Percutaneous biliary drainage catheter insertion in patients with extensive hepatic metastatic tumor burden
Introduction
In patients with advanced metastatic disease of the liver, tumors located adjacent to the biliary tree can exert mass effect resulting in biliary obstruction. Decompression of malignant biliary obstruction can be crucial for preventing acute cholangitis with accompanied sepsis, symptomatic relief of hyperbilirubinemia-associated pruritus (1), and in some cases, can facilitate a patient’s access to further chemotherapy (2,3). However, this patient population may be prone to intrinsic liver dysfunction related to extensive hepatic parenchyma replacement with tumoral tissue or hepatotoxic chemotherapies, which can also result in hyperbilirubinemia (4). Thus, the actual contribution of obstruction versus intrinsic hepatic dysfunction can be difficult to ascertain.
While percutaneous biliary drainage (PBD) catheter insertion has been shown to be an effective and safe method for decompressing an obstructed biliary tree (5), the presence of metastatic disease of the liver can impose particular challenges. Multifocal biliary obstruction can be difficult or impossible to adequately drain with a reasonable number of catheters. Furthermore, there may be risk for direct catheter traversal through malignant tissue prior to entering the biliary tree, with potentially increased risk of acute and chronic hemorrhagic complications, inadequate pericatheter tract formation with persistent pericatheter leakage, or bacterial seeding of necrotic tumor given the PBD catheter continuity with the GI tract. Thus, while palliative reduction in bilirubin levels and mitigation of cholangitis risk are important goals of PBD insertion in this patient population, the risk of complications with PBD insertion through tumoral tissue may be elevated, and the overall benefit of drainage in this patient population with end-stage malignancy may be questionable. Therefore, the purpose of this study was to analyze outcomes of PBD catheter insertion in patients with extensive hepatic metastatic tumor burden.
Methods
Study population
Institutional review board approval and a waiver of informed consent were obtained for this retrospective study. Review of the interventional radiology procedural database revealed 746 patients who underwent PBD catheter insertion between December 2005 and April 2013. Of these, 266 were performed in patients with metastatic disease of the liver in the setting of hyperbilirubinemia and radiologic evidence of biliary ductal dilation. Patients were included in the study if they had more than 20% hepatic tumor burden based on review of the pre-procedure computed tomography (CT) or magnetic resonance imaging (MRI). Patients with extrahepatic cholangiocarcinoma were not included.
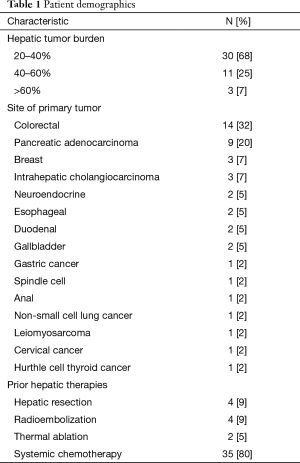
A total of 44 patients (24 males, 20 females, mean age 57.4 years, range, 34–80 years) were included for analysis. Thirteen patients (30%) had clinical and laboratory findings concerning for cholangitis at initial presentation. Colon cancer (n=14, 32%) and pancreatic cancer (n=9, 20%) were the two most common sites of primary tumor (Table 1). Previous oncologic treatments in the patient population are detailed in Table 1. The average number of chemotherapy regimens used for each patient was 2.2 (range, 0–7). Nine patients (20%) did not receive any oncologic treatment prior to PBD placement due to advanced presentation of disease. In 11 patients (25%), an ERCP was attempted or performed prior to PBD placement. Seven patients (16%) presented with an occluded endoscopic biliary stent.
Full table
PBD insertion technique
All procedures were performed by or under the direct supervision of an attending interventional radiologist. Moderate sedation and prophylactic antibiotics were utilized in all cases. Patients with an international normalized ratio (INR) greater than 1.5 or platelet count less than 50,000 were transfused with fresh frozen plasma or platelets, respectively, until the threshold value was achieved. Based on review of the pre-procedure CT or MRI, the appropriate biliary access site was chosen at the discretion of the operator. For right-sided biliary decompression, biliary ductal access was performed using fluoroscopic guidance. Using a 22-gauge needle, blind passes were made until a bile duct was successfully accessed based on injection of dilute iodinated contrast. A cholangiogram was then performed to assess the biliary access point. If the access point was deemed suboptimal due to an excessively central puncture or non-continuity with a specifically desired distribution, a second access to a more optimal site was performed with a second 22-gauge needle using fluoroscopic guidance. For left-sided biliary decompression, biliary ductal access was achieved using fluoroscopic or sonographic guidance at the operator’s discretion. Once an acceptable access point was achieved for catheter insertion, the tract was then dilated with a 6 French triaxial dilator system (Accustick, Boston Scientific, Natick, MA or Aprima, Cook Medical, Bloomington, IN, USA) to allow advancement of a guidewire across the common bile duct into the duodenum. An 8 or 8.5 French locking pigtail PBD catheter (Flexima, Boston Scientific or Ultrathane, Cook Medical) was then inserted with the pigtail loop positioned in duodenum. In cases where the site of obstruction could not be traversed, an 8 French pigtail drainage catheter (Flexima, Boston Scientific) was inserted above the site of obstruction. After several days of drainage, traversal of the obstruction was reattempted.
Data acquisition
Medical records were retrospectively reviewed for patient demographics, procedural detail, relevant laboratory values, and clinical course. The total serum bilirubin and alkaline phosphatase values were recorded immediately pre-procedure and at its lowest point within a month after PBD insertion. For patients with a CT scan or MRI performed subsequent to PBD insertion, imaging findings were reviewed as described below. Treatment-related complications were classified according to the reporting standards of the Society of Interventional Radiology (6). Given the poor life expectancy of the study population, only complications specifically attributable to the PBD insertion were included, such as persistent pericatheter leakage requiring a catheter check under fluoroscopy, hemorrhage related to PBD insertion as evidenced by need for packed red blood cell transfusions or additional intervention, prolonged catheter-related pain, and episodes of cholangitis requiring hospitalization. The date of death for each patient was obtained from medical or public records for all patients.
Image analysis
All patients had a CT or MRI prior to the procedure. Imaging was reviewed for assessment of tumor burden and categorized as 20–40% hepatic volume replacement by tumor, 41–60%, or greater than 60% replacement. Patients with a post-PBD insertion CT scan or MRI underwent additional analysis. The first imaging study after PBD insertion was reviewed to ascertain whether the PBD catheter traversed tumor tissue prior to entering the biliary tree. Additionally, the presence of hemorrhagic complications such as hemoperitoneum or subcapsular hematoma was assessed.
Statistical analysis
Changes in laboratory values prior to and after PBD insertion were compared using the student’s paired t-test. Mean values presented with standard deviation (SD). The initial bilirubin level, number of chemotherapy lines, alkaline phosphatase levels, INR, and tumor burden (20–39%, 40–59% or >60%) were assessed as potential predictors of the percentage change in total bilirubin level after PBD insertion with linear regression analysis. The overall survival, defined as the period from the date of PBD placement to the date of death, was calculated using the Kaplan-Meier technique. Univariate Cox regression analysis was performed to assess predictors of survival based on the following characteristics: age, number of prior chemotherapy regimens, tumor burden, initial bilirubin level, initial alkaline phosphatase level, INR, and percent change in bilirubin level after PBD insertion. Multivariate Cox regression analysis was performed using the variables found to be significant on univariate analysis. A P value of less than or equal to 0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software v22 (IBM, Chicago, IL, USA).
Results
Technical success rate
In two patients, access through the biliary system into the duodenum was initially not able to be achieved. In both patients, a drainage catheter was inserted peripheral to the occlusive lesion; after 2 days of drainage, the lesion was able to be traversed in both patients. All other procedures were technically successful. Twenty-nine patients (64%) underwent insertion of one PBD, 14 (29%) received two, and 1 patient (2%) received three PBD catheters.
Serum laboratory outcomes
The median and mean pre-procedural total serum bilirubin levels were 10.6 and 11.7±6.7 mg/dL (range, 2.7–27.6 mg/dL). Of the 37 patients with postprocedural serum liver function tests available, the mean serum bilirubin levels decreased significantly from 10.9±6.4 to 7.1±5.6 mg/dL (P<0.001) within one month post PBD insertion. The serum total bilirubin decreased by at least 20% in 25 patients (68%). Of these 25 patients, the mean decrease in bilirubin levels was 52%±23% (5.5±3.1 mg/dL). Four patients (11%) demonstrated normalization of bilirubin levels to less than 1.6 mg/dL. In those four patients, the median initial bilirubin level was 5.8 mg/dL (range, 4.2–9.2 mg/dL).
The mean alkaline phosphatase levels dropped significantly from 592±270 to 345±171 (P<0.001) after PBD insertion. Seventy-eight percent of patients (n=29) demonstrated a 20% or greater decrease in serum alkaline phosphatase level after PBD insertion. The mean pre-procedure INR was 1.36±0.28 (range, 1.0–2.4). Neither initial total bilirubin levels, alkaline phosphatase level, INR, number of chemotherapy regimens, nor tumor burden were predictive of the change in bilirubin levels after PBD insertion.
Tumor tissue traversal
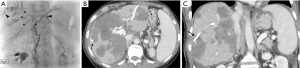
Fourteen patients had a post-PBD insertion CT scan. In 11 cases (79%), the PBD was demonstrated to traverse tumoral tissue prior to entering the biliary tree (Figure 1). One of these patients had a small subcapsular hematoma associated with the catheter. One patient had recurrent pericatheter leakage and catheter occlusion, requiring five catheter exchanges over the ensuing 2 weeks, with progressive catheter upsizing to 14 French. One patient required a packed red blood cell transfusion 3 days after the procedure due to a hematocrit level that drifted from 0.33 L/L to 0.26 over 2 days.
Complications
There were 7 major PBD-related complications (16%) within 30 days of PBD insertion, consisting of five patients with subsequent cholangitis requiring admission and two patients requiring blood transfusions within a week of PBD insertion with an INR of 1.3 and 1.5. Of these, one patient died during the same admission. Nine patients had minor complications including prolonged abdominal pain, dehydration, and catheter exchange due to catheter obstruction or retraction.
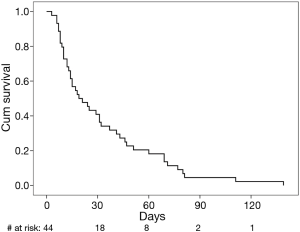
Survival
The 30-day overall survival rate after PBD insertion was 41% (Figure 2). The median survival for the study population was 19 days (95% CI: 8–30 days). The only variables correlated with survival on univariate analysis was the percentage change in serum total bilirubin level after PBD insertion (P=0.005) and INR (P=0.020). On multivariate analysis, both percent change in bilirubin levels and INR were independently predictive (OR =3.7, P=0.010 and OR =4.9, P=0.028 respectively).
Discussion
A majority of patients in the current study demonstrated greater than 20% decrease in serum levels of total bilirubin and alkaline phosphatase after PBD insertion, which is compatible with some degree of biliary tree decompression. However, normalization of serum bilirubin levels occurred rarely. It has been previously reported that liver function is unlikely to normalize following PBD insertion if there is involvement of greater than 75% of the hepatic parenchyma by tumor (7). The current results suggest that normalization may rarely occur at a much lower threshold of tumor involvement in this population.
Patients with extensive hepatic metastatic tumor burden are typically at the end-stage with a poor prognosis; therefore, death due to PBD insertion versus natural disease course is difficult to ascertain. Patients who are in the process of acute hepatic failure typically have an extremely short life expectancy (8). The median survival in the current study population was only 19 days after PBD insertion. A poor prognosis has previously been reported with PBD insertion for patients with obstructive jaundice in the setting of advanced malignancy (9-11). While previous studies in patients with advanced solid malignancies have demonstrated median overall survival of up to 2.9 months, 90% of patients were eligible for chemotherapy following PBD placement (12). Kasuga et al. reported that multiple liver metastases and multiple prior chemotherapy administrations were both independently associated with a poorer prognosis following PBD insertion, with median survivals of 65 and 34 days in patients with successful and unsuccessful PBD insertion (9). While the degree of hepatic tumor burden was not reported in these other studies, all patients in the current study had extensive hepatic tumor burden that may not be treated at all centers. Furthermore, none of the patients in this study received any chemotherapy after PBD insertion. Finally, the vast majority of studies on PBD insertion in patients with advanced malignancy primarily analyzed patients with common bile duct obstruction by extrahepatic cholangiocarcinoma, primary gallbladder cancer, and primary pancreatic cancer, whereas the current study analyzed only patients with extensive intrahepatic metastatic disease burden and intrahepatic biliary obstruction.
Given the generally poor survival after PBD insertion, pre-procedural predictors of survival can be helpful to optimize patient selection. Previously reported predictors of poor prognosis after PBD insertion include an ECOG performance status score of greater than 2 (10), high pre-procedural total bilirubin levels (12), and a postprocedural serum bilirubin level of less than 2 mg/dL (13). The ECOG status of the current patient population was not consistently known, given its retrospective nature. The absolute post-procedural serum bilirubin level did not correlate with survival in the current study. However, the pre-procedure INR and the percentage change in bilirubin levels after PBD insertion were independently predictive of survival. While an elevated INR can predispose patients to hemorrhagic complications, it can also be reflective of poor hepatic synthetic function related to hepatic failure, which may be present in this patient population due to parenchymal replacement by tumor or prior hepatotoxic chemotherapies. The finding that higher percentage decreases in bilirubin were predictive of longer survival may suggest that PBD decompression of an obstructed biliary system improves survival or may be reflective of the underlying etiology of hyperbilirubinemia, i.e., biliary obstruction versus intrinsic hepatic dysfunction from prior hepatotoxic chemotherapy or parenchymal replacement. However, the generally poor survival suggests that these predictors may not be particularly impactful.
The primary goal of many interventions performed in patients with end-stage malignancy is symptom palliation. While PBD catheter insertion may improve symptoms related to hyperbilirubinemia and mitigate cholangitis in patients with extensive hepatic tumor burden, subjective measures of symptom improvement and other quality of life measures such as PBD-related discomfort were not reliably available in the medical records. In a prospective study investigating quality of life measures in patients with unresectable malignant bile duct obstruction, measures of pruritus were improved following the procedure, but quality of life was not (14). It is clear that the goals of intervention should be discussed with the patient and caregivers (15), since PBD insertion can be a cause of major and minor complications (16).
One of the major limitations of this study is its retrospective nature. A selection bias likely exists, since some patients with advanced malignancy may have been declined for the procedure or may not have been referred for the procedure. The small sample size limits a robust analysis of independent predictors of survival. Additionally, given the end-stage nature of this patient population, continued post-procedural labs and clinical information were not routinely available in some patients who were transitioned to hospice soon after PBD insertion. Therefore, the PBD-related complication rate is likely underestimated and the actual cause of death is sometimes unknown. Furthermore, given the median 19-day survival after PBD insertion, many patients did not survive long enough for stabilization of their bilirubin levels.
In conclusion, insertion of PBD catheters in patients with extensive hepatic metastatic tumor burden is associated with a high 30-day mortality with a modest PBD-related major complication rate. While total serum bilirubin is likely to decrease to some degree in this patient population following intervention, normalization of bilirubin levels is unlikely. Therefore, a careful assessment of risks and benefits must be performed in this setting. While patients with higher percentage decreases in serum bilirubin levels had a longer survival, the survival benefit was marginal considering the poor overall survival in this population. Further investigation on quality of life measures in these terminally ill patients would be helpful to assess the degree of palliation provided.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of Duke University (NO. 52816). For this type of study formal informed consent is not required.
References
- Ballinger AB, McHugh M, Catnach SM, et al. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut 1994;35:467-70. [Crossref] [PubMed]
- Van Laethem JL, De Broux S, Eisendrath P, et al. Clinical impact of biliary drainage and jaundice resolution in patients with obstructive metastases at the hilum. Am J Gastroenterol 2003;98:1271-7. [Crossref] [PubMed]
- Thornton RH, Ulrich R, Hsu M, et al. Outcomes of patients undergoing percutaneous biliary drainage to reduce bilirubin for administration of chemotherapy. J Vasc Interv Radiol 2012;23:89-95. [Crossref] [PubMed]
- Reissfelder C, Brand K, Sobiegalla J, et al. Chemotherapy-associated liver injury and its influence on outcome after resection of colorectal liver metastases. Surgery 2014;155:245-54. [Crossref] [PubMed]
- Saad WE, Wallace MJ, Wojak JC, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol 2010;21:789-95. [Crossref] [PubMed]
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199-202. [Crossref] [PubMed]
- Covey AM, Brown KT. Palliative percutaneous drainage in malignant biliary obstruction. Part 1: indications and preprocedure evaluation. J Support Oncol 2006;4:269-73. [PubMed]
- Boone MD, Celi LA, Ho BG, et al. Model for End-Stage Liver Disease score predicts mortality in critically ill cirrhotic patients. J Crit Care 2014;29:881.e7-13. [Crossref] [PubMed]
- Kasuga A, Ishii H, Ozaka M, et al. Clinical outcome of biliary drainage for obstructive jaundice caused by colorectal and gastric cancers. Jpn J Clin Oncol 2012;42:1161-7. [Crossref] [PubMed]
- Iwasaki M, Furuse J, Yoshino M, et al. Percutaneous transhepatic biliary drainage for the treatment of obstructive jaundice caused by metastases from nonbiliary and nonpancreatic cancers. Jpn J Clin Oncol 1996;26:465-8. [Crossref] [PubMed]
- Lee JW, Han JK, Kim TK, et al. Obstructive jaundice in hepatocellular carcinoma: response after percutaneous transhepatic biliary drainage and prognostic factors. Cardiovasc Intervent Radiol 2002;25:176-9. [Crossref] [PubMed]
- Crosara Teixeira M, Mak MP, Marques DF, et al. Percutaneous transhepatic biliary drainage in patients with advanced solid malignancies: prognostic factors and clinical outcomes. J Gastrointest Cancer 2013;44:398-403. [Crossref] [PubMed]
- Gwon DI, Ko GY, Sung KB, et al. Clinical outcomes after percutaneous biliary interventions in patients with malignant biliary obstruction caused by metastatic gastric cancer. Acta Radiol 2012;53:422-9. [Crossref] [PubMed]
- Robson PC, Heffernan N, Gonen M, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol 2010;17:2303-11. [Crossref] [PubMed]
- House MG, Choti MA. Palliative therapy for pancreatic/biliary cancer. Surg Oncol Clin N Am 2004;13:491-503. [Crossref] [PubMed]
- Günther RW, Schild H, Thelen M. Percutaneous transhepatic biliary drainage: experience with 311 procedures. Cardiovasc Intervent Radiol 1988;11:65-71. [Crossref] [PubMed]