
Severe febrile neutropenia and pancytopenia in a patient with advanced hepatocellular carcinoma treated with atezolizumab and bevacizumab: a case report
Highlight box
Key findings
• We observed temporary severe febrile neutropenia and pancytopenia during systemic immunotherapy in a patient with unresectable hepatocellular carcinoma.
What is known and what is new?
• Immune checkpoint inhibitors (ICIs) can result in toxicities and unique adverse effects. This is the first case-report whereby the co-administration of atezolizumab and bevacizumab led to a case of pancytopenia.
What is the implication, and what should change now?
• Healthcare providers should consider hem-immune-related adverse events (hem-irAEs), in patients after the administration of ICIs. Patients with pancytopenia, hem-irAEs, and immune-related colitis should be treated with cefepime, filgrastim, metronidazole, and intravenous methylprednisolone.
Introduction
The emergence of immune checkpoint inhibitors (ICIs) paved the way for improved outcomes in the treatment of unresectable hepatocellular carcinoma (HCC) (1). For advanced disease, sorafenib was the only approved systemic therapy in the first-line setting for HCC by the United States Food and Drug Administration (FDA) until 2017 (2). Atezolizumab, a programmed cell death ligand-1 (PD-L1) antagonist and bevacizumab, a monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A) [atezolizumab plus bevacizumab (atezo/bev)] was established as the first-line standard of care for advanced HCC after the results of IMBrave150, which showed a 6-month improvement in survival when compared to sorafenib (3). Despite the survival advantages, these inhibitors can result in unique adverse events and toxicities classified as immune-related adverse events (irAEs) (4). While rare, the hematopoietic system can also be affected, leading to hematological irAEs (hem-irAEs), most commonly anemia and immune thrombocytopenia (5,6). Hem-irAEs, such as immune thrombocytopenia, pancytopenia, neutropenia, and cytokine-release syndrome, could occur at any time after ICIs therapy and do so in 5% of patients taking a PD-L1 antagonist (6). There is no agreed upon treatment regimen for patients with febrile neutropenia related to hem-irAEs. Only two case reports of febrile neutropenia attributed to atezolizumab monotherapy have been previously documented (7,8); and no cases attributed to the coadministration of atezo/bev have been reported.
Here, we report a case of febrile neutropenia and pancytopenia in a patient with HCC who received one dose of atezo/bev, which was successfully treated with filgrastim and antibiotic therapies. We present this case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-290/rc).
Case presentation
History and diagnosis
A male patient in his early 50s, with history of metabolic syndrome, alcoholism, and hypertension was diagnosed with unresectable HCC after experiencing a syncopal episode following a few days of right-upper quadrant pain. A computed tomography (CT) scan of the chest, abdomen, and pelvis showed a 4.0 cm × 3.5 cm hemorrhagic exophytic liver lesion in segment III as well as cirrhosis (Figure 1). Diagnostic laparoscopy showed numerous liver nodules and multifocal liver disease deeming the patient a non-surgical candidate. His initial labs showed normal bilirubin, albumin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels. The patient had a baseline white blood cell (WBC) count of 3,200 cells/µL with a lymphocyte percentage of 26% and a neutrophil percentage of 54%. His alpha-fetoprotein (AFP) level was 3.6 ng/mL and remained normal throughout. He was referred to the HCC clinic and was recommended to start first-line therapy with the combination of atezo/bev. Figure 2 summarizes the timeline of the patient’s clinical course. Ten days after his first and only infusion with atezo/bev, the patient developed a fever of 39.0 ℃, along with chills, vomiting, headache, fatigue, generalized pain, mild abdominal discomfort, and a burning rash on his face and neck. He presented to the emergency department 5 days after his symptoms began.
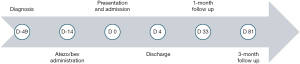
Presentation and admission
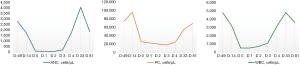
Upon presentation, vital signs indicated a mild fever of 37.6 ℃, a heart-rate of 127 beats per minute and a blood pressure reading of 145 mmHg/92 mmHg. On physical examination, the patient was observed to be well-developed, exhibited tachycardia while cardiovascular, pulmonary, and abdominal examinations were otherwise normal. Skin exam revealed erythematous macules and papules coalescing into patches and plaques across the anterior and posterior trunk, and face (Figure 3). The patient was admitted for further workup and treatment. Laboratory results showed severe neutropenia with an absolute neutrophil count (ANC) of 90 cells/µL, which dropped to 70 cells/µL on the second day of admission (Figure 4). This qualifies as a grade IV hem-irAE. Similarly, the patient had thrombocytopenia, with a platelet count (PC) of 23,000 cells/µL dropping to 18,000 cells/µL on the second day of admission, and anemia, with a hemoglobin level declining from 13.4 to 12.6 gm/dL in the same timeframe. The patient also had leukopenia with a WBC count of 500 cells/µL which remained steady on the second day of admission. Blood and urine cultures as well as respiratory viral panel were negative. CT scan of chest, abdomen, and pelvis, performed on first day of admission, showed diffuse submucosal edema in the region of the ascending and proximal transverse colon with evidence of stranding in the pericolonic fat, suggestive of colitis.
Treatment
The infectious disease team suggested that the fever and chills were most likely explained by cytokine release syndrome, induced by the immunotherapy treatment. Additionally, colitis was also explained as an immune-induced side-effect. The patient was given cefepime (2 g every 8 hours) and metronidazole (500 mg every 8 hours) for the febrile neutropenia until discharge. He was also given filgrastim (480 mcg), a recombinant form of the naturally occurring granulocyte colony-stimulating factor (G-CSF), for 3 consecutive days until neutropenia resolved. Additionally, he was given intravenous methylprednisolone (60 mg) to resolve the non-hematologic immunotherapy-related toxicities as well as to improve his ANC. This was changed to prednisone (50 mg) twice a day for 1 month after his discharge from the hospital. To resolve his burning rash, he was recommended triamcinolone (0.1%) topical cream on the neck twice a day as well as hydrocortisone (2.5%) topical cream on the face. On his fifth and final day at the hospital, the WBC count improved to 3,100 cells/µL while ANC improved to 1,830 cells/µL (Figure 3). Additionally, his PC increased to 25,000 cells/µL and his hemoglobin count to 13.8 gm/dL. Both cefepime and metronidazole were discontinued upon discharge, and he was prescribed ciprofloxacin (750 mg every 12 hours) for 5 days. Importantly, his clinical symptoms greatly improved, with resolution of his rash, fatigue, myalgias, and colitis symptoms.
Follow-up
On his 1-month follow-up, the patient reported doing well and had a normal physical examination and a complete resolution of his rash. He reported no adverse events from the treatments received at the hospital. The WBC count was 4,800 cells/µL, ANC 4,080 cells/µL, hemoglobin 14.3 g/dL, and PC 55,000 cells/µL. Finally, his scans showed further significant decrease in the segment III liver tumor. Three months after his only dose of atezo/bev, the patient’s tumor measured 3 cm, signifying a reduction of 25% (Figure 5). His blood counts and clinical assessment were within normal range at his 3-month follow-up as well. The patient was deemed a surgical candidate and successfully underwent curative resection. His surgical pathology report showed a tumor necrosis of approximately 85%, with visible tumor size approximately 1 cm and no definitive lympho-vascular invasion.

The patient gave informed consent via the MyChart Application, as well as through the phone when obtaining his patient perspective verbatim. Additionally, the patient is included in our chart review/retrospective review protocol ‘Prognostic Factors Analysis of Hepatocellular Carcinoma: Epidemiological Assessment’ (RCR03-0289). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Patient perspective
Before coming to the hospital, I felt like I had the flu. My mouth was super dry, and I had a complete loss of appetite. I felt completely beat-down and every joint I had was hurting. After a few days of treatment, I felt much better and the minute I regained my appetite, no longer had a dry mouth, and had my saliva back, I knew I was back to normal. The treatment, and overall, my stay at the hospital was very helpful, and it led me to where I am today, so I am very grateful for that.
Discussion
Hem-irAEs associated with ICIs
In patients receiving ICIs, skin, endocrine, and gastrointestinal disorders are the most common irAEs (8). In a review assessing several ICI clinical trials, the frequency of hem-irAEs was found to be 3.6% for all grades and 0.7% for grades III–IV (6). The review found that hem-irAEs could occur at any time after ICI therapy, and they are generally highly severe adverse reactions with a mortality rate of 14% (6). ICIs cause irAEs as a result of overactive T lymphocytes, autoantibody production, and cytokine dysregulation. Meanwhile, hematologic toxicities are rare and of uncertain mechanism (9). Several case reports of ICI-induced febrile neutropenia have been documented and were recently summarized in another review (10). Specifically, 8 cases were attributed to pembrolizumab, a programmed cell death protein-1 (PD-1) inhibitor, six cases to nivolumab, also a PD-1 inhibitor, three cases to ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, one case to nivolumab + ipilimumab combination therapy, and one case to durvalumab, a PD-L1 inhibitor (10). None of the patients had HCC; instead, the majority were diagnosed with lung cancer, comprising more than half of the cases, with melanoma and bladder cancer being the subsequent most prevalent diagnoses.
Hem-irAEs in atezolizumab monotherapy or atezo/bev combination therapy
Two case reports in lung cancer have highlighted the potential for atezolizumab monotherapy to induce febrile neutropenia, with both cases demonstrating severe neutropenia, reaching 0 cells/µL in one instance (7) and 367 cells/µL in the other (8). Notably, neutropenia developed approximately 3 weeks after immunotherapy treatment in both situations. In contrast, our patient experienced fever 10 days later, with laboratory results revealing neutropenia 2 weeks following the administration of his sole therapy dose. During the IMBrave150 trial, none of the patients receiving atezo/bev for treatment of advanced HCC were reported to develop neutropenia or pancytopenia. The only hem-irAE reported was thrombocytopenia in 10.6% of patients. The most common adverse events reported were hypertension, fatigue, proteinuria, and pruritis (3).
Treatment of hem-irAEs
Our case showed that, in addition to halting immunotherapy, antibiotic, and filgrastim therapies were effective in the treatment of febrile neutropenia caused by atezo/bev administration. This was similar to the management reported in other cases of atezolizumab-induced febrile neutropenia (7,8). While the American Society of Clinical Oncology recommends discontinuing ICIs when grade IV irAEs are encountered (11) and administering antibiotics for febrile neutropenia (11,12), the use of therapeutic G-CSF remains controversial. A meta-analysis of fourteen randomized trials highlighted that the use of CSFs did not significantly improve mortality as compared with the use of antibiotics alone; however, individuals who received CSFs had a significantly shorter duration of hospitalization, neutropenia, fever, and antibiotics use (13). Our case was notable for the use of additional systemic corticosteroids, both during hospitalization and for a month post-discharge, effectively managing immunotherapy-related toxicities such as rash and colitis.
Correlation between irAEs and response from ICIs
This case added to the existing literature whereby the development of irAEs appears to be associated with better outcomes in cancer patients (14-17). Remarkably, in our case, only one dose of atezo/bev led to 85% necrosis. It has been reported that patients with grade ≥3 irAEs were more likely to have response to treatment than those with no or low-grade irAEs (14). Several studies have noted the trend in HCC (18,19).
Strengths and limitations
The strengths of this case stem from its unique nature and rarity. Notably, there have not been any previously reported cases of febrile neutropenia and pancytopenia in HCC patients receiving atezo/bev combination therapy. With HCC cases rising and with the recent approval of more immunotherapies in HCC, healthcare providers should be wary of the development of hem-irAEs. The data presented was thorough as the medical history, symptoms, diagnostic tests, treatment, and outcomes were all available in the patient’s charts. The significant and swift improvement of the patient in this case report can guide healthcare professionals to treat febrile neutropenia due to ICIs with antibiotics and filgrastim until ANC recovery.
Considering that such clinical setting is not frequent, we acknowledge that the findings may not apply to all patients with similar conditions. The presence of immunotoxicities such as rash and colitis that were treated with systemic corticosteroids add to the complexity + of our findings after treating febrile neutropenia and pancytopenia. Several other case reports of febrile neutropenia and pancytopenia acknowledged that corticosteroids were not given to the patients to avoid the suppression of their immune systems (7,8), but in our case, corticosteroids were given to decrease the symptoms caused by the immunotherapy-related toxicities.
Conclusions
In conclusion, we report an unusual case of a patient with unresectable HCC who developed severe febrile neutropenia and pancytopenia caused by atezo/bev combination therapy. The patient had an ANC count of 90 cells/µL at his nadir but this level improved after treatment with antibiotics and filgrastim, as well as cessation of immunotherapy. At his 1- and 3-month follow-ups, the patient reported feeling well and his blood counts had returned to pre-treatment levels.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-290/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-290/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-290/coif). A.O.K. reports that he received the NCI SPORE grant (No. NCI R01, CA260872-01). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Doorn DJ, Takkenberg RB, Klümpen HJ. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: An Overview. Pharmaceuticals (Basel) 2020;14:3. [Crossref] [PubMed]
- Coffman-D'Annibale K, Xie C, Hrones DM, et al. The current landscape of therapies for hepatocellular carcinoma. Carcinogenesis 2023;44:537-48. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Oliveira C, Mainoli B, Duarte GS, et al. Immune-related serious adverse events with immune checkpoint inhibitors: Systematic review and network meta-analysis. Eur J Clin Pharmacol 2024;80:677-84. [Crossref] [PubMed]
- Moore DC, Elmes JB, Arnall JR, et al. PD-1/PD-L1 inhibitor-induced immune thrombocytopenia: A pharmacovigilance study and systematic review. Int Immunopharmacol 2024;129:111606. [Crossref] [PubMed]
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer 2019;122:72-90. [Crossref] [PubMed]
- Seguchi K, Nakashima K, Terao T, et al. Febrile neutropenia in a patient with non-small-cell lung cancer treated with atezolizumab: A case report. Respir Med Case Rep 2021;33:101439. [Crossref] [PubMed]
- Kanno R, Saito Y, Takekuma Y, et al. Temporary Severe Neutropenia during Administration of Atezolizumab: A Novel Case Report. Case Rep Oncol 2023;16:372-7. [Crossref] [PubMed]
- Kroll MH, Rojas-Hernandez C, Yee C. Hematologic complications of immune checkpoint inhibitors. Blood 2022;139:3594-604. [Crossref] [PubMed]
- Jalil A, Zaffar J, Waqas A, et al. Isolated Neutropenia Due to Immune Checkpoint Inhibitors. Cureus 2023;15:e45674. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Lucas AJ, Olin JL, Coleman MD. Management and Preventive Measures for Febrile Neutropenia. P T 2018;43:228-32. [PubMed]
- Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev 2014;2014:CD003039. [PubMed]
- Sung M, Zer A, Walia P, et al. Correlation of immune-related adverse events and response from immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. J Thorac Dis 2020;12:2706-12. [Crossref] [PubMed]
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016;22:886-94. [Crossref] [PubMed]
- Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology 2016;5:e1231292. [Crossref] [PubMed]
- Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825-30. [Crossref] [PubMed]
- Xu S, Lai R, Zhao Q, et al. Correlation Between Immune-Related Adverse Events and Prognosis in Hepatocellular Carcinoma Patients Treated With Immune Checkpoint Inhibitors. Front Immunol 2021;12:794099. [Crossref] [PubMed]
- Zhou JM, Xiong HF, Chen XP, et al. Correlation between immune-related adverse events and long-term outcomes in pembrolizumab-treated patients with unresectable hepatocellular carcinoma: A retrospective study. World J Gastrointest Oncol 2023;15:689-99. [Crossref] [PubMed]




