
Clinical outcome of non-curative endoscopic submucosal dissection for early gastric cancer
Highlight box
Key findings
• The study analyzes 10-year outcomes of non-curative resections (NCRs) for early gastric cancer (EGC) at Beijing Friendship Hospital, leveraging clinical and pathological data.
What is known and what is new?
• Additional surgical treatment following NCR of EGC including partial gastrectomy or lymph node dissection has shown lower recurrence/metastasis in previous studies.
• This study included 127 patients clinically and pathologically diagnosed with EGC who underwent non-curable resection after endoscopic submucosal dissection (ESD)/endoscopic mucosal resection (EMR) treatment and obtained specific results. Lymph node invasion as an important risk factor for poor prognosis requiring further surgery.
What is the implication, and what should change now?
• Current research underscores lymphatic involvement as critical in deciding additional surgery post-ESD/EMR in EGC, necessitating multi-faceted evaluation and acknowledging the future role of molecular diagnostics.
Introduction
Gastric cancer is the fourth most prevalent cancer globally and holds the second rank in cancer-related mortality worldwide (1-3). The clinical staging of gastric cancer is crucial in determining its outcome and prognosis. Early gastric cancer (EGC), when managed via endoscopic or surgical resection, can attain a 5-year survival rate exceeding 90%. Conversely, patients with advanced gastric cancer often experience a low rate of curative resection (CR), diminished quality of life, and a 5-year tumor-related survival rate below 30% (4).
In recent years, the paradigm for gastric cancer treatment has shifted. Traditionally, surgical resection was the mainstay of gastric cancer treatment. However, with the advent and widespread adoption of endoscopic resection techniques, these are now deemed the standard approach for treating EGC in patients with minimal risk of lymph node or distant metastasis. Endoscopic resection encompasses endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Advances in endoscopic diagnostic and therapeutic technologies have markedly enhanced the early detection rates of EGC. Extensive multi-center studies indicate that endoscopic resection, compared to traditional surgery, offers benefits such as reduced trauma, fewer complications, and improved postoperative survival quality, making it the preferred treatment for EGC patients.
EGC assessment for curability after endoscopic resection is linked to local factors and the risk of lymph node metastasis. The 2021 Japanese Gastric Cancer Association (JGES)’s guidelines (5) for EGC ESD/EMR suggest comparable or improved long-term outcomes with endoscopic resection over surgical resection. These guidelines redefined the concept of “endoscopic curative/non-curative resection” (NCR) to “endoscopic curability (eCura)”. Under this, eCura A and eCura B are categorized as CRs, while eCuraC, including eCuraC-1 and eCura-C2, are categorized as NCRs. eCura A includes: (I) predominantly differentiated type cancer, pT1a, UL0, unlimited tumor diameter, no lymphatic vessels and vascular invasion (Ly0, V0), en bloc resection, HM0, VM0; (II) mainly differentiated type, pT1a, UL1, tumor diameter ≤3 cm, Ly0, V0, en bloc, HM0, VM0. eCura B comprises of: (I) predominantly undifferentiated type, pT1a, UL0, tumor diameter ≤2 cm, Ly0, V0, en bloc, HM0, VM0; (II) mainly differentiated type, pT1b (SM1), ≤3 cm diameter, Ly0, V0, en bloc, HM0, VM0. eCuraC-1 includes differentiated type cancer meeting all criteria except en bloc resection or HM1. Treatments meeting eCura A/B but not eCuraC-1 criteria are classified as eCuraC-2 (5). For the definition of NCR, for example, there is a lack of clinical evidence for cases of differentiated type cancer combined with undifferentiated components. The goal of endoscopic resection for EGC is “CR”, If NCR is achieved, additional treatment is necessary due to local recurrence and lymph node metastasis, typically involving surgical resection and lymph node dissection. However, in cases like refusal of surgery due to extended life expectancy, comorbidities, poor overall health, or increased elderly patient proportion, NCR patients can opt to redo ESD, argon plasma coagulation (APC), or have regular follow-up without further treatment (6).
Although numerous studies have examined the long-term clinical outcomes of NCR patients undergoing ESD for EGC, research on a large population remains scarce. This retrospective study aims to investigate the risk factors influencing recurrence-free survival in NCR patients, with the objective of offering valuable insights for clinical assessment and preventive strategies. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-168/rc).
Methods
Inclusion/exclusion criteria
This study analyzed EGC cases treated with endoscopic therapy at the Digestive Endoscopy Center of Beijing Friendship Hospital, Capital Medical University from September 2012 to January 2020. We adhered to the criteria outlined in the latest Japanese Early Gastric Cancer ESD/EMR Guidelines (2021 JGES Guidelines, 2nd edition) (5). The inclusion criteria were: (I) positive horizontal/vertical margin; (II) submucosal infiltration depth ≥500 µm; (III) lymphatic/blood vessel invasion; (IV) undifferentiated type (undifferentiated cancer component, mucinous adenocarcinoma, or signet ring cell carcinoma); (V) non-en-bloc resection. The exclusion criteria included: (I) residual gastric cancer; (II) concurrent malignant tumors elsewhere; (III) incomplete clinical or endoscopic data; (IV) follow-up <6 months.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by Beijing Friendship Hospital, Capital Medical University (No. 2018-P2-058-01). The patients/participants provided their written informed consent to participate in this study.
Data collection
Patient demographics, endoscopic findings [lesion size, location, macroscopic appearance per Paris classification (7) and 2005 Paris Classification Standard Update (8)], procedural features (ESD approach: standard vs. hybrid ESD, en bloc resection), and pathologic outcomes {Vienna classification (9), depth of invasion [mucosa (M) and submucosa (SM), including SM1 (depth of submucosa infiltration of tumor tissue <500 µm), SM2 (depth of submucosa infiltration of tumor tissue ≥500 µm)] (7), margins [vertical margins (VM) and horizontal margins (HM)], lymphatic vessels invasion [lymph (Ly) and vascular (V)]} (7) from endoscopic specimens were gathered. For surgical patients, pathologic outcomes including residual neoplasia at the resection site, lymph node involvement, and post-surgical stage were recorded. Follow-up data, such as local recurrence, metastatic disease, and vital status at the last follow-up, were also collected. The end of follow up was marked by the last endoscopy and/or imaging study [computed tomography (CT) scan or others]. Recommended post-endoscopic treatment follow-up included gastroscopy at 3-, 6-, and 12-months post-surgery, then annually. For cases indicated as eCuraB resection, follow-up entailed esophagogastroduodenoscopy, along with ultrasound or abdominal-pelvic CT for metastatic tumors.
Definitions and outcomes
Under white light endoscopy, EGC typically presents with distinct features such as clear boundaries, irregular surface (10), changes in local mucosal color (red sign or fading), interrupted mucosal folds, and concentrated shallowness (11). An en bloc resection is defined as the removal of a lesion in one piece, and a complete resection (R0) is an en bloc resection with negative margins (deep or lateral) upon histological examination. Post-ESD, NCR patients were either part of the surgery group, undergoing operations with curative intent, or the follow-up group, based on multi-disciplinary treatment (MDT) recommendations and patient choice. Primary outcomes included disease recurrence, death, or disease-related death rates post non-curative ESD in both groups. Recurrence was categorized as: (I) local recurrence, identified as tumor lesions at the original resection site or within 1 cm of the surrounding area after more than 6 months post-surgery; (II) recurrence-free survival, defined as no residual tumor lesions within 6 months or absence of recurrence/metastasis post 6 months; (III) recurrence-free survival period, defined as the duration from ESD surgery to local/distant cancer recurrence, death, or last follow-up.
Statistical analysis
Data were managed and analyzed using SPSS 24.0 software. Normally distributed continuous variables were represented as , and categorical variables as frequency and percentage. Chi-square test or Fisher’s exact test were used for inter-group comparisons. Factors with statistical significance were further analyzed using multiple-factor logistic regression, presented as odds ratio (OR) values with their 95% confidence interval (CI). Kaplan-Meier method was used to plot survival curves, with differences compared using the log-rank test. Cox univariate regression identified factors affecting non-recurrent survival, and multiple-factor Cox analysis pinpointed independent risk factors, with P<0.05 indicating statistical significance.
Results
Baseline characteristics
Table 1 details the clinical and pathological features of NCR cases. Of the 127 NCRs, 10 were lost to follow-up, leaving 117 cases for the prognosis factor analysis. Among these, non-whole block piecemeal resections were observed in 20 cases (17.1%); SM2 tumor infiltration in 19 cases (16.2%), and SM1 in 25 cases (21.4%). Positive horizontal and vertical margins were found in 16 cases (13.7%), positive vertical margins only in 33 cases (28.2%), and positive horizontal margins only in 19 cases (16.2%). Recurrence/metastasis occurred in 27 cases (23.1%). Post-surgery, 42 out of 117 NCR patients with EGC (35.9%) underwent additional surgical procedures. eCura score distribution included 47 cases in the low-risk group (40.1%), 46 in the moderate-risk group (39.3%), and 23 in the high-risk group (19.6%).
Table 1
| Parameter | NCR patients (n=117), n (%) |
|---|---|
| Resection | |
| En bloc | 97 (82.9) |
| Piecemeal | 20 (17.1) |
| Depth of invasion | |
| Mucosa | 73 (62.4) |
| SM1 | 25 (21.4) |
| SM2 | 19 (16.2) |
| Margin | |
| Clear margin | 49 (41.9) |
| Positive lateral margin only | 19 (16.2) |
| Positive vertical margin only | 33 (28.2) |
| Positive lateral & vertical margin | 16 (13.7) |
| Lymphatic invasion | 45 (38.5) |
| Vascular invasion | 29 (24.8) |
| eCura score | |
| Low risk | 47 (40.1) |
| Medium risk | 46 (39.3) |
| High risk | 23 (19.6) |
| eCura | |
| eCuraC-1 | 43 (36.8) |
| eCuraC-2 | 74 (63.2) |
| Recurrence/metastasis | 27 (23.1) |
| Post-surgery | 42 (35.9) |
NCR, non-curative resection; SM, submucosa; eCura, endoscopic curability.
Follow-up results of enrolled cases
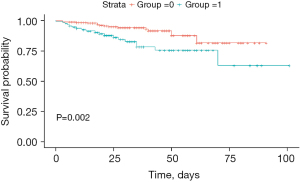
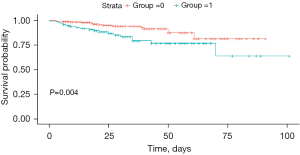
The recurrence rate in the NCR group was significantly higher compared to the CR group, as indicated in Table 2 (P<0.001). Kaplan-Meier analysis was used to construct survival curves, shown in Figure 1, with the red curve representing the CR group, and green curve representing the NCR group. We observed that recurrence rate of CR group was lower than NCR group, with P=0.002. Additionally, 42 of the 117 NCR patients (35.9%) underwent further surgical operations post-surgery. The study also compared the recurrence rates between the surgical and non-surgical groups, as shown in Figure 2, revealing a significant statistical difference (P=0.004).
Table 2
| Parameter | Total (n=117) | Surgery (n=42) | P value |
|---|---|---|---|
| Gender, male, n (%) | 81(69.2) | 35(83.3) | 0.02 |
| Age, ≥60 years, n (%) | 89 (76.1) | 25 (59.5) | 0.004 |
| Pathology, undifferentiated, n (%) | 27 (23.1) | 16 (38.1) | 0.008 |
| Ulcer, n (%) | 46 (39.3) | 18 (42.9) | 0.70 |
| Depth of invasion, SM, n (%) | 43 (36.8) | 22 (52.4) | 0.02 |
| Lymphatic invasion, n (%) | 45 (38.5) | 17 (40.5) | 0.90 |
| Vascular invasion, n (%) | 29 (24.8) | 12 (28.6) | 0.63 |
| Positive lateral margin, n (%) | 35 (29.9) | 11 (26.2) | 0.09 |
| Positive vertical margin, n (%) | 49 (41.9) | 23 (54.8) | 0.06 |
| Gross type, n (%) | 0.007 | ||
| Elevated | 58 (49.6) | 14 (33.3) | |
| Flat | 19 (16.2) | 6 (14.3) | |
| Depressed | 40 (34.2) | 22 (52.4) | |
| Tumor size (cm), n (%) | 0.052 | ||
| <2 | 51 (43.8) | 13 (31.2) | |
| ≥2 & <3 | 38 (32.6) | 13 (31.2) | |
| ≥3 | 27 (23.6) | 16 (37.6) | |
| eCura, n (%) | <0.001 | ||
| eCuraC-1 | 43 (36.8) | 6 (14.3) | |
| eCuraC-2 | 74 (63.2) | 36 (75.7) | |
| eCura score, n (%) | 0.10 | ||
| Low risk | 48 (41.0) | 12 (28.6) | |
| Medium risk | 46 (39.3) | 19 (45.2) | |
| High risk | 23 (19.7) | 11 (26.2) |
ESD, endoscopic submucosa dissection; SM, submucosa; eCura, endoscopic curability.
EGC patients who underwent NCR with or without additional surgical procedures have clinical pathological characteristics
As shown in Table 3, in the NCR cohort, 42 patients, including 35 males and 7 females, underwent additional surgical procedures, which showed a statistical difference between genders (P=0.02). In terms of age, patients ≥60 years were more prevalent in the non-surgical than the surgical group (P=0.004). The surgical group had a higher proportion of undifferentiated pathology compared to the non-surgical group (P=0.008). Additionally, a greater proportion of patients in the surgical group had submucosal infiltration (P=0.02), and a higher prevalence of eCuraC-2 was observed in the surgical group (P<0.001). There were no statistical differences between surgical and non-surgical groups in terms of ulceration, lymphatic vessels/vascular infiltration, and positive horizontal and vertical margins.
Table 3
| Parameter | Muti-logistic regression | P value | |
|---|---|---|---|
| OR | 95% CI | ||
| Gender, male | 0.314 | 0.096–1.029 | 0.06 |
| Age, ≥60 years | 0.230 | 0.074–0.712 | 0.01 |
| Undifferentiated | 2.263 | 0.764–6.706 | 0.14 |
| Depth of invasion, SM | 2.542 | 0.813–7.950 | 0.11 |
| Positive lateral margin | 1.539 | 0.459–5.156 | 0.49 |
| Positive vertical margin | 1.844 | 0.676–5.032 | 0.23 |
| Gross type | 0.02 | ||
| Flat | 1.282 | 0.313–5.257 | 0.73 |
| Depressed | 4.746 | 1.554–14.495 | 0.006 |
| eCura | |||
| eCuraC-1 | 0.059 | 0.009–0.374 | 0.003 |
| eCuraC-2 | 0.172 | 0.032–0.929 | 0.04 |
SM, submucosa; OR, odds ratio; CI, confidence interval; eCura, endoscopic curability.
Logistic multivariate regression analysis, including the aforementioned statistically significant factors, identified age <60 years (P=0.01), gross pathology type as concave (P=0.006), and eCuraC-2 (P=0.04) as independent risk factors for additional surgical procedures after NCR.
NCR prognostic factors (Cox univariate and multivariate regression)
Kaplan-Meier analysis was performed on the prognosis of patients with EGC who underwent NCR. Factors including surgical operation status, intra‑operative persistent bleeding, family history of tumors, resection method, lymphatic and vascular invasion, pathological differentiation, and endoscopic observation of white moss, as well as horizontal/vertical margin status, showed statistical differences in the prognosis following NCR. These factors were then included in univariate Cox regression analysis as detailed in Table 4. The analysis revealed that the family history of tumors, lesion size, presence of ulcers, white moss observed during endoscopy, persistent bleeding during surgery, treatment method, lymphatic invasion, positive vertical margin, and pathological gross type (flat type) significantly impacted the 6-month disease-free survival of patients with EGC who underwent NCR via endoscopy. Furthermore, multivariate Cox regression analysis identified lesion size postoperative ≥2 & <3 cm, presence of ulcers, lymphatic invasion, positive vertical margin, and pathological gross type (flat type) as independent risk factors affecting the disease-free survival of these patients. Detailed findings are available in Table 4.
Table 4
| Parameter | Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender, female | 1.05 (0.45–2.43) | 0.92 | |||
| Age, ≥60 years | 1.83 (0.63–5.29) | 0.27 | |||
| Lesion size (cm) | |||||
| <2 | 1 | ||||
| ≥2 & <3 | 0.24 (0.07–0.84) | 0.03 | 0.12 (0.02–0.67) | 0.02 | |
| ≥3 | 0.49 (0.14–1.69) | 0.26 | 2.07 (0.4–10.84) | 0.39 | |
| Tumor histopathology | |||||
| Differentiated | 1 | ||||
| Undifferentiated | 1.71 (0.74–3.91) | 0.21 | |||
| Ulcer | 6.88 (2.59–18.28) | <0.001 | 5.48 (1.23–24.33) | 0.03 | |
| Remarkable redness | 1.92 (0.88–4.2) | 0.10 | |||
| Gross morphology | 1.74 (0.41–7.42) | 0.45 | |||
| Margin elevation | 0.46 (0.16–1.36) | 0.16 | |||
| Enlarged folds | 0.60 (0.08–4.45) | 0.62 | |||
| Uneven surface | 0.43 (0.2–0.96) | 0.04 | 0.3 (0.06–1.59) | 0.16 | |
| Nodularity | 0.27 (0.04–1.98) | 0.20 | |||
| White moss | 2.68 (1.05–6.87) | 0.04 | 6.16 (0.58–65.74) | 0.13 | |
| Bleeding | 5.49 (1.24–24.35) | 0.03 | 0.41 (0.02–8.91) | 0.57 | |
| Resection | |||||
| En bloc | 1 | ||||
| Piecemeal | 0.88 (0.3–2.55) | 0.81 | |||
| Treatment | |||||
| ESD | 1 | ||||
| EMR | 6.14 (0.83–45.72) | 0.08 | |||
| Lymphatic invasion | 6.67 (2.66–16.73) | <0.001 | 17.51 (1.07–286.23) | 0.045 | |
| Vascular invasion | 1.62 (0.72–3.65) | 0.24 | |||
| Positive horizontal margin | 1.45 (0.76–2.78) | 0.26 | |||
| Positive vertical margin | 14.6 (4.41–48.93) | <0.001 | 3.77 (0.81–17.53) | 0.09 | |
| Depth of invasion (μm) | |||||
| <500 | 1 | ||||
| ≥500 | 0.81 (0.28–2.36) | 0.70 | |||
| Gross type | |||||
| Elevated | 1 | ||||
| Flat | 3.16 (1.26–7.92) | 0.01 | 4.8 (1.01–22.73) | 0.05 | |
| Depressed | 1.29 (0.52–3.23) | 0.60 | 0.62 (0.15–2.59) | 0.51 | |
| eCura score | |||||
| Low risk | 1 | ||||
| Medium risk | 1.45 (0.57–3.73) | 0.44 | 0.24 (0.02–2.63) | 0.24 | |
| High risk | 2.78 (1.05–7.36) | 0.04 | 0.23 (0.02–2.97) | 0.26 | |
HR, hazard ratio; CI, confidence interval; ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection; eCura, endoscopic curability.
Discussion
The advancement and widespread adoption of early screening and minimally invasive endoscopic techniques have led to the increased detection and treatment effectiveness of EGCs (12). Previous large-scale clinical study has demonstrated that endoscopic resection offers higher long-term survival rates and lower recurrence rates compared to traditional surgical methods, thus enhancing patients’ quality of life (13). If the patients with early gastric cancer treated by endoscopy cannot meet the standard of CR after the standard pathological evaluation of the resected lesion, it is considered as NCR (14,15). This study focuses on the long-term follow-up and analysis of clinical outcomes for patients with NCR of EGC.
This study analyzed the recurrence rate and its risk factors in NCRs. The local recurrence rate post NCR was 23.1%, with a median follow-up of approximately 24 months, which was significantly higher than the CR group’s 5.3% recurrence rate. Factors influencing local recurrence included submucosal infiltration, positive vertical margins, and lymphatic vessel invasion, aligning with previous research findings. A study of 152 non-curatively resected EGC cases indicated a higher risk of local recurrence after piecemeal endoscopic resection, especially in cases exceeding ESD criteria with lymphatic vessel invasion (16). Although many studies consider lymph node invasion as an important risk factor for poor prognosis requiring further surgery, a recent study that surveyed 15 lesions regarding lymph node metastasis rate for lymphovascular infiltration, which only detected one lesion (6.7%), showed that lymphovascular invasion does not necessarily indicate lymph node metastasis (17). Patients diverging from CR norms solely on lymphovascular invasion may have lesions unnecessary for further surgery. Additional confirmation is needed in treating deviations based solely on lymphovascular criteria. In our research, postoperative lesion size ≥3 cm, ulceration, lymphatic vessel invasion, and positive vertical margins emerged as independent risk factors impacting recurrence-free survival in NCR patients (18). Previous researches indicated that submucosal invasion, lymphovascular presence, and undifferentiated histology are valid surgical management indicators (19-23). Hence, we compared the survival of surgical and non-surgical groups. The survival curve indicated a significant statistical difference in the recurrence rate between these two groups (P=0.004). In the long-term follow-up of 71 cases diagnosed with EGC by endoscopy, who did not undergo surgical resection or were diagnosed more than 6 months post-surgery, the cumulative risk of progression to advanced cancer after 5 years was 63.0% (95% CI, 48–78%). Additionally, a statistical analysis was conducted between the surgical and non-surgical groups. Univariate analysis revealed significant gender differences, with a higher prevalence of males (P=0.02). More individuals aged ≥60 years were found in the non-surgical group compared to the surgical group (P=0.004). Pathologically, the surgical group had a higher incidence of undifferentiated cases than the non-surgical group (P=0.008). Regarding invasion depth, the surgical group had more submucosal layer cases than the non-surgical group (P=0.02), and a higher proportion of eCuraC-2 (P<0.001). No statistical differences were observed between the groups in terms of ulceration, lymphatic vessel invasion, and positive horizontal and vertical margins. Aligning with latest guidelines, multivariate regression analysis identified age ≥60 years (P=0.01), depressed gross type (P=0.006), and eCuraC-2 (P=0.04) as independent risk factors for NCR necessitating further surgery. In addition to these factors evaluated in this study, a recent study by Japanese scholars pointed out that additional gastrectomy after non-curative ESD for pT1 EGC could provide an oncologically safe outcome (24). With the continuous development of endoscopic technology, an increasing number of expanded ESD are being performed. Although this study only compared patient information for selecting surgical procedures, a recent meta-analysis of all available and most updated studies comparing ESD versus surgery for the treatment of uEGC found that all-cause mortality was similar in both groups after adjusted analyses (25). A recent South Korean study delved into the risks and advantages of extra surgery for upper third EGC post non-curative ESD, identifying lymphovascular invasion as the sole significant predictor of lymph node metastasis (P<0.001) (26). And a Japanese study that included 151 patients diagnosed with noncurative lesions after ESD treatment over the past decade recommended gastrectomy and lymph node dissection for patients with lymphatic vessel invasion and/or undifferentiated types (27). But Katsuragi et al. point out that clinicians advise additional gastrectomy if pathological analysis of ESD specimens shows lymphovascular infiltration by tumors. However, subsequent gastrectomy based on this pathological assessment often fails to reveal any lymph node metastasis (28). In our previous study, we have showed the size of NCR lesions were 2.32±1.30 cm (29), and the pathological evaluation was carried out in accordance with the guidelines, the specimen was cut in parallel at an interval of 2–3 mm perpendicular to the nearest incisal edge of the specimen. In this cohort, there are 5/42 (11.90%) cases with lymph node involvements revealed by surgical resection after non-curative ESD. Consistent with previous studies (13,26), our results show that the additional surgery group has a better prognosis in the long run. In clinical practice, the choice of treatment strategy is influenced by efficacy and risk of recurrence/metastasis, age, baseline physical condition, postoperative quality of life, patient or family preferences, and financial status. Additionally, several articles have indicated that high-risk comorbidities are given greater consideration from the patient’s perspective in treatment decisions (30,31). A retrospective study demonstrated that high-risk comorbidity [Charlson Comorbidity Index (CCI) ≥3] was an independent prognostic factor (30). In conclusion, for EGC patients at high risk for recurrence/metastasis after non-curative ESD, additional surgical treatment should be determined through multi-faceted evaluation by the attending pathologist, endoscopist, surgeon and patients and molecular diagnosis should not be ignored.
This study has several limitations: (I) being a single-center study with a relatively small sample size, it may not fully represent the broader population. Additionally, some endoscopy and pathology reports from the early years [2012–2014] lack the standardized content mentioned in this article. The results could be influenced by the limited case number and the uneven distribution of pathological types, necessitating further validation through multi-center studies with larger sample sizes. (II) As a retrospective study spanning ten years that is based on gastric endoscopy cases from the Digestive Endoscopy Center of Beijing Friendship Hospital, Capital Medical University, there are inherent limitations. Despite careful data selection, achieving consistency among surgical operators and endoscopy report standards is challenging. Furthermore, not all pathological results were evaluated by the same pathologist, which may introduce bias and affect the study outcomes. (III) Some data incompleteness may lead to bias in statistical analysis.
Conclusions
In conclusion, the recurrence/metastasis rate in the NCR group is significantly higher than that in the control group. Prognostic risk factors include tumor size ≥2 & <3 cm, positive vertical margins, lymphatic invasion, and flat type (one of pathological gross classification). Patients with NCR and eCuraC-2 should consider additional surgical intervention.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-168/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-168/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-168/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-168/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by Beijing Friendship Hospital, Capital Medical University (No. 2018-P2-058-01). The patients/participants provided their written informed consent to participate in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700-13. [Crossref] [PubMed]
- Jung KW, Won YJ, Oh CM, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2014. Cancer Res Treat 2017;49:292-305. [Crossref] [PubMed]
- Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol 2020;18:534-42. [Crossref] [PubMed]
- Lee E, Kim SG, Kim B, et al. Metachronous gastric neoplasm beyond 5 years after endoscopic resection for early gastric cancer. Surg Endosc 2023;37:3901-10. [Crossref] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc 2021;33:4-20.
- Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023;26:1-25.
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [Crossref] [PubMed]
- Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251-5. [Crossref] [PubMed]
- Yada T, Yokoi C, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc 2013;2013:241320. [Crossref] [PubMed]
- Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. [Crossref] [PubMed]
- Abe S, Oda I, Suzuki H, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy 2013;45:703-7. [Crossref] [PubMed]
- Lian J, Xu A, Chen T, et al. Unexpected gastric perforation during endoscopic submucosal tunnel dissection for early circumferential esophageal cancer. Endoscopy 2023;55:E833-4. [Crossref] [PubMed]
- Chung MW, Jeong O, Park YK, et al. Comparison on the long term outcome between endoscopic submucosal dissection and surgical treatment for undifferentiated early gastric cancer. Korean J Gastroenterol 2014;63:90-8. [Crossref] [PubMed]
- Yeh JH, Huang RY, Lee CT, et al. Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis. Therap Adv Gastroenterol 2020;13:1756284820964316. [Crossref] [PubMed]
- Han JP, Hong SJ, Kim HK, et al. Risk stratification and management of non-curative resection after endoscopic submucosal dissection for early gastric cancer. Surg Endosc 2016;30:184-9. [Crossref] [PubMed]
- Takano K, Ashikari K, Tamura S, et al. Clinicopathological features of endoscopically treated early gastric cancer with lymphovascular infiltration. J Cancer Res Clin Oncol 2023;149:5781-90. [Crossref] [PubMed]
- Lee JY, Cho KB, Kim ES, et al. Risk factors for local recurrence after en bloc endoscopic submucosal dissection for early gastric cancer. World J Gastrointest Endosc 2016;8:330-7. [Crossref] [PubMed]
- Benites-Goñi H, Palacios-Salas F, Carlin-Ronquillo A, et al. Endoscopic submucosal dissection versus surgery for patients with undifferentiated early gastric cancer. Rev Esp Enferm Dig 2023;115:3-9. [PubMed]
- Ryu KW, Choi IJ, Doh YW, et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol 2007;14:3428-34. [Crossref] [PubMed]
- Nagano H, Ohyama S, Fukunaga T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer 2005;8:149-54. [Crossref] [PubMed]
- Jung H, Bae JM, Choi MG, et al. Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg 2011;98:73-8. [Crossref] [PubMed]
- Shindo K, Castillo J, Ohuchida K, et al. Influence of endoscopic resection on additional laparoscopic distal gastrectomy: a propensity score-matching analysis. Surg Today 2020;50:1290-6. [Crossref] [PubMed]
- Shimada S, Sawada N, Oae S, et al. Impact of non-curative endoscopic submucosal dissection on short- and long-term outcome of subsequent laparoscopic gastrectomy for pT1 gastric cancer. Surg Endosc 2022;36:3985-93. [Crossref] [PubMed]
- Yang HJ, Kim JH, Kim NW, et al. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated-type early gastric cancer meeting the expanded criteria: a systematic review and meta-analysis. Surg Endosc 2022;36:3686-97. [Crossref] [PubMed]
- Park SH, Yoon HM, Ryu KW, et al. Risks and benefits of additional surgery for early gastric cancer in the upper third of the stomach meeting non-curative resection criteria after endoscopic submucosal dissection. World J Surg Oncol 2022;20:311. [Crossref] [PubMed]
- Makimoto S, Mushiake Y, Takami T, et al. Evaluation of additional gastrectomy after noncurative endoscopic submucosal dissection for early gastric cancer. BMC Surg 2022;22:352. [Crossref] [PubMed]
- Katsuragi SY, Otsuki Y, Unno S, et al. Evaluation of the widths of the mucosal strips in pathological examination of specimens of endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 2023;26:755-62. [Crossref] [PubMed]
- Ma X, Zhang Q, Zhu S, et al. Risk Factors and Prediction Model for Non-curative Resection of Early Gastric Cancer With Endoscopic Resection and the Evaluation. Front Med (Lausanne) 2021;8:637875. [Crossref] [PubMed]
- Li S, Tian X, Wei J, et al. Long-term outcomes of additional surgery versus non-gastrectomy treatment for early gastric cancer after non-curative endoscopic submucosal dissection: a meta-analysis. Chin Med J (Engl) 2023;136:528-35. [Crossref] [PubMed]
- Toya Y, Endo M, Nakamura S, et al. Long-term outcomes and prognostic factors with non-curative endoscopic submucosal dissection for gastric cancer in elderly patients aged ≥ 75 years. Gastric Cancer 2019;22:838-44. [Crossref] [PubMed]


