
Prognosis comparison between hepatocellular carcinoma patients with microvascular invasion who received hepatectomy alone and those who underwent early PA-TACE: a retrospective cohort study
Highlight box
Key findings
• Early transcatheter arterial chemoembolization (TACE) treatment after hepatectomy plays an important role in improving hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI).
What is known and what is new?
• TACE is recognized as one of the most effective non-surgical treatments for advanced liver cancer.
• We have identified that postoperative adjuvant TACE not only effectively enhances tumor recurrence and survival rates but also ameliorates the imbalanced immune status in HCC patients with MVI following hepatectomy.
What is the implication, and what should change now?
• HCC patients with MVI should be treated with early TACE, even after hepatectomy.
Introduction
Hepatocellular carcinoma (HCC), accounting for 90% of hepatic malignancies, is one of the leading causes of cancer-related mortality worldwide, with a rapidly rising global incidence rate (1,2). Statistically, approximately 80% of newly diagnosed HCC patients pass away within one year (3), due to the aggressive nature of the disease and the lack of effective treatment alternatives. Although various clinical therapies, such as surgical resection (SR), ablation, and liver transplantation, were widely applied to cure HCC, approximately 70–80% of HCC patients relapsed within five years after receiving curative treatment (4-7).
Recently, microvascular invasion (MVI), the pathological feature reflecting the invasiveness of tumors, has been reported to be involved in the recurrence of HCC (8,9). Recent research has also demonstrated that transcatheter arterial chemoembolization (TACE), a safe intervention strategy, can effectively prevent tumor recurrence and increase the survival of patients with MVI. This is achieved by decreasing blood flow to the tumor and promoting the tumor ischemic necrosis by arterial injection of chemotherapeutic drugs and embolizing agents (10-12). Therefore, to make HCC patients with MVI achieve better clinical outcomes, postoperative adjuvant (PA)-TACE was considered and performed (13-16). Encouragingly, compared with those who did not receive PA-TACE, HCC patients with MVI showed longer overall survival (OS) and disease-free survival (DFS) after receiving PA-TACE (15,16).
With the in-depth exploration of the tumor ecosystem, it has been demonstrated that tumor-associated immune cells participate in the tumorigenesis, development, and invasion of tumors (17-19), suggesting a disorder and dysfunction of the immune system of HCC patients. With the proven effectiveness of TACE in HCC patients with MVI (13,14), we wondered whether this treatment strategy could benefit patients with HCC by mediating the dysfunctional immunological status. However, it was unclear whether early TACE treatment would improve outcomes while also working on immune imbalances. Given the functional role of regulatory T cell (Treg) in shaping immunosuppressive tumor microenvironment (TME) (20), and T helper cell 17 (Th17) in mediating inflammation response (21), we investigated the changes in immune function as measured by the ratio of Treg and Th17 cells in the peripheral blood of HCC patients after TACE. The results of this study provide preliminary evidence that TACE can regulate the already imbalanced immune function in HCC patients, thereby improving the efficacy of HCC treatment. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-282/rc).
Methods
Patients and design
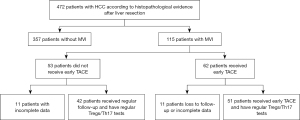
It was designed as a retrospective cohort study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2023-334) and informed consent was taken from all individual participants. This study retrospectively included a total of 472 patients with HCC who were diagnosed for the first time at the First Affiliated Hospital of Soochow University from December 31, 2015 to December 31, 2018. As shown in Figure 1, 115 patients with MVI confirmed by postoperative pathology within 4 weeks were divided into two groups according to whether TACE was performed based on examination results and doctor-patient communication. Finally, after data exclusion, 51 patients undergoing early TACE were included in the TACE group, and 42 patients received hepatectomy alone in the non-TACE group. The diagnosis of HCC was confirmed according to histopathological evidence after liver resection. All the patients’ data were obtained from the same period. MVI was defined as the presence of tumor cells that could only be seen under a microscope in a portal vein, hepatic vein, or significant capsule vessel of the liver tissue close to the tumor edge. The exclusion criteria were as follows: (I) imaging examination indicated insufficient lesions (invasive type, non-arterial enhancement, or maximum lesion <1 cm); (II) other primary malignant tumors in other organs; (III) extrahepatic neoplasms or tumors invading the main portal vein; (IV) Child-Pugh class C; and (V) uncontrolled functional or metabolic disease.
Surgical procedures
Contrast-enhanced ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) were routinely performed before surgery to evaluate tumor status and resectability. Only patients with normal liver function or Child-Pugh grade A or selected grade B liver function received either large or small hepatectomy. CT volume was used to estimate residual liver volume in patients undergoing major excision. Indications of hepatectomy for liver cancer include Child-Pugh grade A or B, adequate residual liver volume (at least 30% in patients with normal liver and at least 40% in patients with cirrhosis or other chronic liver diseases) and overall good health. Intraoperatively, the abdominal cavity was carefully examined to determine the degree of local lesions, extrahepatic metastasis and peritoneal seeding rate, and further diagnosis was made through postoperative pathology.
Early TACE
TACE was performed by qualified professionals with specialized medical training in interventional radiology. Within four weeks after hepatectomy, when the patient’s liver function had recovered, the Seldinger technique was employed to implant the hepatic arterial catheter through the femoral artery to the proper hepatic artery, and TACE was performed on the whole remnant liver. Liver angiography, CT angiography, or both were performed to check for obvious tumor spots in the residual liver. An emulsion of doxorubicin hydrochloride (10 mg), pirarubicin (THP) or pharmorubicin (20–40 mg), and lipiodol (2–10 mL) (Lipiodol Ultrafluide, Guerbet, Aulnay-Sous-Bois, France) was then infused through the catheter. The dosage of lipiodol and doxorubicin was determined by the patient’s body surface area and underlying liver function.
Isolation of lymphocytes from peripheral blood
Fresh heparinized peripheral blood was collected from 51 patients one day before and four weeks after TACE. The blood was deposited into a 5 mL EDTA tube. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood by Ficoll-Paque density gradient centrifugation and frozen until use.
Antibodies used in the study
PBMC was divided into Th17 cells and Treg cells. The following fluorochrome-conjugated monoclonal antibodies were purchased from American BD Company and used for flow cytometry: FITC-labeled CD4 antibody and PC-labeled CD25 antibody were used to identify Treg cells, while PE-CD127, PE-CD3, PC-CD8, and PE-IL-17 antibodies were used to detect Th17 cells. FACS Express 3.0 software was used for data analysis.
Follow-up of the two groups of patients
All patients enrolled in the two groups were followed up through regular outpatient visits and inpatient examinations every 2–4 weeks until death or until the study ended in December 2023. The 12-, 36-, and 60-month OS and progression-free survival (PFS) rates in patients with MVI were compared between patients who received hepatectomy alone and those who underwent early PA-TACE.
Statistical analysis
Continuous variables following a normal distribution were expressed as mean ± standard deviation (SD) and compared using a two-tailed unpaired t-test. For variables inconsistent with a normal distribution, the results were presented as median (range), and compared using the Mann-Whitney U test. Categorical variables were compared using the chi-squared test or Fisher’s exact test. Cumulative OS and PFS rates were estimated using the Kaplan-Meier method and compared using the log-rank test. All data analyses were carried out using SPSS version 22.0. A two-tailed P<0.05 was considered statistically significant.
Results
Patient characteristics and treatment administration
From December 2015 to December 2018, the study enrolled 42 HCC patients with MVI who received PA-TACE (31 males, 11 females) and 51 patients without TACE (37 males and 14 females). The baseline characteristics of HCC patients treated with TACE and without TACE are shown in Table 1. There were no statistically significant differences in the comparison of baseline characteristics of HCC patients between the two groups (all P>0.05), showing comparability between the two groups. The mean age of the enrolled patients was 65.27±12.08 years and hepatitis B virus (HBV) was identified as the main etiology. Tumor characteristics included a mean tumor diameter of 7.6±2.5 cm, with 47.06% presenting as solitary nodules, a median alpha-fetoprotein (AFP) level of 149.31 ng/mL, and extrahepatic metastasis observed in 13 cases (25.49%). Serum biochemical indicators revealed mean levels of total bilirubin at 1.45±0.92 mg/dL, median alanine aminotransferase (ALT) levels at 32.29 U/L, prothrombin time at 14.56±3.78 s, and median platelet counts at 116×109/L. All patients who received early TACE treatment were Child-Pugh grade A (33 cases, 64.71%) and B (18 cases, 35.29%).
Table 1
| Characteristic | TACE group (n=42) | Non-TACE group (n=51) | P value |
|---|---|---|---|
| Gender (male:female) | 31:11 | 37:14 | 0.81 |
| Age, years, mean ± SD | 61.69±9.27 | 62.78±9.97 | 0.44 |
| Etiologies, n (%) | 0.82 | ||
| HBV | 30 (71.43) | 38 (74.51) | |
| Others | 12 (28.57) | 13 (25.49) | |
| AFP, ng/mL, median [IQR] | 143.5 [70.25–315.5] | 192 [71–425.5] | 0.32 |
| TBIL, μmol/L, mean ± SD | 20.43±7.647 | 22.17±6.704 | 0.25 |
| Albumin, g/L, mean ± SD | 32.29±5.637 | 33.82±6.160 | 0.22 |
| ALT, U/L, median [IQR] | 65 [38–81.5] | 54 [40–87] | 0.66 |
| PT, s, mean ± SD | 12.96±2.558 | 12.23±2.69 | 0.19 |
| Platelet, 109/L, median [IQR] | 71 [42–133] | 72 [54–98] | 0.89 |
| Treg/CD4+ T cells (%), mean ± SD | 7.48 ±1.94 | 7.34±3.63 | 0.81 |
| Th17/CD4+ T cells (%), mean ± SD | 0.47±0.25 | 0.49±0.25 | 0.66 |
| Tumor size, cm, mean ± SD | 7.15±3.05 | 6.662±3.61 | 0.48 |
| Tumor number (solitary, %) | 21 (50.00) | 24 (47.06) | 0.84 |
| Extrahepatic metastasis, n (%) | 12 (28.57) | 13 (25.49) | 0.82 |
| Child-Pugh grade, n (%) | |||
| A | 11 (26.19) | 33 (64.71) | 0.38 |
| B | 31 (73.81) | 18 (35.29) | 0.38 |
HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; SD, standard deviation; HBV, hepatitis B virus; AFP, alpha-fetoprotein; IQR, interquartile range; TBIL, total bilirubin; ALT, alanine aminotransferase; PT, prothrombin time; Treg, regulatory T cell; Th17, T helper cell 17.
Comparison of baseline and post-TACE Treg and Th17 in HCC patients within the TACE group
Firstly, flow cytometry was applied to assess the abundance of Treg and Th17 in the PBMC (Figure 2). As shown in Table 2, HCC patients who received TACE had significantly lower levels of Treg among CD4+ T cells (7.34%±3.61% vs. 5.82%±2.76%, P<0.001), but a higher abundance of Th17 among CD4+ T cells (0.49%±0.28% vs. 0.50%±0.25%, P<0.001) compared to the baseline. These findings indicate the lower immunosuppressive and higher inflammatory response of patients who received TACE.
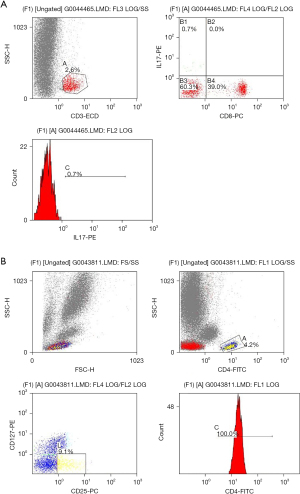
Table 2
| Indexes | Baseline, mean ± SD | Post-TACE, mean ± SD | t value | P value |
|---|---|---|---|---|
| Treg (%) | 7.34±3.61 | 5.82±2.76 | −3.09 | <0.001 |
| Th17 (%) | 0.49±0.28 | 0.50±0.25 | −4.05 | <0.001 |
TACE, transarterial chemoembolization; Treg, regulatory T cell; Th17, T helper cell 17; HCC, hepatocellular carcinoma; SD, standard deviation.
HCC patients who received TACE had better clinical outcomes
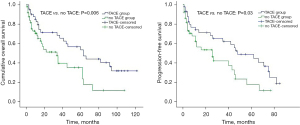
According to the statistics, the median OS of all patients included in the study was 61.8 months, and the 12-, 36-, and 60-month OS rates were 81.2%, 70.3%, and 52.6%, respectively (Table 3). The median OS of patients with TACE was 60 months compared to 37 months in patients without TACE, which was significant in the univariate analysis (P=0.006, Figure 3 and Table 3). The median PFS for all patients was 45.6 months, and the 12-, 36-, and 60-month PFS rates were 79.2%, 60.1%, and 42.5%, respectively. Specifically, compared to patients without TACE after hepatectomy, patients with TACE had longer median PFS time (48 vs. 26 months) and higher 12-, 36-, and 60-month PFS rates (82.1%, 58.8%, and 44.7%, vs. 81.6%, 46.2%, and 17.5%) (P=0.025, Figure 3 and Table 3).
Table 3
| Variable | All patients | TACE group | Non-TACE group | P value |
|---|---|---|---|---|
| Overall survival | ||||
| Median (months) | 61.8 | 60 | 37 | 0.006 |
| 12-month (%) | 81.2 | 82.5 | 68 | |
| 36-month (%) | 70.3 | 71.6 | 51.3 | |
| 60-month (%) | 52.6 | 53.5 | 35.3 | |
| Progression-free survival | ||||
| Median (months) | 45.6 | 48 | 26 | |
| 12-month (%) | 79.2 | 82.1 | 81.6 | 0.03 |
| 36-month (%) | 60.1 | 58.8 | 46.2 | |
| 60-month (%) | 42.5 | 44.7 | 17.5 | |
TACE, transarterial chemoembolization.
Discussion
As a potentially radical therapy for HCC, liver resection can extend the survival of HCC patients compared to other palliative treatments (22,23). However, approximately 70–80% of HCC patients relapse within five years after receiving curative treatment (4-7). Some studies reported that MVI, a pathological characteristic reflecting the invasion of tumor cells, is a major independent prognostic factor for HCC patients (22,23), and might participate in the recurrence and worse outcomes (9,24). Currently, TACE is the first-line therapy for people with unresectable HCC, and is the first course of treatment for HCC patients with MVI (25-27), due to the effective function of TACE to block the nutrient vessels of invisible metastatic liver cancer and maintain the sustainable chemotherapeutic killing of tumor cells (10-12). Recently, some clinical trials reported that PA-TACE may be the most beneficial for HCC patients with high-risk recurrence factors because of its potential to delay cancer recurrence (28,29), while other studies observed the opposite, especially in MVI-negative patients (30,31). We reviewed liver cancer patients who underwent hepatectomy in the First Affiliated Hospital of Soochow University from December 2015 to December 2018. A total of 472 patients received surgical treatment, 115 patients were pathologically confirmed with MVI after surgery (accounting for 24.36% of the total number of cases), and 62 patients received early postoperative TACE therapy. HBV remains the main cause, which is consistent with the results of the cancer epidemic survey in China (32). By injecting chemotherapy drugs and embolic agents into the artery, TACE reduces blood flow to the tumor and induces tumor avascular necrosis (7). TACE can also modulate specific circulating immune cell subsets, including CD4+ T cells (Th1, Th17 and Treg cells), CD8+ T cells, natural killer (NK) cells and Natural killer T (NKT) cells, to delay tumor development (33). Th17 cells are pro-inflammatory Th cells, and Treg cells can inhibit Th17 cells (34). We selected Treg and Th17 cells as representatives, and the results showed that TACE could significantly change the proportion of CD4+T cells and improve their balance (33,35). Further research showed that PA-TACE was related to OS. With and without TACE, the OS rate at 1, 3 and 5 years were 82.5% and 68%, 71.6% and 51.3%, 53.5% and 35.3%, respectively. PA-TACE was also related to PFS. For patients with and without TACE, the PFS rate at 12, 36 and 60 months were 81.2% and 81.6%, 58.8% and 46.2%, 44.7% and 17.5%, respectively. This was consistent with previous studies that PA-TACE after hepatectomy could improve the prognosis of patients with MVI of HCC (13,36,37).
TACE reduces blood flow to the tumor and induces tumor ischemic necrosis, as well as regulates the natural imbalance of the immune microenvironment. The diagnosis of MVI can only be determined by histological examination of the excision specimen. Therefore, adjuvant hepatectomy therapy plays a key role in delayed recurrence. Unfortunately, PA-TACE is not widely used in postoperative adjuvant therapy, and we hope that this trial will be helpful for clinical operations.
Tumorigenesis, metastasis, and drug resistance are related to a variety of factors. Skp2 plays multiple roles in malignant tumors (38). SKP2 is over-expressed in HCC tissues, and its over-expression is associated with poor prognosis (39). Lung cancer is a typical type of cancer resistant to chemotherapy. A study of lung cancer-related resistance mechanisms has identified MARCKSL1-2 as a promising target for improving lung adenocarcinoma chemotherapy (40). Yu et al. conducted reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and found that exosomal LOC85009 inhibits docetaxel (DTX) resistance by regulating ATG5-induced autophagy via USP5/USF1 axis (41), and CPSF6-mediated XBP1 3’UTR shortening attenuates cisplatin-induced ER stress and elevates chemo-resistance in lung adenocarcinoma (42). Triple-negative breast cancer (TNBC) is another representative of liver metastasis and chemotherapy resistance, and is considered the most hazardous subtype of breast cancer owing to its accelerated progression, enormous metastatic potential, and refractoriness to standard treatments (43). It has been shown that the METTL3/YTHDF1 axis up-regulates GPRC5A expression by m6A methylation. GPRC5A activates the Mtorc1/p70s6k signaling pathway by recruiting Mtorc1 to lysosomes and consequently promotes DTX resistance and liver metastasis (44,45). Tumorigenesis, metastasis, and drug resistance of HCC have their own characteristics and complex mechanisms, and this study mainly involves the preliminary exploration of MVI therapy. Our study has some limitations. As this was a small-sample retrospective cohort study, the results may be susceptible to unmeasured confounding factors. Further multicenter randomized controlled studies should be conducted with longer follow-ups to validate and extend the results of this study.
Conclusions
This study retrospectively suggests that PA-TACE may have roles in improving survival outcomes, and restoring immune homeostasis in HCC patients with MVI after hepatectomy.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-282/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-282/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-282/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-282/coif). All authors report that this study was supported by the Chinese Foundation for Hepatitis Prevention and Control-Tian Qing Liver Disease Research Fund Subject 20200098 (TQGB20200098); and Beijing iGandan Foundation (iGandanF-1082022-RGG044). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2023-334) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020;159:335-349.e15. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-491.e1. [Crossref] [PubMed]
- Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960-74. [Crossref] [PubMed]
- Colecchia A, Schiumerini R, Cucchetti A, et al. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol 2014;20:5935-50. [Crossref] [PubMed]
- Qian Y, Teufel A. Regional differences: clinical practice guidelines on the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2022;11:161-3. [Crossref] [PubMed]
- Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr 2020;9:452-63. [Crossref] [PubMed]
- Pommergaard HC, Rostved AA, Adam R, et al. Vascular invasion and survival after liver transplantation for hepatocellular carcinoma: a study from the European Liver Transplant Registry. HPB (Oxford) 2018;20:768-75. [Crossref] [PubMed]
- Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. [Crossref] [PubMed]
- Tsilimigras DI, Pawlik TM. Adjuvant hepatic arterial infusion chemotherapy in patients with resected hepatocellular carcinoma with microvascular invasion. Chin Clin Oncol 2024;13:16. [Crossref] [PubMed]
- Sun JJ, Wang K, Zhang CZ, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol 2016;23:1344-51. [Crossref] [PubMed]
- Chinese Society of Hepatology, Chinese Medical Association. The consensus on tertiary prevention of primary liver cancer (2022 version). Zhonghua Gan Zang Bing Za Zhi 2022;30:832-45. [PubMed]
- Qiu Y, Yang Y, Wang T, et al. Efficacy of Postoperative Adjuvant Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma Patients With Microscopic Portal Vein Invasion. Front Oncol 2022;12:831614. [Crossref] [PubMed]
- Wang L, Ke Q, Lin N, et al. Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: a meta-analysis. Scand J Gastroenterol 2019;54:528-37. [Crossref] [PubMed]
- Winder WW, Loy SF, Burke DS, et al. Liver glycogenolysis during exercise in adrenodemedullated male and female rats. Am J Physiol 1986;251:R1151-5. [PubMed]
- Ye JZ, Chen JZ, Li ZH, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol 2017;23:7415-24. [Crossref] [PubMed]
- He Q, Yang J, Jin Y. Immune infiltration and clinical significance analyses of the coagulation-related genes in hepatocellular carcinoma. Brief Bioinform 2022;23:bbac291. [Crossref] [PubMed]
- Liu Y, Xun Z, Ma K, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol 2023;78:770-82. [Crossref] [PubMed]
- Lu Y, Yang A, Quan C, et al. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun 2022;13:4594. [Crossref] [PubMed]
- Suthen S, Lim CJ, Nguyen PHD, et al. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology 2022;76:1329-44. [Crossref] [PubMed]
- Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis 2019;39:26-42. [Crossref] [PubMed]
- Yang Y, Dang Z, Lu P, et al. Impact of pathological response after preoperative transcatheter arterial chemoembolization (TACE) on incidences of microvascular invasion and early tumor recurrence in hepatocellular carcinoma: a multicenter propensity score matching analysis. Hepatobiliary Surg Nutr 2022;11:386-99. [Crossref] [PubMed]
- Vogel A, Martinelli EESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5. [Crossref] [PubMed]
- Hsieh CH, Wei CK, Yin WY, et al. Vascular invasion affects survival in early hepatocellular carcinoma. Mol Clin Oncol 2015;3:252-6. [Crossref] [PubMed]
- Chang PY, Huang CC, Hung CH, et al. Multidisciplinary Taiwan Consensus Recommendations for the Use of DEBDOX-TACE in Hepatocellular Carcinoma Treatment. Liver Cancer 2018;7:312-22. [Crossref] [PubMed]
- Jeong J, Park JG, Seo KI, et al. Microvascular invasion may be the determining factor in selecting TACE as the initial treatment in patients with hepatocellular carcinoma. Medicine (Baltimore) 2021;100:e26584. [Crossref] [PubMed]
- Yang Y, Lin K, Liu L, et al. Impact of preoperative TACE on incidences of microvascular invasion and long-term post-hepatectomy survival in hepatocellular carcinoma patients: A propensity score matching analysis. Cancer Med 2021;10:2100-11. [Crossref] [PubMed]
- Paik KY. Concerns about how to simultaneously determine microvascular invasion and pathological response after transarterial chemoembolization before hepatocellular carcinoma surgery. Hepatobiliary Surg Nutr 2023;12:815-7. [Crossref] [PubMed]
- Zeng G, Zou B, Li Y, et al. Efficacy of Adjuvant Transarterial Chemoembolization after Radical Hepatectomy in Solitary Hepatocellular Carcinoma Patients: A Retrospective Study. J Invest Surg 2022;35:1208-16. [Crossref] [PubMed]
- Wang L, Ke Q, Deng M, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma after radical hepatectomy: a real world study. Scand J Gastroenterol 2019;54:1403-11. [Crossref] [PubMed]
- Xu Z, Xie H, Zhou L, et al. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst) 2019;2019:8619096. [Crossref] [PubMed]
- Zhou J, Sun HC, Wang Z, et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018;7:235-60. [Crossref] [PubMed]
- Liao Y, Wang B, Huang ZL, et al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLoS One 2013;8:e60444. [Crossref] [PubMed]
- Liu L, Yang J, Zu B, et al. Acacetin regulated the reciprocal differentiation of Th17 cells and Treg cells and mitigated the symptoms of collagen-induced arthritis in mice. Scand J Immunol 2018;88:e12712. [Crossref] [PubMed]
- Ginès P, Krag A, Abraldes JG, et al. Liver cirrhosis. Lancet 2021;398:1359-76. [Crossref] [PubMed]
- Kräusslich HG, Schneider H, Zybarth G, et al. Processing of in vitro-synthesized gag precursor proteins of human immunodeficiency virus (HIV) type 1 by HIV proteinase generated in Escherichia coli. J Virol 1988;62:4393-7. [Crossref] [PubMed]
- Zhang J, Peng H, Wang B, et al. Efficacy of Postoperative Adjuvant Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma Patients with Mesenchymal Circulating Tumor Cell. J Gastrointest Surg 2021;25:1770-8. [Crossref] [PubMed]
- Qiao C, Huang F, He J, et al. Ceftazidime reduces cellular Skp2 to promote type-I interferon activity. Immunology 2023;170:527-39. [Crossref] [PubMed]
- Zhong Z, Xie F, Yin J, et al. Development of a prognostic model for anoikis and identifies hub genes in hepatocellular carcinoma. Sci Rep 2023;13:14723. [Crossref] [PubMed]
- Jiang M, Qi F, Zhang K, et al. MARCKSL1-2 reverses docetaxel-resistance of lung adenocarcinoma cells by recruiting SUZ12 to suppress HDAC1 and elevate miR-200b. Mol Cancer 2022;21:150. [Crossref] [PubMed]
- Yu Z, Tang H, Chen S, et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist Updat 2023;67:100915. [Crossref] [PubMed]
- Zhu C, Xie Y, Li Q, et al. CPSF6-mediated XBP1 3'UTR shortening attenuates cisplatin-induced ER stress and elevates chemo-resistance in lung adenocarcinoma. Drug Resist Updat 2023;68:100933. [Crossref] [PubMed]
- Tang Y, Tian W, Zheng S, et al. Dissection of FOXO1-Induced LYPLAL1-DT Impeding Triple-Negative Breast Cancer Progression via Mediating hnRNPK/β-Catenin Complex. Research (Wash D C) 2023;6:0289.
- Ou X, Tan Y, Xie J, et al. Methylation of GPRC5A promotes liver metastasis and docetaxel resistance through activating mTOR signaling pathway in triple negative breast cancer. Drug Resist Updat 2024;73:101063. [Crossref] [PubMed]
- Wu S, Lu J, Zhu H, et al. A novel axis of circKIF4A-miR-637-STAT3 promotes brain metastasis in triple-negative breast cancer. Cancer Lett 2024;581:216508. [Crossref] [PubMed]
(English Language Editor: JY Yeo)


