Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience
Introduction
Gastric cancer represents a common cancer and one of the leading causes of cancer death worldwide, with an estimated 723,100 cancer deaths in 2012 (1). The incidence of gastric cancer varies with geographical region; its incidence rates are high in Eastern Asia, Central and Eastern Europe, and South America in comparison with the rates in North America and parts of Africa (1). Most gastric cancer patients present with advanced, inoperable disease, and the 5-year survival rate for all stages together is 30% (2).
Treatment options are limited for patients with metastatic gastric cancer. The standard approach is chemotherapy ± targeted therapy. Trastuzumab is a humanized anti-human epidermal growth factor receptor 2 (HER2) monoclonal antibody given in combination with chemotherapy in metastatic gastric cancer patients with positive HER2 overexpression. HER2, a transmembrane tyrosine kinase receptor encoded by the ERBB2 oncogene, is a member of the epidermal growth factor receptor (EGFR) family (3). EGFRs play a major role in controlling cellular growth and differentiation (4,5). In gastric cancer patients, the incidence of HER2 overexpression varies widely, from 8.2% to 53.4% (6,7). Evaluation of HER2 status in advanced gastric cancer setting is essential, as it is a predictive marker for benefit of anti-HER2 therapy. The effectiveness of adding trastuzumab to conventional chemotherapy was tested in a large phase III study, the Trastuzumab for Gastric Cancer (ToGA) trial; The trial demonstrated an improvement in the median overall survival (OS) in patients who received trastuzumab plus chemotherapy (13.8 months) compared with those who received chemotherapy alone (11.1 months) (8).
HER2 overexpression and trastuzumab treatment has been investigated widely in patients with advanced breast cancer. A pooled analysis of 2,618 HER2-positive metastatic breast cancer patients showed that treatment with trastuzumab beyond failure of first-line therapy containing trastuzumab was an effective option in the second-line setting (9). The benefit of trastuzumab continuation beyond progression in advanced gastric cancer is unknown.
Our study retrospectively reviewed gastric cancer patients who had been treated with trastuzumab as part of first-line treatment and then continued to receive trastuzumab beyond disease progression. Our goals were to determine their clinical course and to report any serious or unusual side effects seen with the prolonged administration of trastuzumab. Secondary, we assessed their progression-free survival (PFS) and OS in the second-line with the continuation of trastuzumab. Where PFS was defined as time from administering second-line of chemotherapy with trastuzumab until disease progression or death, OS defined as time from administering second-line of chemotherapy with trastuzumab until death from any cause.
Methods
Patient selection
We searched the University of Texas MD Anderson Cancer Center’s electronic database for patients who were treated for advanced gastric cancer from January 2010 to December 2014. Patients were included in our study if they had HER2 overexpressed gastric adenocarcinoma and had received trastuzumab in combination with cytotoxic chemotherapy in both first- and second-line therapy. A total of 43 patients were identified; all were included in the final analysis. All patients were confirmed to have adenocarcinoma-type gastric cancer. HER2 positivity was assessed in the primary tumor at the time of initial presentation by immunohistochemistry (IHC). As per our institution criteria for testing for HER2, If the IHC score was 3+, the tumor was considered HER2 positive; if the result was 2+, the positive status was confirmed using fluorescence in situ hybridization (FISH).
The electronic records of patients were used to collect and retrospectively analyze demographic, clinical, radiological, histopathological characteristics; chemotherapy agents received; PFS, OS, and documented toxicity. The study was approved by the Institutional Review Board.
Statistical analysis
We used the Kaplan-Meier method to measure patients’ OS, defined as the time from starting second-line chemotherapy until the date of death or last date of follow-up, and PFS, defined as the time from starting second-line chemotherapy until the date of disease progression or the date of death.
Results
Forty-three patients from 2010 to 2014 fulfilling the eligibility criteria were identified. Selected patient and tumor characteristics are shown in (Tables 1,2). The median age was 58 years (range, 34–83 years). Twenty-seven patients (62.8%) were male. The stage at initial presentation was stage 4 in 35 patients (81.4%). Thirty-five patients (81%) had 3+ HER2 overexpression, and 7 (16%) had 2+ HER2 overexpression confirmed with FISH. One patient had his testing at an outside facility, and the IHC/FISH result was not available, but HER2 positivity had been documented in the chart. In 24 patients (56%), the tumor was moderately differentiated; in 16 (37.1%), the tumor was poorly differentiated. The most common sites of metastasis were liver (35 patients, 81.4%) and lung (14 patients, 32.5%). The most commonly used first-line regimen was oxaliplatin, 5-fluorouracil (5-FU), and trastuzumab in 22 (51.1%) patients. Twenty-five (58.1%) patients received irinotecan, 5-FU and trastuzumab in the second-line.
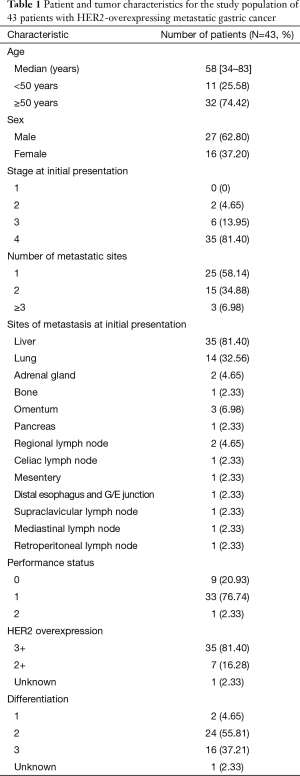
Full table
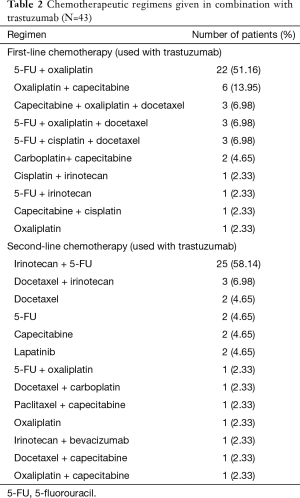
Full table
PFS and OS
For our 43 gastric cancer patients who received trastuzumab as first-line in combination with chemotharpy the median PFS for the first-line treatment was 7.1 months and OS 15.2 months. In the second-line treatment in combination with chemotherapy, Kaplan-Meier analysis showed a median PFS of 5 months (95% CI: 4.01–5.99 months) (Figure 1A). Five patients are still alive and were excluded from calculating the median OS which was 11 months (range, 5–53 months) (Figure 1B) for the remaining 20 subjects. Trastuzumab was not discontinued due to side effects in any of the study subjects.
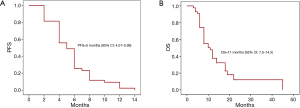
Toxicity
The toxic effects observed in our patients were mostly attributable to the chemotherapy used in combination with trastuzumab. An echocardiogram was done in 36 (83%) of the patients as a baseline prior to the first line treatment; none of the patients developed any cardiac toxicity (symptomatic heart failure, cardiomyopathy, or arrhythmia) during the entire treatment course. Nausea (all grades) was the most common side effect, reported in 26 (60%) of patients, and peripheral neuropathy (all grades) was reported in 18 (41%) of patients likely attributed to the chemotherapy. None of the patients had any infusion reaction to trastuzumab or any pulmonary toxicity (pneumonitis or acute respiratory distress syndrome).
Discussion
This retrospective study of 43 patients with HER-positive advanced gastric cancer treated with trastuzumab in the first-line and continued trastuzumab beyond disease progression indicates that continuation of trastuzumab beyond disease progression is safe and feasible and may improve PFS and survival in advanced HER-positive gastric cancer patients.
In the chemotherapy era, OS in patients with advanced gastric cancer has been increasing steadily. Before the ToGA trial, the Randomized ECF for Advanced and Locally Advanced Esophagogastric Cancer 2 (REAL-2) trial assessed four different three-drug chemotherapy regimens for advanced gastric cancer; the combination of epirubicin, oxaliplatin, and capecitabine was shown to have the best OS rate (11.2 months) (10). In the ToGA trial and the Trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1) study, the median OS improved (13.8 months and 16 months, respectively) after trastuzumab was added to the first-line treatment regimens (8,11).
Although adding trastuzumab to chemotherapy has improved OS in patients with HER2 positive advanced gastric cancer, most patients develop progression after first-line treatment. Kim et al. reported a meta-analysis that provided evidence to support second-line chemotherapy in patients with advanced gastric cancer (12). Table 3 summarizes recent trials of second-line treatment regimens in advanced gastric cancer.
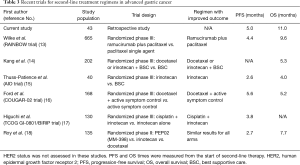
Full table
Additional agents that have been tested for second-line treatment include ramucirumab, a fully humanized anti-vascular endothelial growth factor receptor 2 (VEGFR-2) monoclonal antibody. Wilke et al. reported a double-blind phase III trial (the RAINBOW trial), in which 665 patients with gastric or gastroesophageal junction adenocarcinoma (included both HER-positive and negative) were randomly assigned to receive ramucirumab plus paclitaxel or placebo plus paclitaxel. The median OS and PFS durations were significantly longer in the ramucirumab plus paclitaxel group (OS, 9.6 vs. 7.4 months, respectively; PFS, 4.4 vs. 2.9 months) (13). Other recently published trials of second-line treatment in gastric cancer showed an increase in median OS and in PFS duration with single-agent therapy such as irinotecan and docetaxel (15,16).
Trastuzumab emtansine, or T-DM1, is an antibody-drug conjugate comprising trastuzumab and the microtubule inhibitory drug mertansine (known also as DM1). T-DM1 has shown clinical benefits in patients with metastatic breast cancer who were previously treated with trastuzumab (19). T-DM1 was demonstrated to be more effective than trastuzumab in xenograft gastric cancer models (20). An ongoing multicenter, randomized trial (NCT01641939; ClinicalTrials.gov) is currently being conducted to compare the T-DM1 therapy with the standard taxane therapy (docetaxel or paclitaxel) in patients with HER2 positive advanced gastric cancer who were treated previously.
In our current study, the median PFS of 5 months was comparable to the PFS in the COUGAR-02 docetaxel trial (5.6 months) but longer than those of other trials (Table 3). The median OS of 11 months in current study was longer than any of the historical data for second-line treatment (Table 3).
However, limitations of such comparison should be noted as with cross-trial comparisons there exists limitations including different populations and interventions; for example, in our present study, all subjects had adenocarcinoma-type gastric cancer, whereas Kang et al.’s study had a mixed population with adenocarcinoma and squamous gastric cancers (14). Second, HER2-positive gastric cancer was one of the criteria for selection in our study, but the other trials likely had mixed HER2-positive and -negative gastric cancers; HER2 status was not reported/tested prior to the ToGA trial.
The potential benefit of continuation of trastuzumab after disease progression in patients with advanced gastric cancer needs to be proven in future prospective randomized studies. If confirmed, this treatment strategy will enable more treatment lines by delaying the utilization of other agents (e.g., ramucirumab); allowing those agents to be used later rather than earlier during the treatment course which may ultimately improve OS.
Our study has a number of limitations within which our findings need to be interpreted carefully. Our study was a single-institution, retrospective analysis. The sample size of our study was small (43 patients), which will limit the generalizability of our study and predispose it to selection biases. Another limitation is that our study was a single-arm study with no controlled group.
Conclusions
In conclusion, this retrospective study suggests that continuation of trastuzumab in the second-line beyond disease progression in patients with HER2 positive advanced gastric cancer is feasible, safe and may improve PFS and OS in this population. Prospective randomized studies are warranted.
Acknowledgements
The authors would like to thank Sunita Patterson, Department of Scientific Publications, MD Anderson Cancer center for providing editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee/ethics board (No. PA12-1063) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985;229:974-6. [Crossref] [PubMed]
- Karunagaran D, Tzahar E, Beerli RR, et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J 1996;15:254-64. [PubMed]
- Klapper LN, Glathe S, Vaisman N, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A 1999;96:4995-5000. [Crossref] [PubMed]
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [Crossref] [PubMed]
- Jørgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology 2010;78:26-33. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Petrelli F, Barni S. A pooled analysis of 2618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer 2013;13:81-7. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 2014;110:1163-8. [Crossref] [PubMed]
- Kim HS, Kim HJ, Kim SY, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol 2013;24:2850-4. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Kang JH, Lee SI. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Higuchi K, Tanabe S, Shimada K, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer 2014;50:1437-45. [Crossref] [PubMed]
- Roy AC, Park SR, Cunningham D, et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann Oncol 2013;24:1567-73. [Crossref] [PubMed]
- Peddi PF, Hurvitz SA. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: latest evidence and clinical potential. Ther Adv Med Oncol 2014;6:202-9. [Crossref] [PubMed]
- Barok M, Tanner M, Köninki K, et al. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett 2011;306:171-9. [Crossref] [PubMed]


