
Regorafenib for patients with progression of advanced hepatocellular carcinoma after treatment with atezolizumab plus bevacizumab: a case series
Highlight box
Key findings
• Second-line treatment with regorafenib may benefit patients with intermediate to advanced hepatocellular carcinoma (HCC) that has progressed after treatment with atezolizumab plus bevacizumab (TA) regimens.
What is known and what is new?
• TA regimens are the standard first-line therapy for advanced HCC.
• Our study provides a novel finding that HCC patients receiving second-line regorafenib after TA failure have good disease control.
What is the implication, and what should change now?
• For patients with intermediate- and advanced-stage HCC who have experienced disease progression after treatment with TA, and regorafenib can be used as a second-line regimen to delay progression.
• The potential for regorafenib to be the optimal choice among other molecularly targeted agents raises new questions regarding the second-line treatment of intermediate and advanced HCC.
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant tumor of the liver and the second leading cause of cancer-related death (1). The majority of patients with HCC are already in the middle to late stages at the time of diagnosis, and the 5-year overall survival (OS) rate for patients with advanced HCC is only 14.1% (2); thus, more effective treatment options need to be developed. The clinical application of immunotherapy has greatly enriched the options for the systemic treatment of advanced HCC. The combination regimen of atezolizumab plus bevacizumab (TA) was the first targeted combination immunotherapy to be recommended as a first-line treatment option in the management of advanced HCC, with the IMbrave150 study reporting a median OS of 19 months for patients treated with this regimen (3,4). Although both targeted therapy and immunotherapy are options in the systemic treatment of HCC, the combinations of different drugs are associated with varying degrees of safety and efficacy. Not all patients with advanced HCC benefit from immunotherapy, and there is a lack of clear guidance regarding the choice of second-line therapy for patients with advanced HCC after the TA regimen treatment fails or HCC progresses.
As a second-line standard of care for HCC after sorafenib treatment, regorafenib is a target-rich regimen capable of profoundly blocking multiple levels of cross-talk, effectively overcoming tyrosine kinase inhibitor (TKI) resistance, comprehensively inhibiting angiogenesis, and improving the immune microenvironment of suppressive tumors (5,6). However, to date, few reports exist regarding regorafenib as a second-line therapy for patients whom have already been treated with a TA regimen. Therefore, in this study, we reviewed the second-line treatment with regorafenib after administration of the TA regimen in Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College by examining the clinical outcomes of five cases of intermediate- to advanced-stage HCC. The aim of this case series was to provide a reference for the choice of a second-line treatment regimen after targeted combination immunotherapy for HCC in clinic. We present this article in accordance with the AME Case Series reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-128/rc).
Case presentation
We retrospectively reviewed a total of 58 patients with intermediate and advanced HCC who received TA regimens at the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between December 2019 and June 2023. We ultimately included five patients who received regorafenib as second-line treatment after progression on first-line treatment with TA regimens; Figure 1 summarizes the flowchart of the treatment of all patients. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The institutional review board of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College waived the requirement for patient consent for the publication of this case series and accompanying images due to the retrospective nature of the study (ethics number: 24/002/4282).
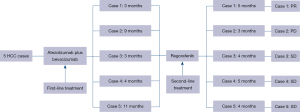
Regorafenib is administered at a dose of 160 mg, orally (po), once a day (qd). During regorafenib treatment, patients were reviewed every 4–6 weeks via computed tomography and magnetic resonance imaging (MRI) to assess tumor response, which was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). A recent clinical outcome in this study was defined as one recorded 8–12 weeks after regorafenib treatment. Progression-free survival (PFS) was defined as the time from the start of regorafenib treatment to the onset of tumor progression. The follow-up period ended in July 2023 for all patients.
In this case series, we retrospectively analyzed and report the clinical data, treatment process, MRI images, recent efficacy, and adverse effects of the five patients whom were ultimately included in the study. Table 1 summarizes the baseline demographic characteristics of the five patients, and Table 2 summarizes the course of treatment and clinical outcomes of the five patients.
Table 1
| Case ID | Age (years) | Sex | HBV | Cirrhosis | AFP level (ng/mL) | Child-Pugh class | BCLC stage | Tumor size (cm) | Distant metastasis | Macroscopic vascular invasion | Treatment response of the TA regimen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | Male | Yes | Yes | 2,745 | A | C | 6.8 | Bone metastasis | No | PD |
| 2 | 63 | Male | No | Yes | 12.7 | A | B | 2.0 | No | No | PD |
| 3 | 56 | Female | No | No | 136,119 | A | B | 2.5 | No | No | PD |
| 4 | 60 | Male | Yes | Yes | 133.6 | A | C | 14 | No | Yes | PD |
| 5 | 64 | Male | Yes | Yes | 34.3 | A | C | 4.0 | No | Yes | PD |
HBV, hepatitis B virus; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; TA, atezolizumab plus bevacizumab; PD, progressive disease.
Table 2
| Case ID | Previous treatment | First-line drugs | Second-line drug | Clinical efficacy | Adverse events | PFS (months) | Death (months post-regorafenib) |
|---|---|---|---|---|---|---|---|
| 1 | HAIC | TA regimen | Regorafenib | PR | None | 5 | Survival, 12 |
| 2 | Surgical resection TACE | TA regimen | Regorafenib | PD | Scrotal edema | 3 | Survival, 7 |
| 3 | Surgical resection TACE | TA regimen | Regorafenib | SD | None | 4 | Survival, 9.5 |
| 4 | HAIC | TA regimen | Regorafenib | SD | None | 5 | Death, 10 |
| 5 | HAIC | TA regimen | Regorafenib | SD | None | 4 | Survival, 14.5 |
PFS, progression-free survival; HAIC, hepatic arterial infusion chemotherapy; TA, atezolizumab plus bevacizumab; PR, partial response; TACE, transarterial chemoembolization; PD, progressive disease; SD, stable disease.
Table 1 includes the clinical data of the patients, including indicators of age, gender, past medical history, Barcelona Clinic Liver Cancer stage, and tumor size. Four patients were male and one was female, their ages ranged from 56 to 64 years old, and three patients had hepatitis B virus (HBV) infection, cirrhosis, and elevated levels of alpha-fetoprotein (AFP) at baseline; two patients had received previous surgical treatment; three patients had received hepatic arterial infusion chemotherapy (HAIC) treatment; and two patients had received transarterial chemoembolization (TACE) treatment. The first-line treatment regimen for all patients was the TA regimen and the second-line treatment was regorafenib. In terms of recent clinical outcomes, one patient achieved partial response (PR), three patients achieved stable disease (SD), and one patient developed progressive disease (PD), representing a disease control rate (DCR) of 80% and an objective response rate (ORR) of 20%. One patient experienced an adverse event of scrotal edema.
Case 1
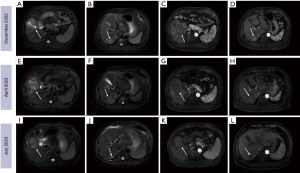
A 58-year-old male patient was found to have an HBV infection for 7 months. The patient underwent MRI at our hospital in December 2022, which showed a tumor size of 6.8 cm with bone metastasis, and through combination with pathological examination, the patient was diagnosed with advanced HCC (Figure 2A-2D). Two HAIC sessions were subsequently administered in January and February 2023. The patient underwent four cycles of the TA regimen from January to April 2023. April 2023 MRI scan in the arterial phase showed tumor enhancement with a markedly enlarged tumor (compared to December 2022 imaging) with partial fusion of lesions and intrahepatic progression of multiple tumors (Figure 2E-2H). The patient’s AFP level was 2,745 ng/mL, and the tumor response was evaluated as PD. From April to July 2023, the patient was treated with regorafenib. MRI scan in July 2023 showed that tumor arterial phase enhancement was significantly reduced, and the tumor response was assessed as PR (compared to April 2023 imaging) (Figure 2I-2L).
Case 2
A 63-year-old male patient was admitted to hospital with no previous history of HBV infection. The patient underwent hepatectomy for HCC in September 2021. A follow-up MRI scan in January 2022 showed intrahepatic tumor recurrence, and the patient underwent one session of TACE. The patients underwent eight cycles of a TA regimen beginning in February 2022, along with three TACE sessions. The MRI findings from June to November 2022 indicated that the lesions were increasing in size and that the tumor was progressing. Regorafenib treatment was performed in November 2022. The patient’s AFP level was 12.7 ng/mL, and the patient had an adverse reaction of scrotal edema during the treatment. Tumor progression was noted in March 2023, and tumor response was assessed as PD. This patient experienced an adverse event of scrotal edema.
Case 3
A 56-year-old female patient was admitted to hospital with no prior history of HBV. The patient underwent hepatectomy for HCC in January 2022 and received one cycle of apatinib and camrelizumab postoperatively, which was discontinued due to dermal and mucosal adverse effects. The patient underwent four sessions of TACE from August to December 2022. A TA regimen was administered from December 2022 to March 2023, and the MRI review showed tumor progression. The patient was switched to regorafenib, which was administered from April to July 2023. Subsequently, MRI revealed the lesion to be approximately the same as before, and the tumor response was assessed as SD.
Case 4
A 60-year-old male patient who had a previous HBV infection and cirrhosis was admitted to the hospital. In November 2021, MRI showed a liver tumor size of 14 cm with multiple cancer emboli in the main trunk and left and right branches of the portal vein. In combination with the findings of a pathological examination, the patient was diagnosed with advanced HCC. The patient underwent one HAIC session in December 2021 and continued a synchronous combined TA regimen until March 2022. Examination in March 2022 revealed tumor progression, and thus the treatment was switched to regorafenib therapy which continued until September 2022. The patient’s AFP level was 133.6 ng/mL, and the tumor was roughly the same size as that observed on the March 2022 MRI image. The tumor response was assessed as SD.
Case 5
A 64-year-old male patient with previous hepatitis C virus infection for 1 year and cirrhosis was admitted to hospital. An MRI scan in December 2021 showed multiple tumors in the right lobe of the liver, with the largest being 7.2 cm. Multiple cancerous emboli could be observed in the main trunk of the portal vein, the right branch of the left branch, and its branches. The AFP level was 81,370 ng/mL in December 2021, which when considered in combination with the AFP and MRI findings, indicated a diagnosis of advanced HCC. This patient underwent six sessions of HAIC from December 2021 to September 2022 and 15 cycles of TA from December 2021 to November 2022, with the tumor response assessed as PD. In January 2023, the baseline AFP levels of patients treated with regorafenib were 34.3 ng/mL. Regorafenib treatment was administered from January to June 2023, with tumor response assessed as SD.
Discussion
In this retrospective case series, we evaluated the recent clinical outcomes of five patients with intermediate to advanced HCC treated with regorafenib after disease progression on first-line TA treatment. In our study, one patient achieved PR, three patients achieved SD, and one patient developed PD according to the mRECIST, representing a DCR of 80% and an ORR of 20%. These results suggest that the second-line therapeutic agent regorafenib may be effective in delaying progression in patients with advanced HCC after progression on TA treatment and may be associated with better disease control in patients.
The mRECIST were chosen for efficacy assessment in our study rather than the RECIST. This is because the RECIST uses the maximum diameter of the tumor to determine efficacy (including the surviving tumor and necrotic area) which does not reflect the tumor necrosis caused by the targeted drug. On the other hand, the mRECIST evaluates the surviving tumor and excludes the interference of necrotic tumors. Dynamic enhancement MRI or CT can clearly detect the disappearance of arterial enhancement within the tumor, and tumors or tumor areas with no contrast uptake can be regarded as necrotic tissues. The mRECIST adopts arterial enhancement to clearly distinguish between surviving and necrotic tumors and therefore can correctly evaluate the changes of tumors before and after treatment. In case 1, the summed diameter reduction of the MRI arterial enhancement for the target lesion was ≥30%, and the efficacy was evaluated as PR.
TA treatment has become the standard first-line therapy for advanced HCC. The results of a phase III clinical study, IMbrave150, showed that the 12-month OS rate of TA for the first-line treatment of advanced HCC reached 67.2%, which was superior to the 54.6% yielded by sorafenib (7). However, secondary resistance is an unavoidable challenge in immunotherapy, in which some patients who begin to show benefit from immunotherapy eventually experience tumor progression after a period of treatment (8). Immune checkpoint inhibitor (ICI) therapy for HCC involves a number of therapeutic options after disease progression, including continuation of ICI treatment, a switch to TKI, and discontinuation of antitumor therapy (9). Several studies have evaluated the second-line treatment options for HCC after progression on TA regimens. Yoo et al. demonstrated that second-line treatment with sorafenib and lenvatinib showed similar efficacy for patients with advanced HCC after progression on TA regimens (10). Storandt et al. examined 26 patients with advanced HCC who had progressed after immunotherapy with the TA regimen and were treated with cabozantinib. These patients had a median PFS of 2.1 months and a median OS of 7.7 months after cabozantinib treatment, suggesting that patients may benefit from cabozantinib treatment after progression following immunotherapy (11). A recent study showed that regorafenib provided better OS than did other TKIs; the first-line treatment with TA did not affect efficacy of second-line TKI therapy, and patients’ PFS was not affected by the TKI type (12).
The above-described findings support the benefit of a variety of multitargeted drugs as second-line treatment following first-line TA therapy. The RESORCE study (13) confirmed the value of regorafenib in the second-line treatment of HCC, leading to regorafenib being the first recommended drug for second-line systemic therapy of HCC. Compared with other TKIs, regorafenib has more targets of action and is thus capable of blocking cross-talk between different signaling pathways and delaying the onset of drug resistance (14,15). In addition, regorafenib promotes normalization of tumor vasculature in modulating the tumor microenvironment and contributes to synergistic effects when combined with ICIs (16,17). Whether the second-line drug regorafenib is superior to other molecularly targeted drugs after treatment with a TA regimen needs to be further confirmed by additional clinical case observations and studies. In our case series, we found that the antitumor effect of regorafenib was only evident in patients who had been treated with sorafenib. This may be due to the small number of patients in our study. Our current results do not provide sufficient evidence to support the effectiveness of regorafenib in treating tumor after sorafenib treatment has failed. Further studies with larger patient cohorts are needed to confirm the findings.
Conclusions
For progression of intermediate- and advanced-stage HCC after treatment with TA, regorafenib can be used as a second-line regimen to delay progression. However, it remains unclear whether regorafenib is the best choice among other molecularly targeted agents for the second-line treatment of intermediate and advanced HCC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-128/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-128/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-128/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The institutional review board of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College waived the requirement for patient consent for the publication of this case series and accompanying images due to the retrospective nature of the study (ethics number: 24/002/4282).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345-62. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151-72. [Crossref] [PubMed]
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. [Crossref] [PubMed]
- Arai H, Battaglin F, Wang J, et al. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev 2019;81:101912. [Crossref] [PubMed]
- Oura K, Morishita A, Hamaya S, et al. The Roles of Epigenetic Regulation and the Tumor Microenvironment in the Mechanism of Resistance to Systemic Therapy in Hepatocellular Carcinoma. Int J Mol Sci 2023;24:2805. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Kluger HM, Tawbi HA, Ascierto ML, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J Immunother Cancer 2020;8:e000398. [Crossref] [PubMed]
- Talbot T, D'Alessio A, Pinter M, et al. Progression patterns and therapeutic sequencing following immune checkpoint inhibition for hepatocellular carcinoma: An international observational study. Liver Int 2023;43:695-707. [Crossref] [PubMed]
- Yoo C, Kim JH, Ryu MH, et al. Clinical Outcomes with Multikinase Inhibitors after Progression on First-Line Atezolizumab plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma: A Multinational Multicenter Retrospective Study. Liver Cancer 2021;10:107-14. [Crossref] [PubMed]
- Storandt MH, Gile JJ, Palmer ME, et al. Cabozantinib Following Immunotherapy in Patients with Advanced Hepatocellular Carcinoma. Cancers (Basel) 2022;14:5173. [Crossref] [PubMed]
- Falette-Puisieux M, Nault JC, Bouattour M, et al. Beyond atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: overall efficacy and safety of tyrosine kinase inhibitors in a real-world setting. Ther Adv Med Oncol 2023;15:17588359231189425. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Grothey A, Blay JY, Pavlakis N, et al. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat Rev 2020;86:101993. [Crossref] [PubMed]
- Heo YA, Syed YY. Regorafenib: A Review in Hepatocellular Carcinoma. Drugs 2018;78:951-8. [Crossref] [PubMed]
- Liu J, Tao H, Yuan T, et al. Immunomodulatory effects of regorafenib: Enhancing the efficacy of anti-PD-1/PD-L1 therapy. Front Immunol 2022;13:992611. [Crossref] [PubMed]
- Juengpanich S, Topatana W, Lu C, et al. Role of cellular, molecular and tumor microenvironment in hepatocellular carcinoma: Possible targets and future directions in the regorafenib era. Int J Cancer 2020;147:1778-92. [Crossref] [PubMed]


