
Survival outcomes and prognostic factors of advanced gastrointestinal stromal tumors: in the era of multiple tyrosine kinase inhibitors
Highlight box
Key findings
• Younger age, a single lesion at enrollment, no previous use of tyrosine kinase inhibitors (TKIs), a smaller tumor burden, good Eastern Cooperative Oncology Group performance status, and lesions limited to the liver were associated with better survival of patients with advanced gastrointestinal stromal tumors (GISTs).
What is known and what is new?
• Some prognostic factors of advanced GIST are known in the era of imatinib.
• We additionally found that a single lesion at enrollment, no previous use of TKIs, a smaller tumor burden, and lesions limited to the liver were associated with better survival.
What is the implication, and what should change now?
• In the era of multiple TKIs, the prognostic factors for advanced GIST may have undergone alterations, and more exploration is needed.
Introduction
Gastrointestinal stromal tumors (GISTs), which arise from pluripotent mesenchymal cells and undergo differentiation into interstitial cells of Cajal, represent the most prevalent malignant mesenchymal tumors affecting the gastrointestinal tract (1). Nevertheless, they are infrequent neoplasms, with a global annual incidence ranging from 10 to 15 cases per million population. The median age at diagnosis is approximately 60 years (2). At initial diagnoses, metastatic disease is observed in approximately 15–47% of patients, commonly involving the liver, peritoneum, and omentum (3).
GISTs exhibit resistance to conventional cytotoxic treatments commonly used for other sarcomas. In 1998, Hirota et al. elucidated the pivotal molecular mechanism in GIST, characterized by the gain-of-function mutations of c-KIT (4). Approximately 85% of those driver alterations are understood to involve activating mutations in either KIT or platelet-derived growth factor receptor alpha (PDGFRA). Nearly 99% of GISTs manifest a discernible, specific driver alteration that imparts unique molecular and biological characteristics. This molecular understanding has led to modifications in the therapeutic strategies for this tumor (5,6).
Imatinib, a small molecule tyrosine kinase inhibitor (TKI) that targets KIT and PDGFRA, was introduced in 2002 for the treatment of advanced GISTs (7). After the introduction of imatinib, the median overall survival (mOS) for patients with advanced GISTs was estimated to increase from 12 months to 4–5 years, with a 10-year OS rate ranging between 10–20% (5). GISTs have since become a paradigm for the successful targeted drug treatment of solid tumors. However, resistance to imatinib is a formidable challenge. Approximately 10% of patients exhibit primary resistance to imatinib, while around 80% of patients experience acquired resistance and disease progression during treatment (1,8). For patients with advanced GISTs who experience imatinib treatment failure, sunitinib and regorafenib have been successively approved as second and third-line treatment drugs. Moreover, different TKIs demonstrated varying efficacy against different gene mutation types. For instance, patients with PDGFRA D842V mutation show primary resistance to imatinib but respond well to avapritinib, making it a first-line treatment option for them (9).
In the new era of multiple TKIs, the prognostic factors for patients with advanced GIST may have undergone alterations, so the identification of prognostic factors for patients is of paramount importance, potentially offering valuable insights for guiding clinical treatment and prognostic assessments. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-63/rc).
Methods
Patients
There are a total of 1,004 GIST patients in our database which were obtained from the First Affiliated Hospital of Chongqing Medical University (CMU; Chongqing, China) from January 2010 to July 2023, and a total of 194 patients were finally included in the cohort after screening. All patients had received a confirmed histopathologic diagnosis of advanced GISTs and remained tumor-bearing at the end of follow-up. The criteria for inclusion of patients were as follows: (I) patients with primary unresectable or recurrent (metastatic/non-metastatic) advanced GISTs; (II) complete clinical records and follow-up information available; (III) age at diagnosis >18 years. Exclusion criteria were as follows: (I) patients with advanced GISTs who underwent surgical resection during follow-up and did not experience recurrence or had a recurrence interval >12 months; (II) patients with concurrent other malignancies; (III) no measurable lesions on imaging. It is worth noting that, patients who had undergone complete surgical resection during follow-up and who had not experienced recurrence or recurrence intervals >12 months during follow-up were excluded because defining their cure status through imaging would be challenging, and clinicians also typically do not recommend discontinuing TKI therapy in such cases. Some patients who have received surgical resection (R0/R1/R2) for progressive disease (PD), partial response (PR), or tumor complications but experienced rapid recurrence postoperatively were still included in the cohort. Surgical decisions were made jointly by the multidisciplinary team and the patients. The inclusion and exclusion process is shown in the flowchart (Figure 1).
Data collection and follow-up
The data collected included the patient’s age and sex, previous surgical treatment (no or yes), stage at diagnosis (single or multifocal lesions), Eastern Cooperative Oncology Group performance status (ECOG PS), prior treatment, primary tumor site, location of disease, tumor burden, genotype mutations, treatment approaches, and survival status. Patient follow-up was handled mainly by the attending physician’s outpatient service or by telephone, or online contact. We established an online database “Weinichangzai”, into which specialized clinical assistants prospectively input patient data. This study received approval from the Institutional Review Board of the First Affiliated Hospital of CMU (approval No. 2022-K364) with an exemption from obtaining written informed consent due to its observational and retrospective nature. The study adhered to the principles of the Declaration of Helsinki (as revised in 2013).
Definitions
The main indicator in this analysis was OS, which was defined as the time from diagnosis of advanced GIST to death resulting from any cause. Progression-free survival (PFS) is defined as the time from diagnosis of advanced GIST to tumor progression or death. Some patients were excluded from the analysis of PFS, mainly because: (I) incomplete information, making it impossible to obtain the PFS time of patients; (II) duration of drug treatment <1 month; (III) patients discontinued medication due to overall deterioration in condition. Patients who discontinued medication due to adverse drug reactions were considered as censored data. “Advanced” was defined as primary unresectable or recurrent unresectable GISTs (with or without metastasis). Unresectable tumors encompassed those accompanied by metastasis or those with no accompanying metastasis, but with involvement of critical blood vessels or organs, for which surgical resection would likely be highly invasive and risky (e.g., requiring a pancreaticoduodenectomy). All the patients with a primary tumor had not received TKIs before being included in the cohort. Of patients with recurrent tumors, some had not received adjuvant TKI therapy after surgery; some had experienced relapse after TKI withdrawal; and others had relapsed while receiving a TKI therapy (“prior treatment”). Patients who had undergone R2 cytoreductive resection for emergencies such as perforation, obstruction, tumor rupture, or bleeding at the first visit were considered to have no “prior surgical treatment”, with the time of follow-up being calculated starting from the first administration of TKI after surgery. Tumor burden was defined using the Response Evaluation Criteria in Solid Tumours 1.1 (the sum of the diameters of all target lesions including no more than five in total and no more than two per organ) (10). All patients had measurable lesions on baseline computed tomography. In terms of disease localization, peritoneal involvement was characterized as tumors situated within the abdominal and pelvic organs (excluding the liver, but including the digestive tract, peritoneum, omentum, and mesentery). “Local treatment” was defined as therapeutic interventions such as radiotherapy, transarterial chemoembolization (TACE), and radiofrequency ablation (RFA).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics (version 27: IBM, Armonk, NY, USA) and R (version 4.2.2. The R Foundation for Statistical Computing, Vienna, Austria). The distributions of variables in the population are presented as descriptive statistics. Normally distributed continuous variables are expressed as means with standard deviation, and categorical variables are expressed as n (%). The median duration of follow-up was calculated using the reverse Kaplan-Meier method, censored at death. OS was analyzed using the Kaplan-Meier survival curves. Log-rank tests were performed to assess statistically significant differences in OS. Cox proportional hazards regression models were used in the independent risk factor analyses. Each potential variable was initially assessed by univariable analysis. To comprehensively investigate relevant prognostic factors, variables with a P value of <0.2 in univariable analysis or those believed to have a significant effect on survival were included in the multivariable analysis. The proportional hazards assumption was tested using Kaplan-Meier curves and negative logarithmic curve diagrams. In our study, sex did not meet the proportional hazards assumption. Despite this, as gender has been identified as a meaningful prognostic factor in several previous studies, we treated it as a potential confounding factor and included it in the final model. Eventually, the variables included in the final model were the patient’s sex, and age, stage at diagnosis, prior treatment, ECOG PS, tumor burden, location of disease, and local treatment. Testing for collinearity revealed no multicollinearity between the variables in the final model. Results were considered statistically significant at P<0.05 and are presented as hazard ratios (HRs), with corresponding 95% confidence intervals (CIs).
Results
Patient characteristics
Table 1 summarizes the baseline characteristics of patients. The mean age in this cohort (38.7% women, 61.3% men) was 57±13 years. Of the patients, 40.7% have not received surgical treatment before, and 59.3% have received surgery before. Regarding the stage at diagnosis, a single lesion was found in 25.8% of patients, and multifocal lesions in 74.2%. Most of the patients (84.0%) had an ECOG PS of ≤1. In terms of tumor burden, 53.6% had a burden of ≤10 cm, while 46.4% had a burden of >10 cm. With respect to prior TKI treatment, 65.5% of the patients had not previously received TKI treatment; 12.4% had experienced tumor recurrence after withdrawal of TKIs; and 22.2% had experienced tumor recurrence during TKI treatment. The most prevalent mutation was c-KIT 11 (63.9%), followed by c-KIT 9 (24.7%); and two patients harbored PDGFRA mutations, one with the PDGFRA 18 mutation and one with the D842V mutation. In this cohort, 59.8% of the patients had peritoneum involvement only; 12.4% had liver involvement only; and 27.8% had both peritoneum and liver involvement. The most common primary tumor site was the small intestine (53.6%), followed by the stomach (27.3%), pelvic/abdominal cavity, and colorectum.
Table 1
| Characteristic | N (%) |
|---|---|
| Age, years, mean ± SD | 57±13 |
| Sex | |
| Female | 75 (38.7) |
| Male | 119 (61.3) |
| Stage at diagnosis | |
| Single lesion | 50 (25.8) |
| Multifocal lesions | 144 (74.2) |
| Previous surgical treatment | |
| No | 79 (40.7) |
| Yes | 115 (59.3) |
| Prior treatment | |
| No prior use of TKIs | 127 (65.5) |
| Recurrence after withdrawal of TKIs | 24 (12.4) |
| Recurrence during the TKIs treatment | 43 (22.2) |
| ECOG PS | |
| ≤1 | 163 (84.0) |
| ≥2 | 31 (16.0) |
| Year of diagnosis | |
| 2010–2016 | 47 (24.2) |
| 2017–2019 | 77 (39.7) |
| 2020–2023 | 70 (36.1) |
| Tumor burden, cm | |
| ≤10 | 104 (53.6) |
| >10 | 90 (46.4) |
| Location of disease | |
| Liver only | 24 (12.4) |
| Peritoneum only | 116 (59.8) |
| Peritoneum + liver | 54 (27.8) |
| Primary tumor sites | |
| Stomach | 53 (27.3) |
| Small intestine | 104 (53.6) |
| Colorectal | 14 (7.2) |
| Pelvic/abdominal cavity | 20 (10.3) |
| Other | 3 (1.5) |
| Manifestations of primary tumors | |
| Abdominal pain | 59 (30.4) |
| Gastrointestinal hemorrhage | 36 (18.6) |
| Abdominal distention | 29 (14.9) |
| Imaging examination | 25 (12.9) |
| Abnormal defecation | 6 (3.1) |
| Paruria | 6 (3.1) |
| Vomit | 5 (2.6) |
| Nausea | 3 (1.5) |
| Other | 6 (3.1) |
| Unknown | 19 (9.8) |
| Mutation | |
| c-KIT 11 | 124 (63.9) |
| c-KIT 9 | 48 (24.7) |
| Other | 14 (7.2) |
| c-KIT 13 | 2 (1.0) |
| Wild | 9 (4.6) |
| PDGFRA (18/D842V) | 2 (1.0) |
| Unknown | 9 (4.6) |
SD, standard deviation; TKIs, tyrosine kinase inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status; PDGFRA, platelet-derived growth factor receptor alpha.
Treatment
All patients received TKI therapy during follow-up, with 72.2% (140/194) receiving systemic treatment solely with TKIs; 14.9% (29/194) receiving surgical intervention in addition to TKIs treatment; 6.2% (12/194) receiving local treatment and TKIs; and only 6.7% (13/194) receiving local treatment, TKIs and surgical resection (Table 2). For a better understanding, we further categorized the data, observing that 21.6% of patients underwent surgery (R0/R1/R2) during follow-up and that 12.9% received local treatment, which consisted of radiotherapy in eight patients. The results showed that 39.2% of patients received only first-line treatment (imatinib) during follow-up, 20.1% received only second-line treatment (imatinib + sunitinib), and 18% received only third-line treatment (imatinib + sunitinib + regorafenib); 43.8%, 20.1%, 24.2%, 9.8%, and 2.1% of the patients used one to five types of TKI drugs respectively, with 56.2% of the patients receiving two or more different TKIs.
Table 2
| Characteristic | N (%) |
|---|---|
| Treatment | |
| TKIs only | 140 (72.2) |
| TKIs + surgical resection | 29 (14.9) |
| TKIs + local treatment | 12 (6.2) |
| TKIs + surgical resection+ local treatment | 13 (6.7) |
| Total TKIs during follow-up | |
| 1 | 85 (43.8) |
| 2 | 39 (20.1) |
| 3 | 47 (24.2) |
| 4 | 19 (9.8) |
| 5 | 4 (2.1) |
| Total TKIs† | |
| ≤1 | 85 (43.8) |
| ≥2 | 109 (56.2) |
| Treatment line | |
| Line 1 (imatinib only) | 76 (39.2) |
| Line 2 (imatinib+ sunitinib only) | 39 (20.1) |
| Line 3 (imatinib+ sunitinib+ regorafenib only) | 35 (18.0) |
| Specific TKIs | |
| Imatinib | 183 (94.3) |
| Sunitinib | 106 (54.6) |
| Regorafenib | 58 (29.9) |
| Ripretinib | 33 (17.0) |
| Famitinib | 11 (5.7) |
| Anlotinib | 6 (3.1) |
| Avaprinib | 3 (1.5) |
| Surgical resection | |
| No | 152 (78.4) |
| Yes | 42 (21.6) |
| Radiotherapy | |
| No | 186 (95.9) |
| Yes | 8 (4.1) |
| Local treatment | |
| No | 169 (87.1) |
| Yes | 25 (12.9) |
| TKIs only | |
| No | 54 (27.8) |
| Yes | 140 (72.2) |
†, regrouped for a clear presentation. TKIs, tyrosine kinase inhibitors.
When the specific TKIs administered were analyzed, 94.3% of the patients were found to have received imatinib; 54.6% sunitinib; 29.9% regorafenib; 17.0% ripretinib; 5.7% famitinib; 3.1% anlotinib; and 1.5% avapritinib, respectively.
Univariable and multivariable analyses
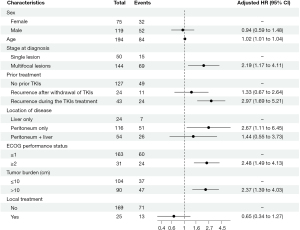
Univariable analysis showed that patient age, prior treatment, ECOG PS, tumor burden, and location of disease were significantly associated with OS. However, sex, patient type, and treatment approach were not associated with significant differences in OS (Table 3).
Table 3
| Characteristic | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex | |||||||
| Female | 1.0 | 1.0 | |||||
| Male | 0.86 | 0.55–1.34 | 0.50 | 0.94 | 0.59–1.48 | 0.78 | |
| Age, years | 1.03 | 1.01–1.04 | 0.002* | 1.02 | 1.01–1.04 | 0.009* | |
| Stage at diagnosis | |||||||
| Single lesion | 1.0 | 1.0 | |||||
| Multifocal lesions | 1.61 | 0.92–2.81 | 0.10 | 2.19 | 1.17–4.11 | 0.02* | |
| Previous surgical treatment | |||||||
| No | 1.0 | ||||||
| Yes | 1.15 | 0.74–1.79 | 0.54 | ||||
| Prior treatment | |||||||
| No prior TKIs | 1.0 | ||||||
| Recurrence after withdrawal of TKIs | 1.28 | 0.66–2.48 | 0.46 | 1.33 | 0.67–2.64 | 0.41 | |
| Recurrence during the TKIs treatment | 1.90 | 1.16–3.10 | 0.01* | 2.97 | 1.69–5.21 | <0.001* | |
| Location of disease | |||||||
| Liver only | 1.0 | 1.0 | |||||
| Peritoneum + liver | 2.18 | 0.94–5.04 | 0.07 | 1.44 | 0.55–3.73 | 0.46 | |
| Peritoneum only | 2.27 | 1.03–5.02 | 0.043* | 2.67 | 1.11–6.45 | 0.03* | |
| ECOG PS | |||||||
| ≤1 | 1.0 | 1.0 | |||||
| ≥2 | 3.64 | 2.24–5.90 | <0.001* | 2.48 | 1.49–4.13 | <0.001* | |
| Tumor burden, cm | |||||||
| ≤10 | 1.0 | 1.0 | |||||
| >10 | 2.22 | 1.43–3.45 | <0.001* | 2.37 | 1.39–4.03 | 0.002* | |
| Treatment | |||||||
| TKIs only | 1.0 | ||||||
| TKIs + surgical resection | 1.16 | 0.67–1.98 | 0.60 | ||||
| TKIs + local treatment | 0.76 | 0.32–1.77 | 0.52 | ||||
| TKIs + surgical resection+ local treatment | 0.65 | 0.29–1.45 | 0.30 | ||||
| TKIs only | |||||||
| No | 1.0 | ||||||
| Yes | 1.10 | 0.70–1.72 | 0.68 | ||||
| Surgical resection | |||||||
| No | 1.0 | ||||||
| Yes | 0.99 | 0.62–1.58 | 0.96 | ||||
| Local treatment | |||||||
| No | 1.0 | 1.0 | |||||
| Yes | 0.67 | 0.37–1.23 | 0.20 | 0.65 | 0.34–1.27 | 0.21 | |
| Radiotherapy | |||||||
| No | 1.0 | ||||||
| Yes | 0.60 | 0.22–1.64 | 0.32 | ||||
*, P<0.05. HR, hazard ratio; CI, confidence interval; TKIs, tyrosine kinase inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status.
Testing for collinearity revealed no multicollinearity between the included variables. The multivariable analysis demonstrated that age (increase per 1 year, HR 1.02, 95% CI: 1.01–1.04, P=0.009), multifocal lesions (HR 2.19, 95% CI: 1.17–4.11, P=0.02), ECOG PS ≥2 (HR 2.48, 95% CI: 1.49–4.13, P<0.001), tumor burden >10 cm (HR 2.37, 95% CI: 1.39–4.03, P=0.002), recurrence during TKI treatment (HR 2.97, 95% CI: 1.69–5.21, P<0.001), and lesions confined solely to the peritoneum ( HR 2.67, 95% CI: 1.11–6.45, P=0.03) were independent risk factors for OS in patients with advanced GISTs (Table 3, Figure 2).
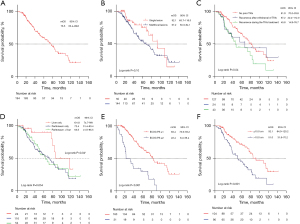
OS and PFS
For the 194 patients enrolled in the analysis, the median follow-up was 59.9 months (range, 2.7–141.7 months), during which 84 (43.3%) deaths occurred. The mOS was 76.5 months (95% CI: 63.4–89.6 months), with 1-, 3-, and 5-year survival rates of 97% (95% CI: 95–100%), 76% (95% CI: 70–83%), and 59% (95% CI: 52–68%), respectively. A total of 184 patients had identified gene mutations. The mOS of patients with c-KIT 11, c-KIT 9, and other mutations were 76.5 (95% CI: 59.0–94.0) months, 61.6 (95% CI: 40.3–82.9) months, and not reached, respectively (log-rank P=0.23). OS curves were plotted for each independent prognostic factor (Figure 3).
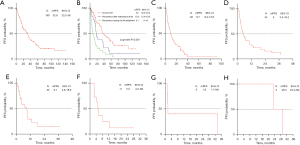
A total of 183 patients received imatinib during the follow-up, among whom 169 patients had evaluable PFS. The median PFS (mPFS) of imatinib was 32.8 (95% CI: 23.2–38) months. Similar to OS, for patients experiencing recurrence during imatinib treatment, continuing imatinib therapy or escalating the dose of imatinib tends to yield worse mPFS compared to those who experienced recurrence after withdrawal of imatinib or in patients who have not previously received imatinib therapy (log-rank P<0.001). For sunitinib, regorafenib, ripretinib, famitinib, anlotinib, and avapritinib, there were 92, 54, 32, 11, 6, and 3 patients available for PFS analysis, respectively. The mPFS for each drug was as follows: sunitinib 9.7 (95% CI: 8.4–13.8) months, regorafenib 5 (95% CI: 3.5–10.2) months, ripretinib 9.1 (95% CI: 4.6–18.6) months, famitinib 5.3 (95% CI: 4.3–NA) months, avapritinib 26.5 (95% CI: 23.4–NA) months, and anilotinib 1.8 (95% CI: 1.7–NA) months (Figure 4).
Discussion
Reports specifically assessing the prognosis for long-term survival in patients with advanced GISTs in the new era of multiple TKIs are limited. Here, we report the results of a single-center study in China.
Epidemiology shows that GIST predominantly occurs in the stomach, followed by the small intestine (2). However, in our study, the most common primary tumor site was found to be in the small intestine. This may be attributed to the fact that our study primarily focused on advanced-stage GIST. Studies have reported a higher risk of recurrence in non-gastric GIST compared to gastric GIST (11). Therefore, it can be speculated that small intestinal GISTs are more likely to progress to an advanced stage. This may explain the findings observed in our study.
Table 4 summarizes some clinical trials and other relevant studies that mention prognosis in advanced GIST in the imatinib era (12-19). The mOS for patients with advanced GIST at our center was 76.5 months (6.4 years), which aligns closely with the findings of Hompland et al. (6.9 years) and is slightly inferior to the results reported by Kim et al. and Patrikidou et al., who reported 8.8 and 8.3 years, respectively. Our mOS is also slightly longer than those reported in several other studies. Collectively, those observations underscore the substantial heterogeneity in survival outcomes for patients with advanced GIST.
Table 4
| Study | Year | Patients (n) | Median follow-up (years) | mOS (years) | Independent prognostic factors for OS (P<0.05)* | HR (95% CI) |
|---|---|---|---|---|---|---|
| Hompland (12) | 2017 | 115 | 9 | 6.9 | Number of metastasis (polymetastatic ≥4 vs. ≤3) | 6.8 (2.6–17.8) |
| Max size of the largest tumor (≥5 vs. <5 cm) | 1.8 (1.0–3.2) | |||||
| Performance status (ECOG >1 vs. ≤1) | 11.4 (4.9–26.6) | |||||
| Blanke (13) | 2008 | 147 | – | 4.8 | Female vs. male | 0.487 (no 95% CI available) |
| KIT exon 11 (yes vs. no) | 0.403 (no 95% CI available) | |||||
| Neutrophils (≥4.5×109/L) | 2.249 (no 95% CI available) | |||||
| Albumin, CTC grade ≥1 | 2.347 (no 95% CI available) | |||||
| Heinrich (14) | 2017 | 551 | – | 4.3 | Age (by decade) | 1.19 (1.08–1.28) |
| Male vs. female | 1.28 (1.06–1.55) | |||||
| Performance status (2–3 vs. 0–1) | 1.79 (1.36–2.35) | |||||
| Maximum tumor diameter | 1.02 (1.01–1.04) | |||||
| White blood cell | 1.69 (1.28–2.23) | |||||
| Albumin (>3.5 vs. ≤3.5 g/dL) | 0.66 (0.53–0.82) | |||||
| Rutkowski (15) | 2013 | 430 | – | 5.8 | Resection of residual disease (no vs. yes) | 0.3179 (0.20001–0.5052) |
| Liver metastases at imatinib start (no vs. yes) | 1.387 (1.01151–1.9029) | |||||
| Tumor genotype (exon11 vs. wild) | 0.4466 (0.24163–0.8254) | |||||
| Baseline albumin level (low vs. normal) | 2.415 (1.48174–3.9363) | |||||
| Performance status (WHO score, poor ≥2 vs. good <1) | 2.427 (1.53092–3.8491) | |||||
| Yeh (16) | 2011 | 171 | 2.8 | 5.9 | ECOG (2, 3 vs. 0, 1) | 5.17 (2.10–12.75) |
| Sum of tumor (≥11.5 vs. <11.5 cm) | 3.21 (1.14–9.04) | |||||
| Response (PD vs. CR/PR) | 17.65 (5.21–59.87) | |||||
| Kim (17) | 2019 | 379 | 6.1 | 8.8 | Age (>60 vs. <60 years) | 1.71 (1.22–2.41) |
| Median diameter of the largest lesions (per 10 mm increase) | 1.08 (1.05–1.12) | |||||
| Exon 11 (no vs. yes) | 2.15 (1.48–3.14) | |||||
| Surgical resection in RD with TKIs (yes vs. no) | 0.40 (0.26–0.61) | |||||
| Casali (18) | 2017 | 913 | 10.9 | 3.9 | Age (50–60 vs. <40 years) | 1.45 (1.03–2.04) |
| Female vs. male | 0.83 (0.70–0.97) | |||||
| KIT exon (9 vs. 11) | 1.87 (1.36–2.57) | |||||
| Baseline diameter of the longest lesion (per 10 mm increase) | 1.03 (1.02–1.04) | |||||
| Performance status (1 vs. 0) | 1.48 (1.25–1.75) | |||||
| Prior chemotherapy (yes vs. no) | 1.31 (1.11–1.55) | |||||
| Patrikidou (19) | 2016 | 322 | 6.1 | 8.3 | Female vs. male | 0.485 (0.294–0.798) |
| Locally advanced tumor (no vs. yes) | 0.515 (0.306–0.865) | |||||
| Performance status (2–3 vs. 0) | 3.696 (1.801–7.583) | |||||
| KIT exon 11 mutation (no vs. yes) | 2.712 (1.657–4.439) | |||||
| Lymphopenia (≥1 vs. <1 g/L) | 0.417 (0.215–0.809) | |||||
| Polymorphonuclear leucocyte (>7.5×109vs. ≤7.5×109/L) | 2.105 (1.119–3.960) |
*, all P<0.05. mOS, median OS; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; CTC, common toxicity criteria; WHO, World Health Organization; PD, progressive disease; CR, complete response; PR, partial response; RD, responsive disease; TKIs, tyrosine kinase inhibitors.
In our cohort, the mPFS for imatinib and sunitinib is longer compared to the corresponding clinical trial data, whereas the mPFS for regorafenib and ripretinib aligns closely with the relevant clinical trial data (20). Given the longer mPFS, this could be attributed, on one hand, to the heterogeneity among patients and, on the other hand, to the benefits derived from the comprehensive process management and multidisciplinary team treatment approach at our center. However, it should also be considered that in the real-world scenario, patients often face challenges in adhering to regular follow-ups, and imaging examinations may lag behind tumor progression. Additionally, in clinical practice, patients’ medication regimens are more complex. Some patients may discontinue or switch medications repeatedly due to adverse reactions or general deterioration in health. Moreover, considering the era of diagnosis, some patients may not have access to additional or new TKIs. Therefore, following resistance and progression on multiple lines of treatment, patients may attempt to rechallenge with imatinib or participate in clinical trials. All these factors may influence drug PFS.
Disease relapse during TKI treatment is considered secondary resistance which has to be a major limiting factor of molecular targeted therapies in advanced solid tumors, and time to development of TKI-resistant is probably a key determinant of survival. The main reason for secondary drug resistance is the emergence of tumor cells carrying secondary resistance genes through polyclonal, mainly clustered in the hotspot regions of the KIT kinase domain: ATP binding pockets (encoded by exons 13 and 14) and activation rings (encoded by exons 17 and 18), and there is extensive heterogeneity in drug resistance mutations and gene amplification intra- and inter-lesions (21-23).
For resectable localized GISTs, tumor size is currently accepted as a predictor of recurrence risk (11). Multiple studies have shown that tumor size is an independent prognostic factor for survival in advanced GISTs in the era of imatinib (Table 4). In the pre-imatinib era, DeMatteo et al. demonstrated that larger tumor diameters were associated with poorer prognosis, and in multivariable analysis, tumor size >10 cm emerged as the most significant factor, carrying a relative risk of 4.4 (95% CI: 2–9.8) (24). Our findings align with the earlier studies. In our study, a tumor burden >10 cm also emerged as an independent risk factor (HR 2.37, 95% CI: 1.39–4.03, P=0.002). Moreover, we contend that, considering that a large proportion had multifocal lesions (74.2%) at initial diagnosis, tumor burden might reflect the impact on OS more effectively than the maximum tumor diameter. We speculate that several factors drive the influence of tumor burden on survival: (I) a larger tumor burden might affect the occurrence of complications, such as tumor rupture, bleeding, perforation, or obstruction, influencing patient’s survival; (II) larger tumors can compress the gastrointestinal tract, leading to symptoms such as nausea and vomiting, which affect the patient’s intake, and defecation, and potentially exacerbate the patient’s general condition due to the associated tumor consumption; (III) some researchers have also proposed that the likelihood of developing TKI resistance is directly related to the number of exposed tumor cells and the duration of TKI exposure, potentially contributing to the occurrence of secondary resistance (25,26). Thus, larger tumors or more lesions might increase the probability of secondary mutations developing. In our study, patients with multifocal disease were found to experience worse OS. Hompland et al. also indicated that prognosis was better for patients with oligometastatic disease (≤3 metastases) than for those with polymetastatic disease because the former patients were more likely to receive curative treatment such as surgical resection (12).
Considering all the foregoing observations, some researchers believe that, because reducing the duration of exposure to TKIs appears unreasonable, surgical resection to reduce the number of exposed tumor cells, when technically feasible, might lower the risk of secondary resistance and extend patient survival (27,28).
A meta-analysis of nine studies including 1,416 patients with advanced GISTs revealed that, compared with TKIs treatment alone, surgery combined with TKIs increased the OS rate in those patients (HR by random-effects model 0.68, 95% CI: 0.54–0.85, I2=44.7%) (29). As the authors of several retrospective studies concluded, patients with advanced GIST who respond to imatinib can benefit from surgery, whereas patients with generalized progression rarely benefit. Nevertheless, some controversies regarding the outcome of surgery for limited progression remain (30-32). In our cohort, the mOS for patients who did and did not undergo surgery were 76.5 months (95% CI: 70.1–82.9) and 78.9 months (95% CI: 59.2–98.6), respectively, with no significance in OS (log-rank P=0.95). We analyzed the potential reasons for this finding: (I) in some patients, surgery was emergent because of tumor complications, which might result in the survival patterns for those patients deviating from the norm; (II) certain patients may have undergone surgical treatment upon disease progression; (III) notably, our study excluded patients who did not experience recurrence after surgical resection or who had a recurrence interval >12 months, which could diminish the role of surgery in prolonging patient survival; (IV) complications from surgery or the cessation of TKIs in the perioperative period might offset the benefits of surgery. We therefore hypothesize that, for selected patients experiencing disease remission after TKI therapy, surgery might confer an OS benefit, provided that general conditions permit. However, for patients experiencing disease progression after TKI therapy, a decision for surgery should be approached with caution.
We demonstrated that survival was poorer for patients with tumors located exclusively in the peritoneum than for those whose lesions were limited to the liver. We posit that, compared with liver lesions, peritoneal lesions are more challenging to discern on imaging, potentially leading to underdiagnosis and delayed treatment during follow-up. Additionally, lesions in the liver might be amenable to treatment modalities beyond systemic therapy, such as surgery, TACE, and RFA. Those modalities might confer additional survival benefits for patients with liver metastases. Studies indicate that, in selected patients (especially those with liver-restricted lesions compared with those with extrahepatic involvement), liver metastasectomy can improve survival (27,28,33). Furthermore, TACE has been found to be effective for patients with liver metastases who have experienced treatment failure with TKIs, with a potentially better prognosis for patients with liver-limited metastases (34,35). Although the long-term effect on OS has not been conclusively established, RFA treatment for liver metastases can achieve symptomatic relief with good tolerability (36-38). The application of radiotherapy in advanced GIST remains controversial. GISTs are generally considered to be resistant to radiation therapy. However, some researchers report that radiotherapy remains effective and well-tolerated, thus providing evidence that GISTs might not universally exhibit radiation resistance; however, substantial prospective research supporting those findings is lacking (39-41). Currently, radiotherapy is recommended only for palliative treatment in selected patients (42).
At our center, only a small number of patients received radiotherapy, and their outcomes did not support significant efficacy. We further observed that additional local treatments or other therapeutic modalities based on TKIs did not significantly extend the survival duration. That lack of any significant extension may be attributed to potential interactions between the various treatment modalities. In addition, given the limitations in our sample size, further subgroup analyses could not be conducted. In clinical practice, we have also observed that patients who achieve long-term disease control are often hesitant to undergo treatments beyond TKIs. Local treatment might be contemplated only after the failure of TKI therapy or in the presence of tumor-related complications.
Previous studies have indicated that exon 11 mutation is an independent prognostic factor for patients in the imatinib era (13,15,17,18). However, our study results did not demonstrate significant differences between genotypes. Studies suggested that different mutation types exhibit varying sensitivity to different TKIs. For instance, sunitinib is more effective in KIT exons 13 and 14 mutations but is less potent for exons 17 and 18 mutations. In contrast to imatinib, sunitinib shows superior efficacy against exon 9 mutations compared to exon 11 mutations (21). Regorafenib exhibits better efficacy against exon 17 mutations (43). Ripretinib also demonstrated good inhibitory effects on major mutations of KIT and PDGFRA, as well as secondary mutations such as KIT exon 13 and 17 (44). Our study findings indicate that 56.2% of patients received more than one TKI during the disease. As mentioned above, these TKIs exhibit different efficacies for the same or different gene mutation sites. Therefore, under the new paradigm of multiple TKIs, the impact of genotype mutations on patients’ OS may not show significant differences.
Our study has certain limitations. As a single-center retrospective study, it has inherent challenges, such as information bias. Moreover, certain baseline data, such as hematological examination results were missing from the medical records of the patients. Further randomized controlled trials are warranted to explore the effect of treatment modalities beyond TKIs.
Conclusions
In this study, we further identified independent prognostic factors for patients with advanced GISTs and made several robust findings. We additionally found that a single lesion at enrollment, no previous use of TKIs, a smaller tumor burden, and lesions limited to the liver were associated with better survival. We also innovatively explored the effect on patient survival of several risk factors from the perspective of secondary drug resistance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-63/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-63/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-63/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-63/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study received approval from the Institutional Review Board of the First Affiliated Hospital of CMU (approval No. 2022-K364) with an exemption from obtaining written informed consent due to its observational and retrospective nature. The study adhered to the principles of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blay JY, Kang YK, Nishida T, et al. Gastrointestinal stromal tumours. Nat Rev Dis Primers 2021;7:22. [Crossref] [PubMed]
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am 2009;23:69-78. viii. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Klug LR, Khosroyani HM, Kent JD, et al. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol 2022;19:328-41. [Crossref] [PubMed]
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 2008;26:5360-7. [Crossref] [PubMed]
- Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 2020;21:935-46. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Hompland I, Bruland ØS, Hølmebakk T, et al. Prediction of long-term survival in patients with metastatic gastrointestinal stromal tumor: analysis of a large, single-institution cohort. Acta Oncol 2017;56:1317-23. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Heinrich MC, Rankin C, Blanke CD, et al. Correlation of Long-term Results of Imatinib in Advanced Gastrointestinal Stromal Tumors With Next-Generation Sequencing Results: Analysis of Phase 3 SWOG Intergroup Trial S0033. JAMA Oncol 2017;3:944-52. [Crossref] [PubMed]
- Rutkowski P, Andrzejuk J, Bylina E, et al. What are the current outcomes of advanced gastrointestinal stromal tumors: who are the long-term survivors treated initially with imatinib? Med Oncol 2013;30:765. [Crossref] [PubMed]
- Yeh CN, Chen YY, Tseng JH, et al. Imatinib Mesylate for Patients with Recurrent or Metastatic Gastrointestinal Stromal Tumors Expressing KIT: A Decade Experience from Taiwan. Transl Oncol 2011;4:328-35. [Crossref] [PubMed]
- Kim JH, Ryu MH, Yoo C, et al. Long-term survival outcome with tyrosine kinase inhibitors and surgical intervention in patients with metastatic or recurrent gastrointestinal stromal tumors: A 14-year, single-center experience. Cancer Med 2019;8:1034-43. [Crossref] [PubMed]
- Casali PG, Zalcberg J, Le Cesne A, et al. Ten-Year Progression-Free and Overall Survival in Patients With Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J Clin Oncol 2017;35:1713-20. [Crossref] [PubMed]
- Patrikidou A, Domont J, Chabaud S, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer 2016;52:173-80. [Crossref] [PubMed]
- Naito Y, Nishida T, Doi T. Current status of and future prospects for the treatment of unresectable or metastatic gastrointestinal stromal tumours. Gastric Cancer 2023;26:339-51. [Crossref] [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. [Crossref] [PubMed]
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [Crossref] [PubMed]
- Serrano C, Mariño-Enríquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer 2019;120:612-20. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Turley RS, Peng PD, Reddy SK, et al. Hepatic resection for metastatic gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Cancer 2012;118:3571-8. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Xue A, Gao X, He Y, et al. Role of Surgery in the Management of Liver Metastases From Gastrointestinal Stromal Tumors. Front Oncol 2022;12:903487. [Crossref] [PubMed]
- Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib -- analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014;40:412-9. [Crossref] [PubMed]
- Cai Z, Yin Y, Shen C, et al. Role of surgical resection for patients with recurrent or metastatic gastrointestinal stromal tumors: A systematic review and meta-analysis. Int J Surg 2018;56:108-14. [Crossref] [PubMed]
- Rubió-Casadevall J, Martinez-Trufero J, Garcia-Albeniz X, et al. Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol 2015;22:2948-57. [Crossref] [PubMed]
- Mussi C, Ronellenfitsch U, Jakob J, et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol 2010;21:403-8. [Crossref] [PubMed]
- Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2018;268:296-302. [Crossref] [PubMed]
- Liu L, Xia X, Ju Y, et al. Effects of surgical management for gastrointestinal stromal tumor patients with liver metastasis on survival outcomes. Front Oncol 2024;14:1289885. [Crossref] [PubMed]
- Kobayashi K, Szklaruk J, Trent JC, et al. Hepatic arterial embolization and chemoembolization for imatinib-resistant gastrointestinal stromal tumors. Am J Clin Oncol 2009;32:574-81. [Crossref] [PubMed]
- Cao G, Li J, Shen L, et al. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol 2012;18:6134-40. [Crossref] [PubMed]
- Jung JH, Won HJ, Shin YM, et al. Safety and Efficacy of Radiofrequency Ablation for Hepatic Metastases from Gastrointestinal Stromal Tumor. J Vasc Interv Radiol 2015;26:1797-802. [Crossref] [PubMed]
- Yoon IS, Shin JH, Han K, et al. Ultrasound-Guided Intraoperative Radiofrequency Ablation and Surgical Resection for Liver Metastasis from Malignant Gastrointestinal Stromal Tumors. Korean J Radiol 2018;19:54-62. [Crossref] [PubMed]
- Liu L, Wang B, Zhang ZY, et al. Percutaneous ultrasound-guided radiofrequency ablation for patients with liver metastases from gastrointestinal stromal tumors. Int J Hyperthermia 2024;41:2292950. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Collan J, et al. Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: A prospective study. Radiother Oncol 2015;116:233-8. [Crossref] [PubMed]
- Gatto L, Nannini M, Saponara M, et al. Radiotherapy in the management of gist: state of the art and new potential scenarios. Clin Sarcoma Res 2017;7:1. [Crossref] [PubMed]
- Cuaron JJ, Goodman KA, Lee N, et al. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat Oncol 2013;8:274. [Crossref] [PubMed]
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20-33. [Crossref] [PubMed]
- Yeh CN, Chen MH, Chen YY, et al. A phase II trial of regorafenib in patients with metastatic and/or a unresectable gastrointestinal stromal tumor harboring secondary mutations of exon 17. Oncotarget 2017;8:44121-30. [Crossref] [PubMed]
- Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:923-34. [Crossref] [PubMed]



