
Totally minimally invasive laparoscopic robot-assisted Ivor Lewis esophagectomy: improved technique and outcomes over 200 cases
Highlight box
Key findings
• Totally minimally invasive Ivor Lewis esophagectomy at a high-volume center is a safe procedure. Operative outcomes improved significantly after 80 cases and continue to improve over time.
What is known and what is new?
• It is well known that surgical resection for esophageal cancer is associated with significant morbidity and mortality. Minimally invasive surgical techniques improve the safety profile of these procedure.
• We found that combining laparoscopic and robotic approaches can be done safely and is associated with progressive improvement in short- and long-term outcomes, but the learning curve is still over 50 procedures.
What is the implication, and what should change now?
• Understanding the intimate technical aspects of a complex procedure is the first step towards continued outcome improvement and learning curve shortening.
Introduction
Surgical resection of malignancies located in the thoracic esophagus or at the gastroesophageal junction (GEJ) require resection and reconstruction of the upper gastrointestinal (GI) tract (1-3). Given the variety of approaches, consensus remains elusive for the most effective technique for resection of esophageal and GEJ malignancies (2,4,5). Most of the clinical trial data reports great overall results for each type of open resection, but what differentiates each approach are some specific short-term outcomes. For example, the transthoracic approach when compared to transhiatal, has been associated with increased mortality due to pulmonary complications and sequela of anastomotic dehiscence (4,6). The transthoracic technique, however, allows for a more complete thoracic lymphadenectomy and increases the rate of complete resection by allowing access to adjacent structures such as pleura, lung, and pericardium (2,5). However, the transthoracic and transhiatal approaches have reported similar long-term oncologic outcomes (5).
Since the first minimally invasive, thoracoscopic esophagectomy was performed by Alfred Cuschieri in 1992 (1), laparoscopic and robotic techniques have come to the forefront (7,8) and were adopted at our institution starting in 2010 (9,10). This is due, in large part, to evidence suggesting that minimally invasive approaches improve surgical outcomes when compared to open approaches (7,11,12). Clinical trials of hybrid esophagectomy, where the thoracic portion of the operation is performed with a minimally invasive technique, have been carried out in a randomized fashion. The significant themes that emerged from these trials were that oncologic outcomes were not compromised, the postoperative complications, especially the pulmonary complications, were significantly reduced (4,11,12), and that utilizing minimally invasive techniques is safe and efficacious (7,13,14). In 2020, we published our experience with 350 transthoracic esophagectomies which included a significant proportion of hybrid procedures (15). The objective of this study is not to advocate for one surgical technique over another, but to review the more salient surgical techniques and short- and long-term outcomes for the totally minimally invasive Ivor Lewis esophagectomy, namely with the abdominal laparoscopic approach and robotic thoracic approach (LRAMIE). We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-923/rc).
Methods
This is a retrospective observational cohort study performed at a specialty cancer center using a prospectively maintained institutional database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional review board with the protocol number MCC #16630- and individual consent for this retrospective analysis was waived. Patients were identified who underwent esophagectomy for malignant or premalignant disease with lesions located in the middle or distal thoracic esophagus, or at the GEJ. We focused our query on patients who underwent robotic-assisted surgery and then further limited the population to only patients who had a minimally invasive abdominal approach by a single surgeon at a free-standing NCI designated Cancer Center. Years of inclusion were 2014–2023. We excluded all patients who underwent three-field esophagectomy, open abdominal and open thoracic procedures. Patients whose procedures were aborted for metastatic disease or impossibility to use a gastric conduit were excluded from analysis (Figure S1). The primary outcomes included immediate surgical outcomes such as operative time, intraoperative complications, estimated blood loss (EBL) and length of hospital and intensive care unit (ICU) stay, postoperative complications including anastomotic, pulmonary, cardiac, need for reoperation and 30–90-day survival. Secondary outcomes were overall survival and local recurrence.
Surgical technique
Abdominal portion
Standard preoperative evaluation for all patients included complete staging, medical clearance when indicated, and extensive pre-anesthesia evaluation. The patient is positioned in the supine position with arms out. Abdominal access is obtained for most patients using a Veress needle in the umbilical region after elevating the abdominal wall with towel clamps. After pneumoperitoneum is established, a 12-mm VisiportTM trocar is used to gain intraperitoneal access under direct vision. Additional laparoscopic ports are placed as depicted in Figure 1A. Next, the patient is positioned in reverse Trendelenburg with the right side slightly down. To retract the liver, an umbilical tape is passed through a window made in the left triangular ligament and passed around the falciform ligament to the right before both ends exit the skin in the epigastrium (Figure 1B).

We start the dissection by mobilizing the gastroepiploic ligament off the greater curve with bipolar LigaSureTM and take extreme care not to injure the right gastroepiploic vascular pedicle. The short gastric vessels are completely divided and the left crus is identified. At the same time, a small omental pedicle flap is harvested at the base of the left gastroepiploic vessels which are divided.
The right gastroepiploic pedicle is further dissected at its origin to free the surrounding peritoneal attachments. We do not routinely Kocherize the duodenum as the reach of the duodenum is directly related to this dissection at the pedicle. We ensure adequate mobilization by bringing the pylorus to the hiatus without tension. The gastrohepatic ligament is then opened and dissected to reveal the right crus. The take-off of the celiac artery is identified at the level of the arcuate ligament initiation at the right crus. The nodal package is dissected off the arcuate ligament and a tunnel is created posterior to the esophagus. This is followed by a complete lymphadenectomy of the splenic artery take off and includes the left gastric and the celiac lymph nodes (Figure 1C). The left gastric artery is then divided with a vascular stapler and the pylorus is injected with 100 U of Botox anteriorly. Indocyanine green (ICG) perfusion evaluation is helpful in cases where gastric perfusion seems compromised after left gastric artery division.
The conduit is then created starting between the 2nd and third crows’ foot on the lesser curve or just proximal to the antrum. If able, the right gastric vessels are spared. An EndoGIATM stapler with a tan staple load (2, 2.5, 3 mm) is used to start the division of the lesser curve vascular arcade. We then continue the division of the stomach towards the fundus with purple staple loads (3, 3.5, 4 mm) all while ensuring a 4.5-cm diameter conduit, following a minimal to no-touch technique with the gastric conduit. We do not divide the conduit fully, unless a cervical anastomosis is planned, to make the conduit easier to “pull” towards the chest. The conduit is marked with ink on the anterior surface to assist with correct positioning as it is brought into the chest (Figure 1D) and a silk suture is used as a crotch stitch to decrease the risk of tearing. The specimen is “stuffed” into the mediastinum and the umbilical tape liver retractor is removed. We routinely place a jejunostomy feeding tube size 14, 15 cm distal to the ligament of Treitz at this point.
Thoracic portion
The patient is positioned in the left lateral decubitus position with a slight left tilt. After one-lung ventilation is ensured, robotic trocars are inserted and a 5-cm extraction site is made as in Figure 2. With the camera located in the extraction port, the robot is docked, and we start by dissecting the azygous vein and dividing it with an Endo GIATM grey load (2 mm) stapler (Figure 3A). The anterior and posterior pleura are opened, and the inferior pulmonary ligament is taken down. We then dissect and remove level 9 lymph nodes (Figure 3B). The dissection is continued superiorly encountering the pericardium and then dissecting the plain posteriorly until the left pleura is found (Figure 3C).
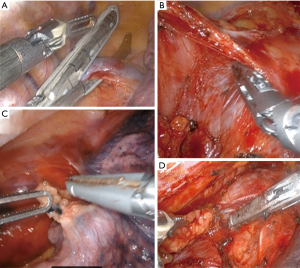
The dissection becomes circumferential, and at this point, we find the end of our dissection inferiorly from the abdomen. Lymph nodes stations are considered per AJCC8 classification (16). We dissect the superficial level 8 lymph nodes and keep them en-bloc with the esophagus, but we divide the deep level 8 and level 7 lymph nodes to expose the membranous portion of the airway which facilitates dissection of the esophagus off the airway (Figure 3D). Above the left side of the trachea, we dissect the paraoesophageal ligament and usually divide the esophagus above this ligament 3–4 cm proximal to the azygous vein. We use an Endo GIATM tan load stapler and we leave a 2 cm pleural flap to cover the anastomosis. This dissection can be taken as high as the thoracic inlet and one can direct the level of transection by endoscopic guidance if needed.
We complete the lymphadenectomy of level 7–8 and are very careful not to injure the airway (especially thermal injuries). The specimen is then fully separated from any residual adhesions and pulled through the extraction site. We pull on the omental pedicle flap, not on the specimen, to avoid injuring the gastric staple line. The specimen is divided extracorporeally with a purple load stapler. Frozen sections are performed on the proximal and distal margins at this point. As we wait, the camera is then moved to the subscapular port and we identify the thoracic duct between the posterior azygous and the aorta and clip it with large robotic clips. If the frozen sections are negative, we measure the length of the conduit based on the distance to the divided esophagus and mark the proposed anastomotic site. The conduit is kept intracorporeal during all these maneuvers to avoid ischemia from manipulation. We place an OrvilTM anvil in the esophagus and secure it with a 2-0 silk purse string. The conduit is brought extracorporeal, a gastrostomy is made, and a size 25 end-to-end anastomosis (EEA) stapler is placed into the stomach, bringing the spear out of the previously marked anastomotic site (Figure 4A). A plastic protector is placed on the tip of the spear and removed under direct vision once inside the thorax. We then use the laparoscopic anvil grasper to aid in coupling the stapler and the anvil then perform the anastomosis avoiding injuring the azygous (Figure 4B). The donuts are inspected and sent as final margins for pathology. The common channel is closed with an Endo GIA™ purple load stapler 2 cm away from the anastomosis. We highlight some lessons learned regarding conduit management (Table 1).
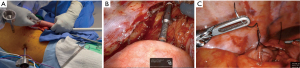
Table 1
| Key technical lessons learned |
| Minimal to no-touch technique for the gastric conduit can limit inadvertent trauma |
| Perfusion evaluation with ICG of the conduit after left gastric artery ligation can be helpful before fashioning the gastric conduit, and to help decide level of transection |
| Standardized step wise approach to the surgical procedure decreases operative time |
| Esophageal tissue that has clear radiation damage should be avoided for anastomosis |
ICG, indocyanine green.
The omental pedicle flap is brought through the space between the esophagus and the airway and draped around the anastomosis. We use 2-0 silk sutures to secure this flap anteriorly and posteriorly (Figure 4C). Next, a nasogastric tube is advanced under direct visualization to the middle of the thoracic conduit. Two size 15 round drains are then placed, one posteriorly and one in the mediastinum. They are kept on bulb suction.
Postoperative care
The patients spend the first postoperative night in the ICU and are usually transferred to a medical-surgical floor the following day. Patients routinely receive thoracic epidurals before surgery. Ambulation starts the morning after surgery. Immunonutrition is started through the jejunal feeding tube on postoperative day (POD) #1 at a trophic rate that is increased after return of bowel function. On POD #4 a swallow study is performed with double contrast to evaluate for an anastomotic leak and delayed gastric emptying. A non-contrast CT of the thorax is also performed before and after the contrasted swallow study to assess for subclinical leaks. If no leak is suspected, the nasogastric tube is removed, and the patient is started on a clear liquid diet. The patients are usually discharged home once they are tolerating enteral nutrition and on a liquid diet. Follow up is conducted as per National Comprehensive Cancer Network (NCCN) guidelines.
Statistical analysis
Descriptive statistics are presented as frequency for categorical variables and median with interquartile range (IQR) for continuous variables. Variables considered for included clinical, pathologic, and treatment factors. Clinical variables were age, sex, medical comorbidities, and surgical history. Tumor variables included histology, primary tumor site, and clinical stage (prior to any neoadjuvant therapy).
Univariate analyses were performed using Chi-square, Fisher exact, or Wilcoxon rank-sum tests, as appropriate. Exploratory analyses were performed to assess for potential associations between outcomes over time (operative time, length of stay, postoperative complications) using scatter plots and local polynomial fit curves for visual inspection. Potential associations that were observed were then tested using linear or logistic regression, as appropriate, in order to test for statistical significance. Overall survival was analyzed using the Kaplan-Meier method. Finally, we compared outcomes between the first half of the cohort and the latter half of the cohort. All statistical analyses were performed using Stata version 14. This study was approved by the Institutional Review Board at Moffitt Cancer Center.
Results
Clinical and pathologic characteristics of the primary cohort are shown in Table 2. Two-hundred patients were identified who underwent minimally invasive surgery (MIS) esophagectomy by a single surgeon (J.M.P.). The median age of the cohort was 65 years (IQR, 58–72 years). The majority of patients were male (n=175, 87.5%), Caucasian (n=183, 91.5%), and overweight or obese [body mass index (BMI): 25–29.9 kg/m2, 79 (39.5%); 30–34.9 kg/m2, 37 (18.5%); ≥35 kg/m2, 24 (12%)]. Medical comorbidities were common (Table 2). Most patients had adenocarcinoma (n=185, 92.5%) of the distal thoracic esophagus (n=102, 51.0%) or GEJ (n=91, 45.5%). The majority of patients received neoadjuvant therapy (n=186, 93.0%). A minimally invasive approach was used for patients with early-stage disease (n=17, 8.5%), locoregional disease (n=176, 88.0%), as well as highly selected patients with initially stage IV disease who had an excellent response to systemic therapy with a long progression-free interval and their primary tumor as the only site of active disease (n=7, 3.5%).
Table 2
| Characteristic | Values |
|---|---|
| Age, years | 65 [58–72] |
| Male | 175 (87.5) |
| Caucasian | 183 (91.5) |
| BMI, kg/m2 | |
| <18.5 | 4 (2.0) |
| 18.5–24.9 | 56 (28.0) |
| 25–29.9 | 79 (39.5) |
| 30–34.9 | 37 (18.5) |
| 35–39.9 | 18 (9.0) |
| ≥40 | 6 (3.0) |
| Smoking history | |
| Never | 63 (31.5) |
| Quit >1 year before surgery | 82 (41.0) |
| Within past year | 55 (27.5) |
| COPD† | |
| None | 151 (75.5) |
| Mild | 41 (20.5) |
| Moderate | 8 (4.0) |
| Coronary artery disease | 35 (17.5) |
| Diabetes | 35 (17.5) |
| Hypertension | 122 (61.0) |
| Dyslipidemia | 77 (38.5) |
| Histology | |
| Adenocarcinoma | 185 (92.5) |
| Squamous cell carcinoma | 15 (7.5) |
| Primary site | |
| Middle thoracic | 7 (3.5) |
| Distal thoracic | 102 (51.0) |
| Gastroesophageal junction | 91 (45.5) |
| Extent of disease | |
| Early stage | 17 (8.5) |
| Locoregional disease | 176 (88.0) |
| Metastatic‡ | 7 (3.5) |
| Preoperative therapy | 186 (93.0) |
Data are presented as median [IQR] or number (percentage). †, based on routine preoperative pulmonary function testing; ‡, highly select cases: patients with excellent response to therapy and long progression-free interval with primary as only site of disease. BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
Intraoperative and perioperative outcomes were observed to improve after 60 cases: inflection points for shorter operative time and less operative blood loss were both observed at the 60-case mark (Figure 5). Additionally, there were two cases converted to open, both of which occurred within the first 30 cases (conversion rate 1%, n=2) during the abdominal portion one due to adhesions and the other for bleeding. Median operative time was 444 min (IQR, 400–494 min) for cases 1–60 and 393 min (IQR, 363–423 min) for cases 61–200 (P<0.001). Improvement in EBL was more modest; median EBL was 200 mL (IQR, 150–300 mL) for cases 1–60 and 100 mL (IQR, 75–200 mL) for cases 61–200 (P<0.001) (Table 3).
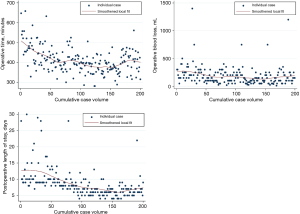
Table 3
| Variables | Overall | Inflection point (Case #) | Before and after inflection | P value |
|---|---|---|---|---|
| Technical outcomes | ||||
| Operative time, min† | 400 [369–443] | Improved at 60 cases | 444 vs. 393 | <0.001 |
| Operative blood loss, mL† | 150 [100–250] | Improved at 60 cases | 200 vs. 100 | <0.001 |
| Conversion to open | 1% | Improved at 30 cases | 7% vs. 0% | 0.02 |
| Anastomotic complications | ||||
| Early postoperative leak‡ | 11.50% | Improved at 80 cases | 24% vs. 3.4% | <0.001 |
| Stricture formation | 16% | Improved at 50 cases | 28% vs. 12% | <0.001 |
| Postoperative outcomes | ||||
| Length of stay, days† | 8 [6–10] | Improved at 50 & 100 cases | 10 vs. 8 vs. 6 | <0.001 for both |
| Postoperative pneumonia | 15% | Improved at 100 cases | 23% vs. 7% | 0.002 |
| 30-day mortality | 1.50% | Reduced at 100 cases | 3% vs. 0% | 0.25 |
| 90-day mortality | 3.50% | Reduced at 100 cases | 6% vs. 1% | 0.12 |
| Oncologic outcomes | ||||
| Number of nodes resected† | 24 [19–30] | Gradual improvement, no cutpoint | 0.08 | |
| R0 resection margin | 96% | Not associated with volume |
†, data are presented as median [interquartile range]. ‡, radiographically detected on postoperative swallow, or clinically apparent prior to discharge.
Anastomotic complications were observed to improve at the same time or slightly later than improvements in intraoperative outcomes. The overall rate of in-hospital leak detected radiographically or clinically was 11.5% (n=23). This was found to decrease significantly after eighty cases (P<0.001). The anastomotic leak rate was 23.8% for the first 80 cases, with 3.4% leak rate for the remaining 120 cases. Among those patients, we observed a 6.5% rate of delayed leak defined as anastomotic leaks more than 2 weeks after discharge and after a negative initial leak evaluation. Among patients with history of smoking within the past year (n=55), overall leak rate was 16.4% (n=9). Rate of stricture was observed to decrease after 50 cases, with a stricture rate of 28% (14/50) for cases 1–50, followed by a rate of 12% for cases 51–200 (P<0.001).
Postoperative LOS was observed to have sequential improvements at cases 50 and 100; median LOS was 10 days (IQR, 9–14 days) for cases 1–50, 8 days (IQR, 7–10 days) for cases 51–100 and 6 days (IQR, 5–7 days) for cases 101–200 (P<0.001 for both break points). Postoperative pneumonia showed significant improvement at the 100-case mark, with a rate of pneumonia of 23% for the first 100 cases and 7% beyond that (P=0.002). The 30-day mortality rate was 1.5% (n=3) and 90-day mortality was 3.5% (n=7) for the entire cohort. Mortality was observed to improve after 100 cases (30-day mortality 3% vs. 0%, P=0.25; 90-day mortality 6% versus 1%, P=0.12), but neither 30- nor 90-day mortality was significantly different after this point, likely due to low overall event rate.
There was a trend toward higher lymph node retrieval rate over time, but this was not statistically significant (P=0.08). The median lymph node count for the first quintile of cases (cases 1–40) was 22 nodes (IQR, 18–29 nodes) compared to 25 nodes (IQR, 19–34 nodes) for the last quintile (cases 161–200). There was also no association between case volume and R0 resection rate (P=0.49). The overall R0 rate was 96%. With median follow-up of 37 months, the 3-year overall survival (OS) rate was 72.6% (95% CI: 64.8–79.0%). Among patients with locoregional disease receiving neoadjuvant chemoradiotherapy, 3-year OS was 70.9% (95% CI: 62.2–77.9%).
Discussion
Surgical therapy for esophageal and gastroesophageal cancer has advanced significantly since its origins (1). The latest iteration of these advances includes the adoption of robotic techniques which over the last 10 years have become more ubiquitous. We have previously published our robotic-assisted (thoracic portion) esophagectomy experience which was made up of a significant proportion of hybrid procedures utilizing an open abdominal approach (9,15). Here we focus on the surgical technique and outcomes for a completely minimally invasive approach. All reported resections were done at a single institution by a single surgeon who performed laparoscopic abdominal mobilization, conduit creation and lymphadenectomy, and a robotic thoracic mobilization, lymphadenectomy, and anastomosis (LRAMIE).
In this cohort, the single-surgeon learning curve demonstrates beginning of improvement in operative performance at approximately 50 cases, with significant improvement in operative time and operative blood loss. The overall conversion rate was 1%, with conversions occurring early within the first 30 cases. Improvements in anastomotic-specific outcomes were seen to follow in parallel, or shortly thereafter, with significant improvement in stricture rate at 50 cases and in-hospital leak rate at 80 cases. Postoperative LOS was observed to improve significantly at 50 cases, with further improvement at the 100-case mark, with a plateau in median LOS at 6 days after that point. Similarly, improvement in postoperative pneumonia rate followed improvements in technical measures, with improved rate seen at 100 cases. Operative mortality was reduced after 100 cases, but this was not statistically significant due to low overall event rate. Most importantly, these outcomes were achieved with acceptable oncologic outcomes with a persistently adequate lymphadenectomy (median 22 lymph nodes during first 40 cases, 25 nodes for last 40 cases) and high R0 resection rate throughout. We provide a detailed, stepwise description of our current technique in hopes that this may be of interest and hopefully shorten others’ learning curve. These results compare favorably with the benchmark provided by van Workum and van der Sluis in their seminal learning curve papers (7,17,18).
This series adds further evidence to existing clinical trial data showcasing the fact that minimally invasive techniques are not inferior to open techniques when considering oncologic outcomes (4,7,14,19). Where most of these clinical trials limited the minimally invasive approach to either the abdomen (19) or the thoracic cavity (4,7), our approach was totally minimally invasive and highlights the fact that the use of minimally invasive techniques, including robotic approaches, can improve short-term surgical outcomes specifically related with length of hospital stay and complication rate, as highlighted by Triantafyllou et al. in their recent review of current evidence supporting MIS approaches for esophagectomy (20). Moreover, our series underscores the need for careful outcome recording to progressively improve perioperative outcome (20), specifically we previously have shown improved lymph node yield and decreased length of postoperative stay for fully MIS robotic techniques and these improvements continue in the current cohort (15). These results are supported by the recent clinical RAMIE that compares MIS transthoracic to robotic transthoracic esophagectomy supporting that robotic technique improved lymph node yield over the thoracoscopic approach (21).
Our learning curve results are similar to previous published data from van Workum et al. who showed that, even at high volume centers, the number of esophagectomies required to minimize complications is around 120 cases (17). Additionally, Vander Suis and colleagues report proficiency after 70 cases when a robotic approach is utilized (18), which is similar to the findings of a recent meta-analysis by Chan and Oo, showing that different learning curves apply to different outcomes (22). Moreover, the number of cases needed to reach better surgical outcomes seems to be closely associated with institutional volume (23), indicating that technical manuscripts like ours can bring attention to maneuvers that could be helpful for novice surgeons. Finally, our results highlight the need to minimize the effect of the personal learning curve for totally minimally invasive esophagectomy to assure best outcomes for patients and this will become even more important as further technological advancements continue to emerge, and in our institution, we are using the improvements learned in these 200 cases to create procedure-specific guidelines for trainee and junior faculty coaching.
Our limitations are related to the fact that this is an analysis of a subset of the patients operated on at our institution, and possible selection bias can make our results and conclusions non-generalizable. The outcome improvements seen over time can be achieved as the number of cases remain high year after year, but these improvements may not be observed with lower volumes over longer periods. Additionally, the institutional experience may have significantly contributed to the outcomes, especially as related to the very low mortality encountered in our series. However, we believe this data may be helpful to understand the need for continuous monitoring of clinical quality and the pursuit of persistent outcome improvements for patients.
Conclusions
Totally minimally invasive esophagectomy at a high-volume center is a safe procedure with good short- and long-term surgical outcomes, and with corresponding excellent oncologic outcomes. The current manuscript also highlights some of the most salient technical points that need to be considered when performing this operation.
Acknowledgments
Funding: The study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-923/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-923/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-923/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-923/coif). R.M. reports consulting for Eli Lilly and participation on the monitoring board of Arcus Biosciences, Merck, BMS, Astellas, Novartis, Seagen, Natera, Guardant Health, Boston Gene and is a member of the scientific advisory board for Debbie’s Dream foundation. J.J.B. participates in Achilles Monitoring Board. J.P.F. reports consulting for AstraZeneca, Intuitive Surgical and Bristiol Myers Squibb. J.M.P. reports Astellas consulting. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by our institutional review board with the protocol number MCC #16630- and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lerut T, Wiesel O. History of esophagectomy for cancer of the esophagus and the gastroesophageal junction. Ann Transl Med 2021;9:897. [Crossref] [PubMed]
- Hung PC, Chen HY, Tu YK, et al. A Comparison of Different Types of Esophageal Reconstructions: A Systematic Review and Network Meta-Analysis. J Clin Med 2022;11:5025. [Crossref] [PubMed]
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72; discussion 372-4. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Tagkalos E, van der Sluis PC, Berlth F, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer 2021;21:1060. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Pickering OJ, van Boxel GI, Carter NC, et al. Learning curve for adoption of robot-assisted minimally invasive esophagectomy: a systematic review of oncological, clinical, and efficiency outcomes. Dis Esophagus 2023;36:doac089. [Crossref] [PubMed]
- Amaral M, Pimiento J, Fontaine JP. Robotic esophagectomy: the Moffitt Cancer Center experience. Ann Cardiothorac Surg 2017;6:186-9. [Crossref] [PubMed]
- de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013;27:3339-47. [Crossref] [PubMed]
- Pimiento JM, Fontaine JP. Minimally invasive transthoracic esophagectomy: pushing the boundaries to improve surgical outcomes. J Thorac Dis 2019;11:S1336-8. [Crossref] [PubMed]
- Nuytens F, Dabakuyo-Yonli TS, Meunier B, et al. Five-Year Survival Outcomes of Hybrid Minimally Invasive Esophagectomy in Esophageal Cancer: Results of the MIRO Randomized Clinical Trial. JAMA Surg 2021;156:323-32. [Crossref] [PubMed]
- de Groot EM, van der Horst S, Kingma BF, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open esophagectomy: long-term follow-up of a randomized clinical trial. Dis Esophagus 2020;33:doaa079. [Crossref] [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Laparoscopic versus open transhiatal esophagectomy for distal and junction cancer. Rev Esp Enferm Dig 2012;104:197-202. [Crossref] [PubMed]
- Pointer DT Jr, Saeed S, Naffouje SA, et al. Outcomes of 350 Robotic-assisted Esophagectomies at a High-volume Cancer Center: A Contemporary Propensity-score Matched Analysis. Ann Surg 2022;276:111-8. [Crossref] [PubMed]
- Schuring N, Matsuda S, Hagens ERC, et al. A proposal for uniformity in classification of lymph node stations in esophageal cancer. Dis Esophagus 2021;34:doab009. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Learning Curve for Robot-Assisted Minimally Invasive Thoracoscopic Esophagectomy: Results From 312 Cases. Ann Thorac Surg 2018;106:264-71. [Crossref] [PubMed]
- Brierley RC, Gaunt D, Metcalfe C, et al. Laparoscopically assisted versus open oesophagectomy for patients with oesophageal cancer-the Randomised Oesophagectomy: Minimally Invasive or Open (ROMIO) study: protocol for a randomised controlled trial (RCT). BMJ Open 2019;9:e030907. [Crossref] [PubMed]
- Triantafyllou T, van der Sluis P, Skipworth R, et al. The Implementation of Minimally Invasive Surgery in the Treatment of Esophageal Cancer: A Step Toward Better Outcomes? Oncol Ther 2022;10:337-49. [Crossref] [PubMed]
- Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: the RAMIE Trial. Ann Surg 2022;275:646-53. [Crossref] [PubMed]
- Chan KS, Oo AM. Exploring the learning curve in minimally invasive esophagectomy: a systematic review. Dis Esophagus 2023;36:doad008. [Crossref] [PubMed]
- Claassen L, Hannink G, Luyer MDP, et al. Learning Curves of Ivor Lewis Totally Minimally Invasive Esophagectomy by Hospital and Surgeon Characteristics: A Retrospective Multinational Cohort Study. Ann Surg 2022;275:911-8. [Crossref] [PubMed]



