), or CAPOX (capecitabine and oxaliplatin) with targeted drugs such as cetuximab (3), bevacizumab (4), and panitumumab (5) as first- or second-line treatments. In China, TAS-102 (6), regorafenib (7), and fruquintinib (8) have been approved as third-line regimens for mCRC. However, no single regimen shows superior survival (9-11). Meanwhile, the current standard treatments also have certain limitations, such as high cost and side effects, including hypertension and hand and foot skin reaction. More reliable treatment options are needed for patients with financial limitations, intolerance to adverse reactions, or with a preference for alternative therapies.
Gemcitabine [2',2'-difluoro-2'-deoxycytidine (dFdC)] is a nucleoside analogue which can be metabolized intracellularly into gemcitabine mono-(dFdCMP), di-(dFdCDP), and triphosphate (dFdCTP) by deoxycytidine kinase and other nucleotide kinases (12). By inhibiting the activity of ribonucleotide reductase (RNR), dFdCDP suppresses the production of deoxyribonucleoside-triphosphate (dCTP), which is essential for DNA synthesis. Moreover, dFdCTP competes with dCTP for increased incorporation into DNA strands which results in DNA strand termination and cellular apoptosis (13).
Raltitrexed, an antimetabolic folate-like analogue, specifically and directly inhibits thymidylate synthase (TS), which is the key enzyme in the synthesis of thymidine triphosphate (TTP), leading to DNA fragmentation and cell apoptosis. Additionally, after its ingestion into cells and conversion into active folylpolyglutamates, raltitrexed folylpolyglutamates promote antitumor activity by enhancing the inhibitory ability of TS and prolonging the inhibition time (14).
S-1 is an oral fluoropyrimidine derivative composed of tegafur (FT), gimeracil (CDHP), and oteracil potassium (Oxo). After oral administration, FT is gradually converted into 5-fluorouracil (5-FU). CDHP enhances the concentration of 5-FU by reversibly inhibiting DPD, which is the catabolic enzyme of fluorouracil present in the liver. Oxo can selectively and reversibly inhibit the activity of the 5-FU distributed in the gastrointestinal tract and thus decreases gastrointestinal toxicity without affecting the antitumor activity of 5-FU (15).
The combination of gemcitabine with raltitrexed or S-1 has been proven effective as a therapy for pancreatic cancer (16-18) and biliary tract cancer (19) with tolerable toxicity. Raltitrexed was demonstrated to have a similar effect like 5-FU (20) while being more suitable for patients with mCRC and cardiologic risk factors or previous cardiotoxicity. Thus, in this study, we selected gemcitabine plus raltitrexed as the preferred regimen. Small-scale research has suggested the effectiveness of S-1 as a third- or later-line regimen for patients with refractory mCRC (21-23). Furthermore, S-1 has been proven to be highly effective in gastric cancer and pancreatic cancer with peritoneal metastasis by virtue of its high rate of transition into the peritoneal cavity (24,25). On the basis of the results of previous studies (24,25), we selected gemcitabine plus S-1 to treat patient with mCRC and peritoneal metastasis.
A randomized controlled trial (RCT) needs to meet stringent conditions including randomization, adequate sample size, unbiased outcomes and blinding and so on. The above conditions could not be met due to the limitations of the hospital size, numbers of patients and participating researchers. In addition, every treatment opportunity for mCRC patients is precious. It is not appropriate to conduct an RCT before the preliminary evaluation of efficacy and tolerable toxicity. Thus, we conducted this retrospective study to assess the feasibility of a future RCT.
In the present study, combinations of gemcitabine plus S-1 or raltitrexed were evaluated as a third- or later-line treatment for its efficacy and safety in mCRC patients whose cancer progressed after at least a second-line treatment. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-76/rc).
Methods
Study design and patient population
We enrolled patients with mCRC who received gemcitabine plus raltitrexed or S-1, TAS-102, regorafenib, or fruquintinib at The Second Affiliated Hospital of Soochow University from April 1, 2018 to October 31, 2022. Limited by the size of the hospital and the number of patients who underwent treatments, we failed to enroll the expected number of cases. Anyway, we included as many patients as possible who met the inclusion criteria in the study. Data on the following clinical characteristics were collected from these patients: sex, age, primary location, primary tumor resection, time to metastasis, metastasis management, number of metastatic organs, gene mutation status, and line of treatment.
The enrolled patients were required fulfill the following criteria: (I) a confirmed diagnosis of colon or rectal cancer via endoscopic biopsy or postoperative pathology; (II) mCRC with one or more measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1; (III) failure or intolerance of at least two lines of standard therapies with oxaliplatin, 5-FU, irinotecan, or capecitabine regardless of targeted drugs; and (IV) age ≥18 years. Patients with other malignancies were excluded (except for cervical carcinoma in situ and skin basal cell carcinoma). Patients were excluded for the following reasons: lost to follow-up, protocol violation, voluntary withdrawal, and involuntary withdrawal. This retrospective study was approved by The Second Affiliated Hospital of Soochow University Ethics Committee (No. JD-HG-2023-016). All eligible patients signed a written informed consent prior to their participation. This study was conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Treatment
The enrolled patients chose therapeutic regimens on the basis of their individual physical and financial status. A combination regimen of chemotherapy was administered in this study, consisting of gemcitabine plus raltitrexed or S-1, and the dose schedules, which were repeated in a 3-week cycle, were as follows: gemcitabine 1,000 mg/m2 on days 1 and 8, raltitrexed 3 mg/m2 on day 1, or S-1 1,250 mg/m2 orally twice per day on days 1–14. The fruquintinib dose was 5 mg per day on days 1–21, which was repeated every 28 days; the regorafenib dose was 160 mg once a day on days 1–21 in a 28-day cycle; and the dose of TAS-102 was 35 mg/m2 (maximum 80 mg/m2), which was given twice a day on day 1–5 and 8–12 in a 28-day cycle. Treatment was maintained until disease progression, intolerance of toxicity, patient rejection, or death.
Efficacy and safety assessment
During treatment, clinical and imaging follow-up with contrast-enhanced computerized tomography (CT) and enhanced magnetic resonance imaging (MRI) were performed every 3 months. Laboratory tests, including blood routine, biochemistry, and serum tumor markers detection, were performed every 3 weeks. The patients with disease progression were followed up by telephone every 1 month until death or the last follow-up date if the patient was still alive. The tumor response was assessed according to RECIST (version 1.1) as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR; ORR = CR + PR) and the disease control rate (DCR; DCR = CR + PR + SD) were analyzed as measurements of efficacy. Adverse reactions were assessed based on the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
The study endpoints included progression-free survival (PFS) and overall survival (OS). The PFS was estimated from the initiation of the regimen to disease progression or death without evidence of progression. The OS was recorded from the initiation of the target regimen application to death or the last follow-up date if the patient was still alive. The chi-square test was applied to compare the constituent ratio among the groups. Survival analysis was performed using GraphPad Prism 8.4.2 (GraphPad Software Inc., La Jolla, CA, USA) with the Kaplan-Meier method for median estimation and the 95% confidence interval (CI) for the incidence of events. The log-rank test was used for subgroup analysis. Cox regression analysis was used to investigate potential predictors of survival. Statistical analysis was conducted with SPSS software 25.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
From April 2018 to October 2022, based on the size of the hospital and the study inclusion criteria, 60 patients with mCRC were enrolled in our study, 38 (63.3%) of whom were male. The median age was 60.6 years old, and the other patient characteristics are summarized in Table 1. Among these patients, 13 received chemotherapy (8 were treated as gemcitabine plus raltitrexed and 5 were treated as gemcitabine plus S-1) (Table 2), 15 received fruquintinib, 17 received regorafenib, and 15 received TAS-102. The primary tumor location was the colon and the rectum in 39 (65%) and 21 (35.0%) patients, respectively. Most of the patients (90%) had undergone radical or palliative surgery of the primary tumor. Moreover, 51 (85%) of the participants had metastases in 3 or more organs, and 9 (15%) had metastases in less than 3 organs. The number of patients with and without gene mutation each accounted for half of the total. The regimens conducted in this study were beyond the third line for most patients (71.7%). More than half (55%) of the patients had synchronous distant metastases at diagnosis. Moreover, metastasis managements were performed on 38 (63.3%) patients and included local radiotherapy, radiofrequency ablation (RFA), transcatheter arterial chemoembolization, metastasis resection, and hyperthermic intraperitoneal chemotherapy. In general, there were differences of baseline comparisons in metastasis management, number of metastatic organs and gene mutation status among the four groups.
Table 1
| Characteristics | All (N=60) | Chemotherapy (N=13) | Fruquintinib (N=15) | Regorafenib (N=17) | TAS-102 (N=15) | P value |
|---|---|---|---|---|---|---|
| Sex | 0.72 | |||||
| Male | 38 (63.3) | 8 (13.3) | 11 (18.3) | 11 (18.3) | 8 (13.3) | |
| Female | 22 (36.7) | 5 (8.3) | 4 (6.7) | 6 (10.0) | 7 (11.7) | |
| Age, years | 60.6 [30–82] | 57.7 [38–76] | 58.1 [30–77] | 63.8 [47–82] | 61.9 [47–78] | 0.94 |
| ≤65 | 43 (71.7) | 10 (16.7) | 11 (18.3) | 12 (20.0) | 10 (16.7) | |
| >65 | 17 (28.3) | 3 (5.0) | 4 (6.7) | 5 (8.3) | 5 (8.3) | |
| Primary location | 0.57 | |||||
| Colon | 39 (65.0) | 8 (13.3) | 12 (20.0) | 10 (16.7) | 9 (15.0) | |
| Rectum | 21 (35.0) | 5 (8.3) | 3 (5.0) | 7 (11.7) | 6 (10.0) | |
| Primary tumor resection | 0.918 | |||||
| Yes | 54 (90.0) | 12 (20.0) | 14 (23.3) | 15 (25.0) | 13 (21.7) | |
| No | 6 (10.0) | 1 (1.7) | 1 (1.7) | 2 (3.3) | 2 (3.3) | |
| Time to metastasis | 0.51 | |||||
| Synchronous | 33 (55.0) | 7 (11.7) | 9 (15.0) | 7 (11.7) | 10 (16.7) | |
| Metachronous | 27 (45.0) | 6 (10.0) | 6 (10.0) | 10 (16.7) | 5 (8.3) | |
| Metastasis management | 0.001 | |||||
| Yes | 38 (63.3) | 8 (13.3) | 10 (16.7) | 10 (16.7) | 10 (16.7) | |
| No | 22 (36.7) | 5 (8.3) | 5 (8.3) | 7 (11.7) | 5 (8.3) | |
| Number of metastatic organs | 0.05 | |||||
| <3 | 9 (15.0) | 1 (1.7) | 5 (8.3) | 0 | 3 (5.0) | |
| ≥3 | 51 (85.0) | 12 (20.0) | 10 (16.7) | 17 (28.3) | 12 (20.0) | |
| Gene mutation status | 0.005 | |||||
| Wild type | 30 (50.0) | 11 (18.3) | 3 (5.0) | 10 (16.7) | 6 (10.0) | |
| Mutant | 30 (50.0) | 2 (3.3) | 12 (20.0) | 7 (11.7) | 9 (15.0) | |
| Line of treatment | 0.51 | |||||
| ≤3 | 17 (28.3) | 4 (6.7) | 2 (3.3) | 6 (10) | 5 (8.3) | |
| >3 | 43 (71.7) | 9 (15.0) | 13 (21.7) | 11 (18.3) | 10 (16.7) |
Data are presented as median [range] or number (percentage).
Table 2
| Characteristics | Gemcitabine plus raltitrexed (N=8) | Gemcitabine plus S-1 (N=5) |
|---|---|---|
| Sex | ||
| Male | 4 (30.8) | 4 (30.8) |
| Female | 4 (30.8) | 1 (7.7) |
| Age, years | 50 [38–57] | 62.5 [50–76] |
| ≤65 | 5 (38.5) | 5 (38.5) |
| >65 | 3 (23.1) | 0 |
| Primary location | ||
| Colon | 4 (30.8) | 3 (23.1) |
| Rectum | 4 (30.8) | 2 (15.4) |
| Primary tumor resection | ||
| Yes | 7 (54.8) | 5 (38.5) |
| No | 1 (7.7) | 0 |
| Time to metastasis | ||
| Synchronous | 3 (23.1) | 4 (30.8) |
| Metachronous | 5 (38.5) | 1 (7.7) |
| Metastasis management | ||
| Yes | 4 (30.8) | 4 (30.8) |
| No | 4 (30.8) | 1 (7.7) |
| Number of metastatic organs | ||
| <3 | 8 (61.5) | 1 (7.7) |
| ≥3 | 0 | 4 (30.8) |
| Gene mutation status | ||
| Wild type | 6 (46.2) | 5 (38.5) |
| Mutant | 2 (15.4) | 0 |
| Line of treatment | ||
| ≤3 | 3 (23.1) | 1 (7.7) |
| >3 | 5 (38.5) | 4 (30.8) |
Data are presented as median [range] or number (percentage).
Survival time and efficacy
Regarding tumor response, none of the enrolled patients achieved CR. As shown in Figure 1 and Table 3, in the chemotherapy group, the median PFS was 4.1 months (95% CI: 1.75–6.45), the ORR was 7.69% (1/13), and the DCR was 61.54% (8/13). Separately, in the fruquintinib group, patients had a median PFS of 3.4 months (95% CI: 0.77–6.03), an ORR of 6.67% (1/15), and a DCR of 60.00% (9/15), with 1 (6.67%) patient achieving PR and 8 (53.33%) patients achieving SD. In the regorafenib group, the median PFS was 4.4 months (95% CI: 2.11–6.69), and the DCR was 70.59% (12/17), with no patients achieving CR or PR. In the TAS-102 group, the PFS was 2.3 months (95% CI: 0–5.51), the ORR was 13.33% (2/15), and the DCR was 60.00% (9/15), with 2 patients achieving PR. The median OS of the chemotherapy, fruquintinib, regorafenib, and TAS-102 groups was 7.4 months (95% CI: 3.77–11.03), 6.1 months (95% CI: 2.75–9.45), 8.3 months (95% CI: 5.16–11.44), and 6.7 months (95% CI: 4.68-8.72), respectively (Figure 2).
Table 3
| Response | All, n (%) | Chemotherapy, n (%) | Fruquintinib, n (%) | Regorafenib, n (%) | TAS-102, n (%) |
|---|---|---|---|---|---|
| Total | 60 (100.00) | 13 (21.67) | 15 (25.00) | 17 (28.33) | 15 (25.00) |
| CR | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| PR | 4 (6.67) | 1 (7.69) | 1 (6.67) | 0 (0.00) | 2 (13.33) |
| SD | 34 (56.67) | 7 (53.85) | 8 (53.33) | 12 (70.59) | 7 (46.67) |
| PD | 22 (36.67) | 5 (38.46) | 6 (40.00) | 5 (29.41) | 6 (40.00) |
| ORR | 4 (6.67) | 1 (7.69) | 1 (6.67) | 0 (0.00) | 2 (13.33) |
| DCR | 38 (63.33) | 8 (61.54) | 9 (60.00) | 12 (70.59) | 9 (60.00) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
To further verify the relationship between the clinical characteristics and prognosis of patients with mCRC, univariate analyses were performed (Table 4). In the univariate analysis, the primary lesion location was revealed to be significantly associated with OS in patients with mCRC (P=0.01). Based on the multivariate Cox regression analysis, primary lesion located in the rectum [hazard ratio (HR) =2.22, 95% CI: 1.08–4.55] was adverse independent prognostic factors for OS.
Table 4
| Characteristic | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Univariate analysis | Multivariate analysis | ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Sex | 1.64 (0.93–2.87) | 0.09 | 0.74 (0.38–1.44) | 0.38 | ||||
| Age | 1.14 (0.63–2.08) | 0.66 | 0.59 (0.31–1.13)* | 0.11* | 0.57 (0.29–1.12) | 0.10 | ||
| Primary location | 0.89 (0.50–1.59) | 0.70 | 2.53 (1.24–5.16)* | 0.01* | 2.22 (1.08–4.55)* | 0.03* | ||
| Primary tumor resection | 1.39 (0.55–3.54) | 0.48 | 0.46(0.16-1.35) | 0.15 | ||||
| Time to metastasis | 0.98 (0.57–1.70) | 0.94 | 0.71 (0.39–1.31) | 0.27 | ||||
| Metastasis management | 0.87 (0.50–1.53) | 0.63 | 0.78 (0.42–1.45) | 0.42 | ||||
| Number of metastatic organs | 1.01 (0.47–2.16) | 0.98 | 0.46 (0.16–1.29)* | 0.14* | 0.42 (0.15–1.24) | 0.11 | ||
| Gene mutation status | 1.25 (0.73–2.17) | 0.41 | 0.83 (0.44–1.56) | 0.55 | ||||
| Line of treatment | 1.49 (0.77–2.88) | 0.23 | 0.83 (0.40–1.71) | 0.61 | ||||
To ensure adequate independent variables were included, we expanded the P value range to <0.15. *, significant values. PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Safety assessment
The adverse reactions are shown in Table 5. The incidence of adverse reactions in the chemotherapy group was higher than that in the other three groups, including neutropenia (69.23%), anemia (38.46%), thrombocytopenia (38.46%), nausea (46.15%), vomiting (23.08%), fatigue (46.15%) and diarrhea (38.46%). The toxicity was manageable with adequate symptomatic supportive care. No grade 4 treatment-related adverse reactions or deaths occurred.
Table 5
| Adverse reaction | Chemotherapy | Fruquintinib | Regorafenib | TAS-102 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | ||||
| Hematological | |||||||||||
| Neutropenia | 7 (53.85) | 2 (15.38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (46.67) | 1 (6.67) | |||
| Anemia | 5 (38.46) | 0 (0) | 0 (0) | 0 (0) | 1 (5.88) | 0 (0) | 8 (53.33) | 0 (0) | |||
| Thrombocytopenia | 5 (38.46) | 0 (0) | 1 (6.67) | 0 (0) | 1 (5.88) | 0 (0) | 4 (26.67) | 0 (0) | |||
| Nonhematological | |||||||||||
| Nausea | 6 (46.15) | 0 (0) | 2 (13.33) | 0 (0) | 2 (11.76) | 0 (0) | 6 (40.00) | 0 (0) | |||
| Vomiting | 3 (23.08) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (20.00) | 0 (0) | |||
| Fatigue | 6 (46.15) | 0 (0) | 3 (20.0) | 0 (0) | 6 (35.29) | 0 (0) | 5 (33.33) | 0 (0) | |||
| Diarrhea | 5 (38.46) | 0 (0) | 2 (13.33) | 0 (0) | 4 (23.53) | 0 (0) | 5 (33.33) | 0 (0) | |||
| Rash | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (17.65) | 0 (0) | 0 (0) | 0 (0) | |||
| Petechiae/purpura | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Hypertension | 0 (0) | 0 (0) | 5 (33.33) | 0 (0) | 3 (17.65) | 0 (0) | 0 (0) | 0 (0) | |||
| Transaminase increased | 2 (15.38) | 0 (0) | 1 (6.67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Hyperbilirubinemia | 1 (7.69) | 0 (0) | 2 (13.33) | 0 (0) | 1 (5.88) | 0 (0) | 1 (6.67) | 0 (0) | |||
| Hand-foot skin reaction | 0 (0) | 0 (0) | 5 (33.33) | 1 (6.67) | 5 (29.41) | 1 (5.88) | 0 (0) | 0 (0) | |||
| Oral mucositis | 0 (0) | 0 (0) | 2 (13.33) | 0 (0) | 4 (23.53) | 1 (5.88) | 0 (0) | 0 (0) | |||
| Fever | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.67) | 0 (0) | |||
| Dysphonia | 0 (0) | 0 (0) | 4 (26.67) | 0 (0) | 3 (17.65) | 0 (0) | 0 (0) | 0 (0) | |||
| Proteinuria | 0 (0) | 0 (0) | 4 (26.67) | 0 (0) | 1 (5.88) | 0 (0) | 2 (13.33) | 0 (0) | |||
Data are presented as number (percentage).
Case example
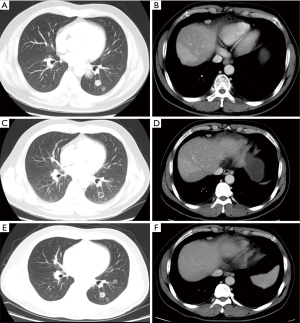
A 64-year-old man was diagnosed with right colon cancer with synchronous liver metastases. The patient underwent radical right hemicolectomy and postoperative adjuvant chemotherapy with FOLFOX. Four months after his surgery, the patient developed a new metastasis in the lesser curvature of the stomach He received FOLFIRI as the first-line treatment. XELOX was replaced as the second-line treatment when the lung metastasis occurred during follow-up. During this period, the patient underwent three CT-guided RFA. First- and second-line treatments lasted for a total of 13 months. After second-line treatment, disease progression recurred in the left lower lobe and the right cardiophrenic angle (Figure 3A,3B). The patient was administered the gemcitabine and raltitrexed regimen (a 3-week cycle of gemcitabine 1.4 g on days 1 and 8 plus raltitrexed 5 mg on day 1). The tumor response was evaluated as PR after three cycles (Figure 3C,3D) and SD after six cycles (Figure 3E,3F) of treatment via contrast-enhanced CT. The regimen lasted for approximately 8.6 months until a new progression occurred. During the course of the treatment, the regular tumor response assessment showed SD. Grade 1 neutropenia occurred during the treatment and improved after the granulocyte colony-stimulating factor (G-CSF) treatment.
Discussion
In our study, gemcitabine plus raltitrexed or S-1 demonstrated some antitumor activity in mCRC. In the chemotherapy group, the median PFS was 4.1 months (95% CI: 1.75–6.45), the ORR was 7.69% (1/13), and the DCR was 61.54% (8/13). According to the DCR (61.54%), median PFS (4.1 vs. 2.3 vs. 4.4 months), and median OS (7.4 vs. 6.1 vs. 8.3 months) of the chemotherapy group, we interpreted that the chemotherapy indicated the similar efficacy compared with those of the other three regimens.
In China, the morbidity and mortality of CRC have been increasing over the years (26). The standard medical treatments for CRC include initial regimens of 5-FU, oxaliplatin, and irinotecan combined with targeted drugs (cetuximab, bevacizumab, and panitumumab), and third-line treatments, such as regorafenib, fruquintinib, and TAS-102. Due to the current multidisciplinary treatments of surgery, radiotherapy, cytotoxic therapies, and target-specific agents, the survival time has been effectively prolonged (27). Nevertheless, the efficiency of the later-line treatment for patients with progression after the receipt of these regimens remains inconclusive, and providing further treatment for these patients remains a challenge for oncologists.
A previous study revealed that the combination of S-1 and raltitrexed can prolong the ORR in the third- or later-line treatment of patients with mCRC (28). In other research, irinotecan plus raltitrexed proved to be promising regimen for patients with oxaliplatin-refractory mCRC (29). A proportion of patients who have previously received the standard and the most effective regimens experienced disease progression. For those patients who cannot receive standard third-line treatments due to an intolerance of side effects or high cost, a more economical and efficient regimen is needed for third- or later-line treatment. Considering the convenience of administration and lack of cardiotoxicity of 5-FU, we excluded those drugs that had been used in the first- and second-line treatments and finally administered the therapy of gemcitabine combined with raltitrexed or S-1.
Additionally, the cost effectiveness of each regimen was evaluated. The regimen of fruquintinib cost CNY ¥2,262 per cycle, that of regorafenib regimen cost CNY ¥2,897 per cycle, and that of TAS-102 ranged from CNY ¥12,848 to CNY ¥13,755 per month. If the above regimens were combined with immunotherapy, an additional cost of CNY ¥2,100 would be incurred per cycle. The cost of the chemotherapy regimen fluctuated between CNY ¥872 and CNY ¥3,645 for each cycle. With similar efficacy, the cost of chemotherapy was much less than that of the other standard third-line therapies.
Toxicity is a particularly important factor in patients with mCRC who have undergone multiple lines of treatment. The most common adverse events of chemotherapy are hematological toxicity, including neutropenia (69.23%), anemia (38.46%), and thrombocytopenia (38.46%). With adequate supportive and symptomatic treatment, the incidence of adverse reactions can be tolerable.
Our study has several limitations. The main drawbacks include the retrospective design and the limited number of participants. Despite the relatively ideal results obtained, the number of patients in the chemotherapy group was insufficient for reclassification and further analysis. A larger population size and rigorous prospective studies are required to further confirm the efficacy of this therapy. Moreover, clinical heterogeneity was present in the interventions and characteristics of the patients. Thus, we attempted to classify the population characteristics and performed Cox regression analysis to investigate independent prognostic factors. Despite the present limitations of our trial, our findings support the viability of a novel clinical option for third- and later-line therapies in patients with mCRC.
Conclusions
In summary, the gemcitabine plus raltitrexed or S-1 regimen achieved a therapeutic effect not worse off than that in the currently practiced standard third-line treatments. With certain therapeutic effect, tolerable adverse reactions and low cost, this regimen represents a potentially therapeutic option for patients with mCRC in clinical work.
Acknowledgments
Funding: This study was funded by grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-76/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-76/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-76/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-76/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Second Affiliated Hospital of Soochow University Ethics Committee (No. JD-HG-2023-016) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci 2023;44:222-36. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [Crossref] [PubMed]
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018;319:2486-96. [Crossref] [PubMed]
- Dasari A, Lonardi S, Garcia-Carbonero R, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet 2023;402:41-53. [Crossref] [PubMed]
- Casadei-Gardini A, Vagheggini A, Gelsomino F, et al. Is There an Optimal Choice in Refractory Colorectal Cancer? A Network Meta-Analysis. Clin Colorectal Cancer 2020;19:82-90.e9. [Crossref] [PubMed]
- Xu J, Xu RH, Qin S, et al. Regorafenib in Chinese patients with metastatic colorectal cancer: Subgroup analysis of the phase 3 CONCUR trial. J Gastroenterol Hepatol 2020;35:1307-16. [Crossref] [PubMed]
- Pandit B, Royzen M. Recent Development of Prodrugs of Gemcitabine. Genes (Basel) 2022;13:466. [Crossref] [PubMed]
- Beutel AK, Halbrook CJ. Barriers and opportunities for gemcitabine in pancreatic cancer therapy. Am J Physiol Cell Physiol 2023;324:C540-52. [Crossref] [PubMed]
- Liu X, Ma X, Ou K, et al. Real-World Results of Raltitrexed Combined with S-1 and Bevacizumab in Heavily Pretreated Metastatic Colorectal Cancer. Cancer Manag Res 2023;15:277-89. [Crossref] [PubMed]
- Huang M, Yang Y, Zhu X, et al. A prospective phase II study of raltitrexed combined with S-1 as salvage treatment for patients with refractory metastatic colorectal cancer. Asia Pac J Clin Oncol 2021;17:513-21. [Crossref] [PubMed]
- Van Laethem JL, Van Maele P, Verslype C, et al. Raltitrexed plus gemcitabine (TOMGEM) in advanced pancreatic cancer. Results of a Belgian multicentre phase II study. Oncology 2004;67:338-43. [Crossref] [PubMed]
- Sugiura T, Toyama H, Fukutomi A, et al. Randomized phase II trial of chemoradiotherapy with S-1 versus combination chemotherapy with gemcitabine and S-1 as neoadjuvant treatment for resectable pancreatic cancer (JASPAC 04). J Hepatobiliary Pancreat Sci 2023;30:1249-60. [Crossref] [PubMed]
- Hua S, Gao J, Xu Q, et al. Pathological complete response in a patient with locally advanced pancreatic adenocarcinoma treated with neoadjuvant gemcitabine and S-1: a case report and literature review. Gland Surg 2022;11:494-503. [Crossref] [PubMed]
- Ioka T, Shindo Y, Ueno M, et al. Current progress in perioperative chemotherapy for biliary tract cancer. Ann Gastroenterol Surg 2023;7:565-71. [Crossref] [PubMed]
- Batra A, Rigo R, Hannouf MB, et al. Real-world Safety and Efficacy of Raltitrexed in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer 2021;20:e75-81. [Crossref] [PubMed]
- Derksen JWG, Smit KC, May AM, et al. Systematic review and non-inferiority meta-analysis of randomised phase II/III trials on S-1-based therapy versus 5-fluorouracil- or capecitabine-based therapy in the treatment of patients with metastatic colorectal cancer. Eur J Cancer 2022;166:73-86. [Crossref] [PubMed]
- Wan Y, Luo D. Using a combination of fruquintinib, raltitrexed, and S-1 as a third-line treatment for metastatic colorectal cancer with co-existence of Hodgkin lymphoma: a case report. J Gastrointest Oncol 2023;14:450-7. [Crossref] [PubMed]
- Dai Y, Sun L, Zhuang L, et al. Efficacy and safety of low-dose apatinib plus S-1 versus regorafenib and fruquintinib for refractory metastatic colorectal cancer: a retrospective cohort study. J Gastrointest Oncol 2022;13:722-31. [Crossref] [PubMed]
- Wei J, Wu ND, Liu BR. Regional but fatal: Intraperitoneal metastasis in gastric cancer. World J Gastroenterol 2016;22:7478-85. [Crossref] [PubMed]
- Yamamoto T, Fujii T, Hirano S, et al. Randomized phase III trial of intravenous and intraperitoneal paclitaxel with S-1 versus gemcitabine plus nab-paclitaxel for pancreatic ductal adenocarcinoma with peritoneal metastasis (SP study). Trials 2022;23:119. [Crossref] [PubMed]
- Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 2021;14:101174. [Crossref] [PubMed]
- Leowattana W, Leowattana P, Leowattana T. Systemic treatment for metastatic colorectal cancer. World J Gastroenterol 2023;29:1569-88. [Crossref] [PubMed]
- Chen Y, Wu J, Cheng K, et al. S-1 plus Raltitrexed for Refractory Metastatic Colorectal Cancer: A Phase II Trial. Oncologist 2019;24:591-e165. [Crossref] [PubMed]
- Cheng K, Zhou YW, Chen Y, et al. Biweekly Raltitrexed Combined With Irinotecan as Second-Line Therapy for Patients With Metastatic Colorectal Cancer: A Phase II Trial. Cancer Control 2022;29:10732748221080332. [Crossref] [PubMed]




