
Efficacy of first-line chemotherapy based on primary site of tumor versus etoposide-platinum in advanced gastroenteropancreatic neuroendocrine carcinoma
Highlight box
Key findings
• Chemotherapy regimens based on primary site of tumor and small cell lung cancer (SCLC) are both optional for advanced gastroenteropancreatic neuroendocrine carcinomas (GEP-NECs) and elevated neutrophil-to-lymphocyte ratio (NLR) is an independent negative prognostic factor.
What is known and what is new?
• When making treatment decisions for advanced GEP-NECs, clinicians often follow the paradigm of SCLC due to morphological similarities. However, the outcomes were unsatisfying and effective prognostic factors are lacking.
• In this retrospective study, we found no statistical difference in treatment outcomes between patients receiving these two regimens. We also proved that elevated baseline NLR is an independent negative prognostic factor for advanced GEP-NECs.
What is the implication, and what should change now?
• Both regimens are optional and future studies are required to establish optimum treatment protocols for GEP-NECs.
Introduction
Neuroendocrine tumors (NETs) are characterized by the expression of neuroendocrine proteins secreting peptide hormones or bio-amines, while highly aggressive and poorly differentiated neuroendocrine carcinomas (NECs) are usually accompanied by rapidly lethal disease (1). The incidence of NECs is increasing due to the emerging precise histopathologic diagnosis. NECs arise most commonly in the lung (91.3%) (2), while extrapulmonary NECs are mainly located along the gastrointestinal tract (3). According to the World Health Organization grading system, neuroendocrine neoplasms with mitotic count >20 per 10 high power fields (HPF) and/or Ki-67 index >20% are classified as NECs (4). Compared with well-differentiated NETs, NECs are more aggressive and are usually diagnosed at advanced stages. A Surveillance, Epidemiology, and End Results (SEER) database analysis of 162,983 cases disclosed that 60.8% have developed distant metastasis at diagnosis and median overall survival (OS) in untreated NEC patients is only 4–6 months (5,6).
Given that some of these neoplasms share similar immunohistochemical characteristics with small cell lung cancer (SCLC), chemotherapy of etoposide-platinum (EP), the standard of care for SCLC, is widely used in gastroenteropancreatic NECs (GEP-NECs) (7,8). Several retrospective studies evaluated the efficacy of EP as first-line chemotherapy in GEP-NECs and found a median response rate of 42.95% (28–62.5%), a median progression-free survival (PFS) of 5.65 months and a median OS of 11.67 months (9-11). However, according to National Comprehensive Cancer Network (NCCN) guidelines (12), the recommendation for EP regimen in GEP-NECs is only grouped into category 2A, which means the adoption of this regimen was based upon only low-level evidence, indicating that there is a lack of optimal treatment regimen for GEP-NECs. And the response rate and prognosis of GEP-NECs are even worse due to the molecular heterogeneity with SCLC (13). Other regimen, including temozolomide based chemotherapy, Folinic acid with fluorouracil and oxaliplatin (FOLFOX), Folinic acid with fluorouracil and irinotecan (FOLFIRI), were only covered in some small cohorts or under clinical trials (14-16). Immunotherapy and target therapy are feasible only in patients harboring certain gene mutations (12).
Meanwhile, it is noteworthy that in terms of cell morphology, not all GEP-NECs appear as pure small cells, adenocarcinoma or squamous cell carcinoma components could also be observed in GEP-NECs. In these so-called mixed neuroendocrine non-NECs (MiNECs) (4), squamous cell carcinoma components may exist in esophageal NECs while adenocarcinoma is found in gastric and colorectal NECs. This subtype and molecular differences between GEP-NECs and SCLC raise the problem of whether to adopt chemotherapy based on primary site of tumor (PST).
Prognostic factors have always been essential parts of clinical studies. Blood markers such as platelet count, neutrophil-to-lymphocyte (NLR) (17), lactate dehydrogenase (LDH) (5), and histopathological data such as Ki-67 proliferation index (5,6) have been confirmed useful in predicting treatment outcomes in lung, stomach and many other malignant tumors (18). However, the application value of these markers in GEP-NECs remains to be explored.
Given the paucity of cases and poor prognosis, the standard management and prognostic factors of GEP-NECs have not yet been defined. To improve our understanding of this rare tumor, we performed a retrospective and real-world study in our center to investigate the clinicopathological features of GEP-NEC patients. We also identified risk factors that influence prognosis and made a comparison of the effects of different first-line treatment regimens. We present this article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-64/rc).
Methods
Study subject and data collection
This retrospective and real-world study was conducted based on the medical records from January 2010 to December 2022 in The First Affiliated Hospital of Nanjing Medical University. The inclusion criteria were as follows: (I) pathologically diagnosed with GEP-NECs from biopsied or surgically resected specimens; (II) diagnosed at an advanced stage or with recurrence at least six months after adjuvant chemotherapy; (III) received chemotherapy with/without target therapy and immunotherapy; (IV) no other primary malignancies. Figure 1 summarizes the inclusion criteria of patients and the processing of data collected. Information on age, gender, symptoms at diagnosis, primary tumor location, stage at diagnosis, sites of metastases, morphology, baseline laboratory blood test results, treatment regimen, and clinical outcomes was reviewed. Tumor node metastasis (TNM) stage was assessed according to the seventh edition criteria of the American Joint Committee on Cancer (AJCC) staging system. Objective response rate (ORR; defined as the percentage of patients with complete or partial response), and disease control rate (DCR; defined as the percentage of patients with objective response and stable disease) were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. PFS was defined as the time from initiation of chemotherapy to confirmation of disease progression.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2022-SR-706) and individual consent for this retrospective analysis was waived.
Statistical analysis
The cut-off value of the NLR, platelet-to-lymphocyte ratio (PLR) and other factors for predicting PFS was calculated using R version 4.2.1 and the “survminer” package. PFS was estimated using the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariate analyses by COX proportional hazard models were performed, and the hazard ratio (HR) and the corresponding 95% confidence interval (95% CI) for PFS were calculated. P<0.05 was considered statistically significant. Statistical analysis was performed using R version 4.2.1 (The R Foundation, Vienna, Austria).
Results
Patient demographics
We retrospectively reviewed 54 patients diagnosed with GEP-NECs. The main clinicopathologic features of all enrolled patients in the study are summarized in Table 1. Forty-three (79.6%) patients were male and the median age at diagnosis was 65.5 (range, 35–81) years. The most common primary sites were esophagus (40.7%), followed by stomach (33.3%), colorectum (13.0%), and pancreas and hepatobiliary tract (13.0%). Forty-seven (87.0%) patients were diagnosed at stage IV and 7 (13.0%) recurred at least 6 months after adjuvant chemotherapy who previously underwent radical surgery. The most frequent site of distant metastasis was liver (51.9%) while brain metastasis was only seen in two patients. Among all the patients, no hormone-related symptoms were detected. Abdominal distention or pain (37.0%), dysphagia (37.0%), gastrointestinal bleeding (9.3%) and other clinical manifestations presented at diagnosis were related to tumor growth or metastases.
Table 1
| Characteristic | Data, N=54 |
|---|---|
| Age, years | 65.5 [35–81] |
| Gender | |
| Female | 11 (20.4) |
| Male | 43 (79.6) |
| Location | |
| Esophagus | 22 (40.7) |
| Stomach | 18 (33.3) |
| Colorectum | 7 (13.0) |
| Liver/gall bladder/pancreas | 7 (13.0) |
| Clinical manifestation | |
| Abdominal pain or distention | 20 (37.0) |
| Dysphagia | 20 (37.0) |
| Gastrointestinal bleeding | 5 (9.3) |
| Others/asymptomatic | 13 (24.1) |
| Hormonal symptoms | 0 (0) |
| Clinical stage at diagnosis | |
| Advanced | 47 (87.0) |
| Recurrenta | 7 (13.0) |
| Distant organ metastasis | |
| Liver | 28 (51.9) |
| Lung | 8 (14.8) |
| Bone | 6 (11.1) |
| Brain | 2 (3.7) |
| Pathology | |
| Pure NEC | 45 (83.3) |
| MiNEC | 9 (16.7) |
| Ki-67(%) | |
| ≥80 | 30 (56.6) |
| <80 | 20 (37.0) |
| NA | 4 (7.4) |
| Neuroendocrine markers | |
| Syn(+) (in 52 pts available) | 51 (98.1) |
| CgA(+) (in 47 pts available) | 27 (57.4) |
| CD56(+) (in 47 pts available) | 40 (85.1) |
Data are presented as n (%) or median [range]. a, at least 6 months after last cycle of adjuvant chemotherapy. NEC, neuroendocrine carcinoma; MiNEC, mixed neuroendocrine non-NEC; Syn, synaptophysin; CgA, chromogranin A.
Histologically, the average and median Ki-67 proliferation index was 78% and 80% (range, 30–95%, available in 50 patients). Neuroendocrine markers were positive for synaptophysin (Syn) in 98.1% of the 52 patients with data, chromogranin (CgA) in 57.4% of the 47 with data, and cluster of differentiation 56 (CD56) in 85.1% of the 47 with data. All three markers were positive in 23 patients. In terms of pathological type, 9 (16.7%) patients had MiNEC.
Treatment patterns and response
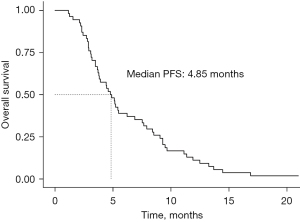
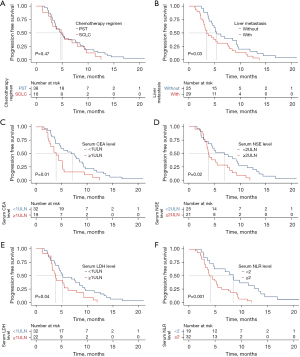
The efficacy of first-line chemotherapy according to treatment regimen is shown in Table 2. The median PFS for all the patients was 4.85 months (Figure 2). Thirty-eight (70.4%) patients were managed with EP that used for reference to first-line chemotherapy in advanced SCLC, while 16 (29.6%) counterparts received first-line chemotherapy based on primary site of the tumor, e.g., capecitabine + oxaliplatin for colorectal NEC patients, S1+oxaliplatin for gastric NEC patients, paclitaxel + platinum for esophageal NEC patients, etc. Median number of chemotherapy cycles was four for both cohorts. There were no statistically significant differences between these two regimens with regard to median PFS (4.75 vs. 4.98 months, P=0.47, Figure 3A), DCR (68.4% vs. 50.0%, P=0.20) and ORR (26.3% vs. 25.0%, P>0.99).
Table 2
| Characteristic | SCLC | PST | P value |
|---|---|---|---|
| Total number | 38 (70.4) | 16 (29.6) | – |
| No. of cycles | 4 [2–10] | 4 [1–7] | 0.75 |
| PFS (months) | 4.75 [1.57–21.5] | 4.98 [1.27–12.5] | 0.47 |
| Response rate | – | ||
| CR | 0 (0) | 0 (0) | – |
| PR | 10 (26.3) | 4 (25.0) | – |
| SD | 16 (42.1) | 4 (25.0) | – |
| PD | 12 (31.6) | 8 (50.0) | – |
| DCR | 68.4% | 50.0% | 0.20 |
| ORR | 26.3% | 25.0% | >0.99 |
Data are presented as n (%) or median [range]. SCLC, small cell lung cancer; PST, primary site of tumor; PFS, progression-free survival; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; DCR, disease control rate; ORR, objective response rate.
Prognostic factors of PFS
Results of univariate and multivariate analyses of prognostic factors of the whole cohort are shown in Table 3. According to univariate analysis, liver metastasis (with vs. without, median 3.50 vs. 5.43 months, HR 1.841, P=0.03, Figure 3B), elevated baseline serum carcinoembryonic antigen (CEA) level [≥1 upper limit of normal value (ULN) vs. <1ULN, median 3.93 vs. 7.22 months, HR 2.081, P=0.01, Figure 3C], elevated baseline serum neuron-specific enolase (NSE) level (≥2ULN vs. <2ULN, median 3.83 vs. 6.90 months, HR 1.430, P=0.02, Figure 3D), elevated baseline serum LDH level (≥1ULN vs. <1ULN, median 3.78 vs. 5.20 months, HR 1.792, P=0.04, Figure 3E) and elevated baseline serum NLR level (≥2 vs. <2, median 4.13 vs. 8.43 months, HR 2.838, P=0.001, Figure 3F) were significantly associated with poorer PFS, while there was no significant difference in PFS in terms of Ki-67 index (≥80% vs. <80, median 4.14 vs. 4.85 months, P=0.69), baseline serum CA199 level (≥1ULN vs. <1ULN, median 4.34 vs. 4.90 months, P=0.055), baseline serum PLR level (≥105 vs. <105, median 4.18 vs. 6.90 months, P=0.62), baseline serum alkaline phosphatase (ALP) level (≥1ULN vs. <1ULN, median 4.63 vs. 5.17 months, P=0.06), and combination of target therapy and/or immunotherapy (with vs. without, median 6.23 vs. 3.80 months, P=0.44).
Table 3
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥70 vs. <70 years) | 1.193 (0.645–2.204) | 0.57 | – | – | |
| Gender (male vs. female) | 1.339 (0.685–2.619) | 0.39 | – | – | |
| Liver metastasis (with vs. without) | 1.841 (1.052–3.223) | 0.03 | 1.595 (0.867–2.932) | 0.13 | |
| Ki-67 index (≥80% vs. <80%) | 1.122 (0.627–2.007) | 0.69 | – | – | |
| Baseline serum CEA level (≥1ULN vs. <1ULN) | 2.081 (1.146–3.780) | 0.01 | 1.415 (0.737–2.716) | 0.29 | |
| Baseline serum NSE levela (≥2ULN vs. <2ULN) | 1.430 (1.039–1.969) | 0.02 | – | – | |
| Baseline serum CA199 level (≥1ULN vs. <1ULN) | 2.063 (0.984–4.323) | 0.055 | – | – | |
| Baseline serum LDH level (≥1ULN vs. <1ULN) | 1.792 (1.007–3.188) | 0.04 | 0.926 (0.453–1.893) | 0.83 | |
| Baseline serum NLR level (≥2 vs. <2) | 2.838 (1.448–5.562) | 0.001 | 2.705 (1.183–6.184) | 0.01b | |
| Baseline serum PLR level (≥105 vs. <105) | 1.156 (0.646–2.067) | 0.62 | – | – | |
| Baseline serum ALP level (≥1ULN vs. <1ULN) | 1.857 (0.972–3.546) | 0.06 | – | – | |
| Combination therapyc (with vs. without) | 1.116 (0.454–1.769) | 0.44 | – | – | |
a, baseline serum NSE level was excluded from multivariate analysis because of lack of data for 8 patients; b, significant; c, combination therapy includes target therapy and/or immunotherapy. HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; ULN, upper limit of normal value; NSE, neuron-specific enolase; CA199, carbohydrate antigen 19-9; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALP, alkaline phosphatase.
A multivariate Cox proportion hazards model disclosed that elevated NLR (HR 2.705, 95% CI: 1.183–6.184, P=0.01) was a significant independent negative prognostic factor for GEP-NECs.
Discussion
The study aimed to analyze clinical and histological characteristics of GEP-NECs, as well as treatment regimens and several prognostic factors in a real-world setting.
Functional NETs have the ability to secret certain hormones like histamine and 5-hydroxytryptamine, thus inducing palpitation, flushing and many other carcinoid syndromes (19,20). However, the majority of NECs are clinically silent and are always presented with abdominal distention, bleeding and other gastrointestinal syndromes. Therefore, many patients may be wrongly treated as benign diseases or diagnosed at an advanced stage. These clinical characteristics also presented in our study: none of the patients complained of any hormone-related manifestations, which also attaches great importance to early diagnosis and surveillance.
Because of the rarity of disease and lack of large-scale prospective studies, there are no standard nor evidence-based chemotherapeutic recommendations for the treatment of GEP-NECs. When making treatment decisions, clinicians often follow the paradigm of SCLC due to morphological similarities. However, according to NCCN guideline (12), recommendation of chemotherapy regimen for EP is only classified as category 2A. Clinical data also show that even with the same regimen, parameters assessing clinical outcomes of NECs are much more unsatisfying than those in SCLC (13). The poor survival rate observed in locally advanced and metastatic settings challenges the treatment strategies, indicating that optimal therapy for GEP-NECs may be site-specific and different from SCLC (21). While in the current cohort, there is no statistical difference in treatment outcome indicators, such as PFS, DCR and ORR between patients receiving both chemotherapy strategies, suggesting that either regimen is optional.
On the other hand, only 2 (3.7%) patients in our study developed brain metastasis at diagnosis. However in SCLC, brain metastasis rates are much higher and carcinoid syndromes are more common (22). These features strongly suggest a certain degree of molecular divergence between GEP-NECs and SCLC.
Genomically, GEP-NECs disclose typical alterations of adenocarcinoma from the same sites of origin (23). For example in colorectal NECs, mutation rates of KRAS and BRAF range from 25% to 60% (24-26), making BRAF inhibitor a promising therapeutic strategy. GEP-NECs are highly vascularized tumors and are able to secrete vascular endothelial growth factor (VEGF), serving as a possible therapeutic target (27). A retrospective study conducted by Mishima et al. (28) focused on ramucirumab, a monoclonal antibody for VEGF receptor 2, which showed promising efficacy in gastric NECs, with ORR of 59% and median PFS of 7.7 months. In our cohort, however, the improvement of treatment outcomes could hardly be observed because only 13 (24.1%) patients received combination therapy of target and/or immunotherapy. Nonetheless, the combination therapy may serve as a promising treatment option according to the theory and mechanism mentioned above. As a result, the efficacy and safety of target therapy based on adenocarcinoma from the site of origin should be explored. Comprehensive tumor molecular profiling should be included in future large-scale prospective studies to identify novel therapeutic targets.
Apart from medical treatment, surgery and radiotherapy have been applied alone or in combination to improve survival in GEP-NECs patients. Surgery has been the primary curative treatment for early-stage malignant tumors (29). Even in metastatic diseases, palliative resection is indicated for preventing complications and reducing tumor burden (30). Liver metastasis is a negative prognostic factor in the current study, suggesting the significance of locoregional-directed therapy, including radiofrequency ablation and chemoembolization in cases with liver-predominant disease (29). To improve prognosis, a tailored treatment regimen is required for every single individual, thus precise pathological and molecular examinations are strongly recommended in the treatment of rare, advanced malignancies.
Furthermore, searching for prognostic factors is essential in the prediction of prognosis in malignant tumors, especially in highly-aggressive GEP-NECs. As a marker of cell proliferation, Ki-67 expression could predict prognosis to some extent. In the NORDIC study (5), PFS and OS in patients with Ki-67>55% significantly decreased while Lamarca et al. (31) identified 80% as a cut-off value for OS. In our cohort, given that the average and median Ki-67 index had already reached 78% and 80%, it was not statistically related to shortened PFS (P=0.607), reflecting the high aggressiveness of GEP-NECs.
Several studies have investigated serum markers as prognostic tools in other aggressive types of cancer (5,17,18,32). In our study, elevated baseline serum CEA, NSE, LDH and NLR levels were associated with shorter PFS. After multivariate analysis, elevated NLR level was the only independent negative prognostic factor. The inflammatory milieu within the tumor microenvironment plays a pivotal role in cancer development and progression, and NLR serves as a surrogate marker reflecting this delicate balance (33). Elevated NLR results from a relatively increased neutrophil count or depleted lymphocyte count and has been consistently linked to increased tumor aggressiveness, poorer response to therapy. Neutrophils may act as tumor-promoting leukocytes by secreting high levels of TGF-β, interleukin-18 (34), and VEGF (35), thus promoting invasion and angiogenesis. Conversely, diminished lymphocyte levels may compromise the anti-tumor immune response (36). The combination of these factors, as captured by NLR, offers a holistic view of the host’s immune status and tumor microenvironment.
While this study has a number of advantages and certainly complements the current evidence base, there were some limitations. First, as this is a small-scale and retrospective study based on a real-world setting, statistics such as OS are not available, thus relationships between first-line chemotherapy and OS are unclear. Second, biological data, including neuroendocrine markers and blood tumor markers, are not available for all patients, which decreases the power of the multivariate analysis. Third, gene test results are absent, indicating that molecular analyses are unable to be performed, which prevents us from evaluating the efficacy of target therapy and/or immunotherapy. The representativeness and accuracy of statistical analysis are compromised given the small sample size of the study. All these deficiencies should be considered in future studies.
Conclusions
GEP-NECs are extremely aggressive tumors with poor prognosis, and elevated NLR is an independent negative prognostic factor. In terms of treatment, chemotherapy regimens based on PST and SCLC are both optional. Future studies are required to establish optimum treatment protocols for GEP-NECs.
Acknowledgments
Funding: This research received financial support from
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-64/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-64/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-64/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-64/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2022-SR-706) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaliszewski K, Ludwig M, Greniuk M, et al. Advances in the Diagnosis and Therapeutic Management of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Cancers (Basel) 2022;14:2028. [Crossref] [PubMed]
- Schmitz R, Mao R, Moris D, et al. Impact of Postoperative Chemotherapy on the Survival of Patients with High-Grade Gastroenteropancreatic Neuroendocrine Carcinoma. Ann Surg Oncol 2021;28:114-20. [Crossref] [PubMed]
- Pellat A, Cottereau AS, Terris B, et al. Neuroendocrine Carcinomas of the Digestive Tract: What Is New? Cancers (Basel) 2021;13:3766. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013;24:152-60. [Crossref] [PubMed]
- Dasari A, Mehta K, Byers LA, et al. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018;124:807-15. [Crossref] [PubMed]
- Hainsworth JD, Spigel DR, Litchy S, et al. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol 2006;24:3548-54. [Crossref] [PubMed]
- Pellat A, Walter T, Augustin J, et al. Chemotherapy in Resected Neuroendocrine Carcinomas of the Digestive Tract: A National Study from the French Group of Endocrine Tumours. Neuroendocrinology 2020;110:404-12. [Crossref] [PubMed]
- Hadoux J, Afchain P, Walter T, et al. FOLFIRINEC: a randomized phase II trial of mFOLFIRINOX vs platinum-etoposide for metastatic neuroendocrine carcinoma of gastroenteropancreatic or unknown origin. Dig Liver Dis 2021;53:824-9. [Crossref] [PubMed]
- Yamaguchi T, Machida N, Morizane C, et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci 2014;105:1176-81. [Crossref] [PubMed]
- Luecke S, Fottner C, Lahner H, et al. Treatment Approaches and Outcome of Patients with Neuroendocrine Neoplasia Grade 3 in German Real-World Clinical Practice. Cancers (Basel) 2022;14:2718. [Crossref] [PubMed]
- Neuroendocrine and Adrenal Tumors. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). 2021.
- Terashima T, Morizane C, Hiraoka N, et al. Comparison of chemotherapeutic treatment outcomes of advanced extrapulmonary neuroendocrine carcinomas and advanced small-cell lung carcinoma. Neuroendocrinology 2012;96:324-32. [Crossref] [PubMed]
- Liu AJ, Ueberroth BE, McGarrah PW, et al. Treatment Outcomes of Well-Differentiated High-Grade Neuroendocrine Tumors. Oncologist 2021;26:383-8. [Crossref] [PubMed]
- Rogowski W, Wachuła E, Gorzelak A, et al. Capecitabine and temozolomide combination for treatment of high-grade, well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma - retrospective analysis. Endokrynol Pol 2019;70:313-7. [Crossref] [PubMed]
- Hadoux J, Kanaan C, Durand A, et al. Prognostic factors of metastatic neuroendocrine carcinoma under first-line treatment with platinum etoposide with a focus on NEC score and Rb expression: Results from the multicentre RBNEC study of the Groupe d'Etude des Tumeurs Endocrines (GTE) and the ENDOCAN-RENATEN network. Eur J Cancer 2021;152:100-15. [Crossref] [PubMed]
- Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med 2020;18:360. [Crossref] [PubMed]
- Freis P, Graillot E, Rousset P, et al. Prognostic factors in neuroendocrine carcinoma: biological markers are more useful than histomorphological markers. Sci Rep 2017;7:40609. [Crossref] [PubMed]
- Schott M, Klöppel G, Raffel A, et al. Neuroendocrine neoplasms of the gastrointestinal tract. Dtsch Arztebl Int 2011;108:305-12. [PubMed]
- Pavlidis ET, Pavlidis TE. Molecular factors, diagnosis and management of gastrointestinal tract neuroendocrine tumors: An update. World J Clin Cases 2022;10:9573-87. [Crossref] [PubMed]
- Garcia-Carbonero R, Anton-Pascual B, Modrego A, et al. Advances in the Treatment of Gastroenteropancreatic Neuroendocrine Carcinomas: Are we Moving Forward? Endocr Rev 2023;44:724-36. [Crossref] [PubMed]
- Megyesfalvi Z, Gay CM, Popper H, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin 2023;73:620-52. [Crossref] [PubMed]
- Frizziero M, Kilgour E, Simpson KL, et al. Expanding Therapeutic Opportunities for Extrapulmonary Neuroendocrine Carcinoma. Clin Cancer Res 2022;28:1999-2019. [Crossref] [PubMed]
- Venizelos A, Elvebakken H, Perren A, et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2021;29:1-14. [Crossref] [PubMed]
- Takizawa N, Ohishi Y, Hirahashi M, et al. Molecular characteristics of colorectal neuroendocrine carcinoma; similarities with adenocarcinoma rather than neuroendocrine tumor. Hum Pathol 2015;46:1890-900. [Crossref] [PubMed]
- Capdevila J, Arqués O, Hernández Mora JR, et al. Epigenetic EGFR Gene Repression Confers Sensitivity to Therapeutic BRAFV600E Blockade in Colon Neuroendocrine Carcinomas. Clin Cancer Res 2020;26:902-9. [Crossref] [PubMed]
- Fang JM, Li J, Shi J. An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol 2022;28:1009-23. [Crossref] [PubMed]
- Mishima S, Kawazoe A, Matsumoto H, et al. Efficacy and safety of ramucirumab-containing chemotherapy in patients with pretreated metastatic gastric neuroendocrine carcinoma. ESMO Open 2018;3:e000443. [Crossref] [PubMed]
- Liu DJ, Fu XL, Liu W, et al. Clinicopathological, treatment, and prognosis study of 43 gastric neuroendocrine carcinomas. World J Gastroenterol 2017;23:516-24. [Crossref] [PubMed]
- Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844-60. [Crossref] [PubMed]
- Lamarca A, Walter T, Pavel M, et al. Design and Validation of the GI-NEC Score to Prognosticate Overall Survival in Patients With High-Grade Gastrointestinal Neuroendocrine Carcinomas. J Natl Cancer Inst 2017; [Crossref] [PubMed]
- Grenader T, Waddell T, Peckitt C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 2016;27:687-92. [Crossref] [PubMed]
- Song M, Zhang Q, Song C, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle 2022;13:2504-14. [Crossref] [PubMed]
- Dharmapuri S, Özbek U, Lin JY, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med 2020;9:4962-70. [Crossref] [PubMed]
- Zhang Q, Song MM, Zhang X, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle 2021;12:1466-76. [Crossref] [PubMed]
- Grenader T, Nash S, Plotkin Y, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol 2015;26:1910-6. [Crossref] [PubMed]



