
Effect of perioperative blood transfusion on complications and prognosis after radical gastrectomy in elderly patients: a retrospective study of 1,666 cases
Highlight box
Key findings
• Perioperative blood transfusion (BTF) was not an independent risk factor affecting the prognosis of elderly patients who underwent radical gastrectomy and had no significant effect on surgical complications except fever.
What is known and what is new?
• The effect of perioperative BTF on postoperative complications and prognosis in gastric cancer patients is still controversial, and there are few relevant reports on elderly patients.
• We found that perioperative BTF may have no significant effect on surgical complications and prognosis of elderly patients with gastric cancer.
What is the implication, and what should change now?
• Perioperative BTF may be feasible in elderly patients undergoing radical gastrectomy.
Introduction
Gastric cancer is a prevalent form of malignancy affecting the digestive tract. Currently, surgery stands as the sole possible cure for resectable gastric cancer (1). Blood transfusion (BTF) is frequently used as a treatment measure for gastric cancer patients experiencing perioperative anemia or bleeding. Numerous studies have explored the impact of perioperative BTF on postoperative complications and the prognosis of gastric cancer patients, yet the findings remain contentious (2-5). Moreover, prior research has highlighted that elderly patients with gastric cancer exhibit distinct clinical characteristics (6,7). Nevertheless, there is a scarcity of studies analyzing the influence of perioperative BTF on postoperative complications and prognosis in elderly patients with gastric cancer. Against this backdrop, the present study aimed to investigate this influence by retrospectively examining the clinical data of 1,666 elderly patients with gastric cancer admitted to our hospital who met the eligibility criteria. The findings are detailed below. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-906/rc).
Methods
Materials
The clinical data of gastric cancer patients who underwent radical surgery from October 2013 to October 2021 at the Department of Gastroenterology, Xijing Hospital were retrospectively collected.
To be eligible for inclusion in this study, the patients had to meet the following criteria: (I) be aged ≥60 years; (II) have been pathologically diagnosed with gastric adenocarcinoma; (III) have no distant metastasis in preoperative examinations; (IV) have undergone radical gastrectomy (R0 resection and D2 lymph node dissection); and (V) have complete clinical and follow-up data.
Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a history of gastric surgery; (II) had a history of other malignancies; (III) died during the perioperative period; (IV) had a history of BTFs within 6 months before the surgery; and/or (V) had undergone emergency surgery. The flow chart of this study is shown in Figure 1.
Study methods
General pathological data
The following general pathological data were collected: sex, site and size of the tumor, extent of gastrectomy, degree of differentiation, T stage, N stage, tumor-node-metastasis (TNM) stage, preoperative hemoglobin (Hb) levels, intraoperative blood loss, perioperative BTF data, and complications in the immediate postoperative period (i.e., ≤30 days post-surgery), including fever (defined as axillary temperature exceeding 37.3 ℃), pulmonary infections, incision infections, anastomotic leakage, chylous leakage, pleural effusion, gastroparesis, intestinal obstruction, and abdominal infections.
The tumor sites and surgical methods were determined in accordance with the Japanese gastric cancer treatment guidelines 2014 (8), and pathological staging was performed according to the 7th edition of the TNM staging system for gastric cancer set by the Union for International Cancer Control (UICC) (9). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Xijing Hospital (No. KY20222190-C-1), and informed consent was obtained from all the patients.
Follow-up
Postoperative follow-up was carried out through outpatient review, telephone calls, and other forms of communication. The patients were followed-up every 3 months in the first postoperative year and every 6 months from the second year onwards. The endpoint was defined as death. The median follow-up time for patients overall was 51 (range, 3.8–75.3) months.
Effect analyses of perioperative BTF on postoperative complications and prognosis
Based on whether the patients received a BTF (erythrocytes or whole blood) during the perioperative period, they were stratified into the BTF group and the non-BTF group. The immediate (≤30 days post-surgery) postoperative complications and the long-term (>30 days post-surgery) prognosis of the patients of the two groups were compared. Next, univariate and multivariate analyses were conducted to ascertain whether perioperative BTF was an independent risk factor associated with postoperative prognosis. Finally, further analyses based on the stratification of tumor stages (stage I/II/III) and transfusion time points (preoperative/intraoperative/postoperative) were performed to determine the effect of perioperative BTF on patient prognosis.
Statistical methods
The statistical analyses were conducted using the Statistical Package for Social Science (SPSS) version 24.0 for Windows (IBM, Chicago, IL, USA). The χ2 test was used for intergroup comparisons of the enumeration data; the Kaplan-Meier method was used for survival curve plotting; the log-rank test was used for intergroup comparisons of the survival rates; and the Cox regression model was used for the multivariate survival analysis. Differences were considered to be statistically significant at P<0.05.
Results
Intergroup comparison of clinical data
A total of 1,666 patients (male, n=1,357; female, n=309) were included in the study. The median age of the patients was 66 (range, 60–86) years. Among the patients, 1,255 (75.3%) were assigned to the non-BTF group and 411 (24.7%) were assigned to the BTF group. Additionally, 32 (1.9%) patients had Hb levels <70 g/L, 216 (13.0%) had Hb levels ranging from 70 to 100 g/L, and 1,418 (85.1%) had Hb levels >100 g/L. As Table 1 shows, there were statistically significant differences between the two groups in terms of sex, site and size of the tumor, extent of gastrectomy, degree of differentiation, T stage, N stage, TNM stage, preoperative anemia, and intraoperative blood loss (P<0.05).
Table 1
| Characteristics | Non-BTF (n=1,255) | BTF (n=411) | χ2 value | P value |
|---|---|---|---|---|
| Sex | 7.532 | 0.006 | ||
| Male | 1,041 (82.9) | 316 (76.9) | ||
| Female | 214 (17.1) | 95 (23.1) | ||
| Tumor location | 8.950 | 0.03 | ||
| Upper third | 517 (41.2) | 139 (33.8) | ||
| Middle third | 189 (15.1) | 60 (14.6) | ||
| Lower third | 454 (36.2) | 171 (41.6) | ||
| Two thirds or more | 95 (7.6) | 41 (10.0) | ||
| Tumor size (cm) | 39.747 | <0.001 | ||
| <5 | 692 (55.1) | 153 (37.2) | ||
| ≥5 | 563 (44.9) | 258 (62.8) | ||
| Resection type | 10.755 | 0.005 | ||
| Proximal | 190 (15.1) | 36 (8.8) | ||
| Distal | 434 (34.6) | 152 (37.0) | ||
| Total | 631 (50.3) | 223 (54.3) | ||
| Differentiation status | 9.789 | 0.02 | ||
| Well | 164 (13.1) | 31 (7.5) | ||
| Moderately | 386 (30.8) | 141 (34.3) | ||
| Poorly | 657 (52.4) | 225 (54.7) | ||
| Mucinous | 48 (3.8) | 14 (3.4) | ||
| T stage | 42.714 | <0.001 | ||
| T1 | 278 (22.2) | 38 (9.2) | ||
| T2 | 190 (15.1) | 58 (14.1) | ||
| T3 | 410 (32.7) | 189 (46.0) | ||
| T4 | 377 (30.0) | 126 (30.7) | ||
| N stage | 11.218 | 0.01 | ||
| N0 | 519 (41.4) | 137 (33.3) | ||
| N1 | 200 (15.9) | 62 (15.1) | ||
| N2 | 223 (17.8) | 82 (20.0) | ||
| N3 | 313 (24.9) | 130 (31.6) | ||
| TNM stage | 24.829 | <0.001 | ||
| I | 368 (29.3) | 70 (17.0) | ||
| II | 334 (26.6) | 120 (29.2) | ||
| III | 553 (44.1) | 221 (53.8) | ||
| Preoperative Hb level (g/dL) | 319.909 | <0.001 | ||
| <120 | 303 (24.1) | 300 (73.0) | ||
| ≥120 | 952 (75.9) | 111 (27.0) | ||
| Intraoperative blood loss (mL) | 95.793 | <0.001 | ||
| <300 | 1,030 (82.1) | 240 (58.4) | ||
| ≥300 | 225 (17.9) | 171 (41.6) |
Data are presented as n (%). BTF, blood transfusion; TNM, tumor-node-metastasis; Hb, hemoglobin.
Effect of perioperative BTF on postoperative complications in elderly patients with gastric cancer
The incidence rates of postoperative complications of the patients in the non-BTF and BTF groups were 24.6% (309/1,255) and 43.8% (180/411), respectively, and the incidence rates of non-surgery-related complications of the patients in the two groups were 20.4% (256/1,255) and 37.2% (153/411), respectively. The differences were statistically significant (P<0.001). Among all the complications, the incidence of postoperative fever of patients in the BTF group (31.6%) was significantly higher than that of patients in the non-BTF group (15.4%), and the difference was statistically significant (P<0.001). However, no statistically significant differences were observed between the two groups in terms of the incidences of postoperative surgery-related complications (i.e., intestinal obstruction, anastomotic leakage, incision infections, chylous leakage, abdominal infections, and gastroparesis), respiratory infections, and pleural effusion (P>0.05). The above findings are outlined in Table 2.
Table 2
| Variables | Non-BTF (n=1,255) | BTF (n=411) | χ2 value | P value |
|---|---|---|---|---|
| Total complications | 309 (24.6) | 180 (43.8) | 27.992 | <0.001 |
| Non-operation-related complications | 256 (20.4) | 153 (37.2) | 26.922 | <0.001 |
| Fever | 193 (15.4) | 130 (31.6) | 52.321 | <0.001 |
| Pneumonia | 52 (4.1) | 19 (4.6) | 0.174 | 0.68 |
| Pleural effusion | 11 (0.9) | 4 (1.0) | 0.032 | 0.86 |
| Operation related complications | 53 (4.2) | 27 (6.6) | 3.349 | 0.07 |
| Ileus | 17 (1.4) | 9 (2.2) | 1.406 | 0.24 |
| Anastomosis leakage | 13 (1.0) | 7 (1.7) | 1.487 | 0.48 |
| Wound infection | 8 (0.6) | 2 (0.5) | 0.118 | 0.73 |
| Chyle leakage | 7 (0.6) | 4 (1.0) | 0.815 | 0.37 |
| Abdominal infection | 5 (0.4) | 4 (1.0) | 1.904 | 0.17 |
| Gastric stasis | 3 (0.2) | 1 (0.2) | <0.001 | 0.99 |
Data are presented as n (%). BTF, blood transfusion.
Effect of perioperative BTF on postoperative survival in elderly patients with gastric cancer
As Figure 2 shows, the Kaplan-Meier survival curve revealed that the postoperative overall survival (OS) of the patients in the non-BTF group was superior to that of the patients in the BTF group (3-year OS rates: 70.1% vs. 53.5%), and the difference was statistically significant (P<0.001). The univariate analysis revealed that the site and size of the tumor, extent of gastrectomy, degree of tumor differentiation, T stage, N stage, TNM stage, preoperative Hb levels, intraoperative blood loss, and perioperative BTF (P<0.001) were risk factors affecting the prognosis of elderly patients with gastric cancer. However, according to the further multivariate analysis, only tumor size, T stage, N stage, and intraoperative blood loss were independent risk factors affecting the prognosis of elderly patients with gastric cancer. The above findings are set out in Table 3.
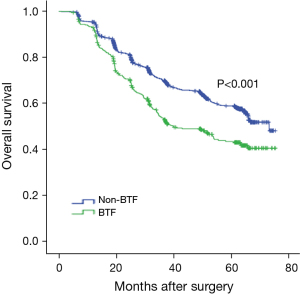
Table 3
| Characteristics | N | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| 3-year survival rate (%) | P value | HR (95% CI) | P value | |||
| Sex | 0.42 | |||||
| Male | 1,357 | 66.2 | ||||
| Female | 309 | 65.1 | ||||
| Tumor location | 0.36 | |||||
| Upper third | 656 | 62.7 | ||||
| Middle third | 249 | 68.9 | ||||
| Lower third | 625 | 72.2 | ||||
| Two thirds or more | 136 | 45.7 | ||||
| Tumor size (cm) | <0.001 | 1.416 (1.183–1.696) | <0.001 | |||
| <5 | 845 | 80.3 | ||||
| ≥5 | 821 | 51.6 | ||||
| Resection type | <0.001 | 1.011 (0.894–1.144) | 0.86 | |||
| Proximal | 226 | 74.5 | ||||
| Distal | 586 | 75.2 | ||||
| Total | 854 | 57.1 | ||||
| Differentiation status | <0.001 | 0.955 (0.846–1.077) | 0.45 | |||
| Well | 195 | 86.9 | ||||
| Moderately | 527 | 70.2 | ||||
| Poorly | 882 | 58.9 | ||||
| Mucinous | 62 | 61.0 | ||||
| T stage | <0.001 | 1.567 (1.353–1.815) | <0.001 | |||
| T1 | 316 | 93.7 | ||||
| T2 | 248 | 82.4 | ||||
| T3 | 599 | 65.5 | ||||
| T4 | 503 | 41.4 | ||||
| N stage | <0.001 | 1.583 (1.394–1.798) | <0.001 | |||
| N0 | 656 | 87.6 | ||||
| N1 | 262 | 75.5 | ||||
| N2 | 305 | 56.2 | ||||
| N3 | 443 | 35.6 | ||||
| TNM stage | <0.001 | |||||
| I | 438 | 93.2 | ||||
| II | 454 | 78.1 | ||||
| III | 774 | 43.9 | ||||
| Preoperative Hb level (g/dL) | <0.001 | 0.901 (0.754–1.077) | 0.25 | |||
| <120 | 603 | 57.4 | ||||
| ≥120 | 1,063 | 70.2 | ||||
| Intraoperative blood loss (mL) | <0.001 | 1.360 (1.146–1.615) | <0.001 | |||
| <300 | 1,270 | 69.8 | ||||
| ≥300 | 396 | 54.0 | ||||
| Perioperative BTF | <0.001 | 1.178 (0.971–1.428) | 0.10 | |||
| No | 1,255 | 70.1 | ||||
| Yes | 411 | 53.5 | ||||
HR, hazard ratio; CI, confidence interval; BTF, blood transfusion; Hb, hemoglobin.
As Figure 3 shows, the stratified analysis demonstrated that patients with stage III tumors in the BTF group had a significantly inferior prognosis compared to that of those in the non-BTF group (3-year OS rates: 33.7% vs. 47.9%), and the difference was statistically significant (P<0.001). Conversely, in patients with stage I/II tumors, there was no statistically significant difference in prognosis between the two groups (3-year OS rates for stage I patients: 94.0% vs. 93.6; stage II patients: 80.7% vs. 70.7%; P>0.05). The factors affecting prognosis in patients with stage III tumors were further analyzed, and the results are set out in Table 4. The univariate analysis indicated that perioperative BTF was one of the risk factors affecting prognosis (P<0.001), but the multivariate analysis demonstrated that it was not an independent risk factor affecting prognosis (P=0.12).
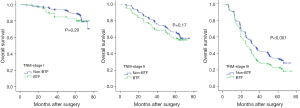
Table 4
| Variables | Regression coefficient | Standard error | Wald χ2 | P value | HR (95% CI) |
|---|---|---|---|---|---|
| Tumor size | 0.338 | 0.111 | 9.201 | 0.002 | 1.402 (1.127–1.743) |
| Resection type | 0.042 | 0.078 | 0.286 | 0.59 | 1.043 (0.894–1.216) |
| T stage | 0.458 | 0.089 | 26.673 | <0.001 | 1.582 (1.329–1.882) |
| N stage | 0.474 | 0.077 | 37.786 | <0.001 | 1.606 (1.381–1.868) |
| Preoperative Hb level | −0.115 | 0.105 | 1.196 | 0.27 | 0.891 (0.725–1.095) |
| Intraoperative blood loss | 0.331 | 0.101 | 10.756 | <0.001 | 1.392 (1.142–1.696) |
| Perioperative BTF | 0.174 | 0.113 | 2.394 | 0.12 | 1.191 (0.955–1.485) |
HR, hazard ratio; CI, confidence interval; BTF, blood transfusion; Hb, hemoglobin.
Effect of perioperative BTF at various time points on postoperative survival in elderly patients with gastric cancer
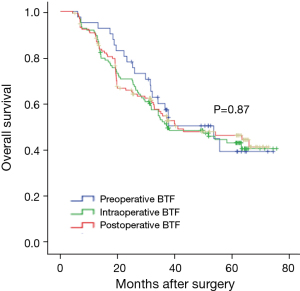
The postoperative survival of patients who were transfused only preoperatively (n=41), intraoperatively (n=123), or postoperatively (n=130) was compared. As Figure 4 shows, the postoperative 3-year OS rates were 57.2%, 51.1%, and 54.6% respectively, and the postoperative 5-year OS rates were 39.1%, 42.9%, and 46.1% respectively. The differences were not statistically significant (P=0.87).
Discussion
It has been reported that about 20% of patients undergoing gastrectomy need a BTF due to perioperative anemia or intraoperative bleeding (10). Multiple studies have revealed the effect of BTF on the postoperative complications and the prognosis of gastric cancer patients, but the conclusions remain controversial. Squires et al. (11) analyzed 765 gastric cancer patients at seven institutions from the United States Gastric Cancer Collaborative and discovered that the postoperative disease-free survival (DFS) and OS rates were significantly reduced in the patients who received perioperative BTFs. Two meta-analysis studies have shown that perioperative BTF is associated with negative survival effects (in terms of OS, DFS, and disease-specific survival) and a higher incidence of perioperative complications in gastric cancer patients (12,13). Another study of 927 patients from six Italian study centers revealed that the effect of BTF on the postoperative 5-year survival rate was not significant in gastric cancer patients (14). However, few studies have examined the effect of perioperative BTF on complications and prognosis after radical gastrectomy in elderly patients. To our knowledge, the present study, which analyzed the clinical data of 1,666 elderly patients who had undergone gastrectomy, is the largest single-center study to examine the effect of perioperative BTF on the postoperative complications and prognosis of elderly patients with gastric cancer.
The baseline data analysis in this study showed that patients in the BTF group were in more advanced tumor stages, and that more of them had preoperative anemia, overall tumor sizes ≥5 cm, and intraoperative blood loss volumes ≥300 mL compared with patients in the non-BTF group. The findings are consistent with the results of multiple previous studies (2-5).
Controversies still exist regarding the relationship between perioperative BTF and postoperative complications in gastric cancer patients. It has been found that BTF might elevate the incidence of perioperative complications among gastric cancer patients (12,15). Conversely, Kouyoumdjian et al.’s study of 9,936 gastric cancer patients showed that perioperative BTF might lower the incidence of postoperative complications and the mortality rate of patients with severe anemia (hematocrit <29) (3). Xiao et al. reported that BTF was an independent risk factor for postoperative infections in gastric cancer patients (10). However, a previous study showed that the incidence rates of postoperative infectious complications were similar between the perioperative BTF and non-BTF groups after propensity-score matching was used to diminish the intergroup differences in clinical tumor characteristics and surgery-related factors (16). In the present study, the overall incidence of postoperative complications in the BTF group (43.8%) was significantly higher than that in the non-BTF group (24.6%), which was mainly due to the significantly increased incidence of postoperative fever in the BTF group (31.6%) compared with that in the non-BTF group (15.4%), as shown by further analyses. However, no significant intergroup differences were observed in the incidences of other complications. The findings suggest that BTF did not elevate the incidences of perioperative complications (other than fever) among elderly patients with gastric cancer. Additionally, it has been reported that fever is the most common complication of BTF and might be associated with the immune response (17).
Tumor staging is a decisive factor affecting the prognosis of gastric cancer patients. However, controversy continues as to whether BTF affects the prognosis of gastric cancer patients. A retrospective study of 1,581 patients by Liu et al. confirmed that perioperative BTF was associated with the poor prognosis of patients with stage III gastric cancer (18). Kanda et al. showed that BTF was also an independent risk factor for a poor prognosis in patients with stage II/III gastric cancer (19). Further, a meta-analysis of 18 studies showed that perioperative BTF was related to a poor prognosis in patients undergoing gastrectomy (20). However, Gong et al. pointed out that the literature included in this meta-analysis study was insufficient and the results were partially contradictory (21). Meanwhile, Hyung et al. revealed that BTF had a non-significant effect on the postoperative survival rates of patients with stage I/II gastric cancer (22). For patients with stage III gastric cancer, Yamashita et al. showed that BTF had no effect on their postoperative survival (23). Zhou et al. carried out an analysis stratified by tumor stages (stage I/II/III) of 605 gastric cancer patients, and found no differences in the postoperative survival rates among the patients in different stages in both the non-BTF and BTF groups, which suggests that BTF was not an independent risk factor affecting the postoperative survival of gastric cancer patients (24).
In the present study, the postoperative 3-year OS rate in patients in the BTF group was significantly lower than that in patients in the non-BTF group, and the further analyses stratified by tumor stages indicated that BTF had a significant effect on prognosis in patients with stage III gastric cancer but not in patients with stage I/II gastric cancer. These findings are identical to the results reported by Xue et al. (25) However, our multivariate analysis demonstrated that BTF was not an independent risk factor affecting the prognosis of gastric cancer patients in all the three stages, and that it might have been closely associated with the more advanced tumor stages and the greater intraoperative bleeding in patients in the BTF group. Further, Rausei et al. also reported that BTF was not an independent risk factor affecting the prognosis of gastric cancer patients but was a confounder that was considerably influenced by other variables (26). In a study of 2,905 patients with stage II/III gastric cancer, Song et al. found no significant correlation between perioperative BTF and the long-term survival of the patients after the intergroup differences in clinical tumor characteristics and surgery-related factors were diminished using propensity score matching (2). These findings are consistent with those of Xiao et al. and Cui et al. (16,27), and similar to the conclusions drawn by studies on other cancers (28,29).
In addition, the definition of perioperative BTF is also a potential factor that could have led to the differences reported by various studies, as the definition may encompass different transfusion time points, transfused volumes, and transfused components. In terms of transfusion time points, the majority of studies have included patients who received BTF 1 or 2 weeks preoperatively, and 1 or 2 weeks or even 1 month postoperatively (19,24), while only one study included patients undergoing intraoperative and/or postoperative BTF (11). Xue et al. noted that gastric cancer patients who underwent BTF intraoperatively exhibited a significantly inferior prognosis compared with that of patients who underwent BTFs preoperatively or postoperatively (25).
In relation to transfused volumes, a previous study reported a negative correlation between transfused volumes and patient prognosis (22). Conversely, Xue et al. stratified gastric cancer patients into three groups based on the volumes of perioperative red blood cell transfusion (<2, 2–4, and >4 U), and found no significant difference in the postoperative prognosis among the patients across all the groups (25).
In relation to the transfused components, Dhar et al. reported that patients who were only transfused with concentrated erythrocytes had notably higher survival rates than those who were transfused with other blood products (30). van Hilten et al. showed that in patients in the erythrocyte group who underwent leucocyte-filtered transfusion, the average hospitalization duration was 2.4 days shorter and the incidence of multi-organ failure was 30% lower than that of patients in the non-filtration group, but the difference in the mortality rates between the two groups was not significant (31). Additionally, another study found no statistically significant differences in the mortality and tumor recurrence rates between filtration and non-filtration groups (32). In the present study, perioperative BTF referred to the transfusion of erythrocytes from admission to discharge (typically 3 days preoperatively to 14 days postoperatively). Finally, no significant difference was discovered in the effect of preoperative, intraoperative, and postoperative BTFs on the prognosis of elderly patients with gastric cancer, which is consistent with the findings of Song et al. (2) obtained in the study on patients with stage II/III gastric cancer.
This study had a number of limitations: (I) it was a single-center retrospective study; and (II) some of the patients included received platelet or plasma transfusions concurrently, and the effects of transfused volumes and components on prognosis were not further investigated through a stratified analysis.
Conclusions
Perioperative BTF may elevate the incidence of postoperative fever in elderly patients with gastric cancer, but it has no significant effect on other complications. Additionally, BTF is not an independent risk factor affecting the postoperative prognosis of elderly patients with gastric cancer. As the definitions of perioperative BTF vary among hospitals and physicians, multi-center studies with larger sample sizes need to be conducted to obtain higher-level evidence.
Acknowledgments
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-906/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-906/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-906/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-906/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Xijing Hospital (No. KY20222190-C-1), and informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li GZ, Doherty GM, Wang J. Surgical Management of Gastric Cancer: A Review. JAMA Surg 2022;157:446-54. [Crossref] [PubMed]
- Song JH, Shin HJ, Lee S, et al. No detrimental effect of perioperative blood transfusion on recurrence in 2905 stage II/III gastric cancer patients: A propensity-score matching analysis. Eur J Surg Oncol 2022;48:2132-40. [Crossref] [PubMed]
- Kouyoumdjian A, Trepanier M, Al Shehhi R, et al. The Effect of Preoperative Anemia and Perioperative Transfusion on Surgical Outcomes After Gastrectomy for Gastric Cancer. J Surg Res 2021;259:523-31. [Crossref] [PubMed]
- Nakanishi K, Kanda M, Kodera Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J Gastroenterol 2019;25:2743-51. [Crossref] [PubMed]
- Kawakami LE, Bonomi PB, Pereira MA, et al. Risk factors for blood transfusion and its prognostic implications in curative gastrectomy for gastric cancer. World J Gastrointest Surg 2023;15:643-54. [Crossref] [PubMed]
- Loizides S, Papamichael D. Considerations and Challenges in the Management of the Older Patients with Gastric Cancer. Cancers (Basel) 2022;14:1587. [Crossref] [PubMed]
- Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev 2020;85:101980. [Crossref] [PubMed]
- Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Kwon SJ. Evaluation of the 7th UICC TNM Staging System of Gastric Cancer. J Gastric Cancer 2011;11:78-85. [Crossref] [PubMed]
- Xiao H, Quan H, Pan S, et al. Impact of peri-operative blood transfusion on post-operative infections after radical gastrectomy for gastric cancer: a propensity score matching analysis focusing on the timing, amount of transfusion and role of leukocyte depletion. J Cancer Res Clin Oncol 2018;144:1143-54. [Crossref] [PubMed]
- Squires MH 3rd, Kooby DA, Poultsides GA, et al. Effect of Perioperative Transfusion on Recurrence and Survival after Gastric Cancer Resection: A 7-Institution Analysis of 765 Patients from the US Gastric Cancer Collaborative. J Am Coll Surg 2015;221:767-77. [Crossref] [PubMed]
- Wang W, Zhao L, Niu P, et al. Effects of perioperative blood transfusion in gastric cancer patients undergoing gastrectomy: A systematic review and meta-analysis. Front Surg 2023;9:1011005. [Crossref] [PubMed]
- Agnes A, Lirosi MC, Panunzi S, et al. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol 2018;44:404-19. [Crossref] [PubMed]
- Pacelli F, Rosa F, Marrelli D, et al. Do perioperative blood transfusions influence prognosis of gastric cancer patients? Analysis of 927 patients and interactions with splenectomy. Ann Surg Oncol 2011;18:1615-23. [Crossref] [PubMed]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016;59:322-9. [Crossref] [PubMed]
- Xiao H, Liu W, Quan H, et al. Peri-Operative Blood Transfusion Does Not Influence Overall and Disease-Free Survival After Radical Gastrectomy for Stage II/III Gastric Cancer: a Propensity Score Matching Analysis. J Gastrointest Surg 2018;22:1489-500. [Crossref] [PubMed]
- Abdallah R, Rai H, Panch SR. Transfusion Reactions and Adverse Events. Clin Lab Med 2021;41:669-96. [Crossref] [PubMed]
- Liu X, Ma M, Huang H, et al. Effect of perioperative blood transfusion on prognosis of patients with gastric cancer: a retrospective analysis of a single center database. BMC Cancer 2018;18:649. [Crossref] [PubMed]
- Kanda M, Kobayashi D, Tanaka C, et al. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer 2016;19:255-63. [Crossref] [PubMed]
- Sun C, Wang Y, Yao HS, et al. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg 2015;13:102-10. [Crossref] [PubMed]
- Gong L, Dong C, Ouyang W. Influence of blood transfusion on clinical outcomes in resected gastric cancer. Int J Surg 2015;18:142. [Crossref] [PubMed]
- Hyung WJ, Noh SH, Shin DW, et al. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol 2002;9:5-12. [Crossref] [PubMed]
- Yamashita K, Sakuramoto S, Kikuchi S, et al. Transfusion alert for patients with curable cancer. World J Surg 2007;31:2315-22. [Crossref] [PubMed]
- Zhou HY, Yi W, Wang J, et al. Association of perioperative allogeneic blood transfusions and prognosis of patients with gastric cancer after curative gastrectomy. Am J Surg 2014;208:80-7. [Crossref] [PubMed]
- Xue L, Chen XL, Wei-Han Z, et al. Impact of Perioperative Blood Transfusion on Postoperative Complications and Prognosis of Gastric Adenocarcinoma Patients with Different Preoperative Hemoglobin Value. Gastroenterol Res Pract 2016;2016:6470857. [Crossref] [PubMed]
- Rausei S, Ruspi L, Galli F, et al. Peri-operative blood transfusion in gastric cancer surgery: prognostic or confounding factor? Int J Surg 2013;11:S100-3. [Crossref] [PubMed]
- Cui J, Deng J, Ding X, et al. Blood transfusion does not affect survival of gastric cancer patients. J Surg Res 2016;200:98-104. [Crossref] [PubMed]
- Warschkow R, Güller U, Köberle D, et al. Perioperative blood transfusions do not impact overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg 2014;259:131-8. [Crossref] [PubMed]
- Yang T, Lu JH, Lau WY, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A Propensity Score Matching Analysis. J Hepatol 2016;64:583-93. [Crossref] [PubMed]
- Dhar DK, Kubota H, Tachibana M, et al. A tailored perioperative blood transfusion might avoid undue recurrences in gastric carcinoma patients. Dig Dis Sci 2000;45:1737-42. [Crossref] [PubMed]
- van Hilten JA, van de Watering LM, van Bockel JH, et al. Effects of transfusion with red cells filtered to remove leucocytes: randomised controlled trial in patients undergoing major surgery. BMJ 2004;328:1281. [Crossref] [PubMed]
- van de Watering LM, Brand A, Houbiers JGA, et al. Perioperative blood transfusions, with or without allogeneic leucocytes, relate to survival, not to cancer recurrence. Br J Surg 2001;88:267-72. [Crossref] [PubMed]



