Clinical, morphologic, and immunophenotypic characteristics of ampullary carcinomas with an emphasis on SMAD4 expression
Introduction
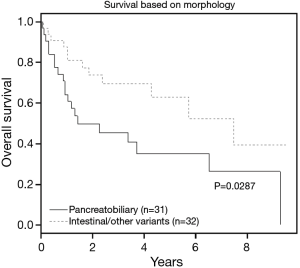
Carcinomas of the ampulla of Vater (AOV) are malignant epithelial neoplasms that are either centered in or completely replace the ampulla according to the World Health Organization (WHO) definition. Two main histologic subtypes of ampullary adenocarcinoma are recognized: pancreatobiliary and intestinal types, with the former carrying a worse prognosis including worse survival, higher stage, and higher incidence of lymph node (LN) involvement (1,2).
Immunohistochemical (IHC) studies have been used to support the categorization of these tumors into a pancreatobiliary or intestinal phenotype, with cytokeratin 7 (CK7), cytokeratin 17 (CK17) and MUC1 expression supporting a pancreatobiliary phenotype (3-5), and cytokeratin 20 (CK20), MUC2, and CDX2 expression supporting an intestinal phenotype (4,6-9). However, not all cases fit into these two categories. Tumors with mucinous, signet-ring cell, solid, and papillary morphology are often placed in a separate “other” category. In addition, some tumors show an IHC profile that does not entirely fit the classic phenotypes described for pancreatobiliary and intestinal type tumors. Because of this, Zhou et al. introduced an IHC category of “other type” for tumors with double positive or double negative CK7/CK20 profile (5). Clinical behavior of this subset of ampullary tumors is difficult to predict given the limited data available. Immunophenotyping using CK7, CK20, CDX2, MUC1 and MUC2 showed more enhanced subtyping of ampullary carcinoma once combined with histologic examination [hematoxylin and eosin (H&E)] (6), but still there are some cases that would not fit into this subtyping.
Carcinomas of the AOV are also a genetically heterogeneous group of tumors that may harbor different types of molecular alterations including TP53 mutations (10), Kras mutations (11,12), APC mutations (10), SMAD4 inactivation (13), and microsatellite instability (14,15). Although the 5-year overall survival lies between that of duodenal adenocarcinoma and pancreatic adenocarcinoma, there is great heterogeneity within this tumor group from both a clinical and histopathological spectrum (12). Identifying specific prognostic biomarkers is thus important to tailor treatment recommendations and future potential targeted therapies.
Inactivation of SMAD4, a tumor suppressor gene, has been shown to be an adverse prognostic factor in pancreatic adenocarcinomas (16). This type of genetic alteration has been identified in 55% of pancreatic adenocarcinomas, and though less frequent, it is also found in roughly a third of adenocarcinomas of the AOV (16-18). Determination of SMAD4 expression by IHC has been shown to have good concordance with genetic status of SMAD4 in pancreatic adenocarcinoma as demonstrated by Tascilar et al. (16), with lack of expression in cases with inactivation of the SMAD4 gene.
There are limited data on SMAD4 expression profiles in ampullary adenocarcinoma. A prior study of SMAD4 expression in 140 adenocarcinomas of the AOV showed complete loss of SMAD4 in 34% of cases with no difference between the different histologic subtypes (36% of intestinal, 37% of pancreatobiliary, and 29% of “other variants”) of ampullary carcinoma (13). Other clinicopathologic variables including pancreatobiliary morphology and histomolecular phenotype (1,3), stage (1,19), loss of CDX2 expression (7), vascular space invasion (7,19), and LN involvement (5,7,19) have been shown to be independent predictors of survival in ampullary carcinomas by various authors.
This study aims to describe the clinical characteristics and outcomes of ampullary carcinomas in regards to histologic subtypes, and evaluate the prognostic implications of SMAD4 expression in these tumors.
Methods
Study cases and tumor specimens
This study was approved by the Institutional Review Board of the University of Florida. The study population comprised all ampullary carcinoma patients who had definitive surgical resection between Jan 2000–Aug 2011 at our institution. The following information was recorded from the Gastrointestinal Oncology Research Database: age, sex, clinical presentation, laboratory results of serum CEA and CA19.9, tumor stage (T), LN status (N), and recurrence. Clinical information about recurrence was available in 33 patients. Death status was obtained through a search of the national death index (NDI) performed on 4/1/2012.
Histologic evaluation
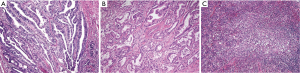
H&E slides available for all cases were reviewed by three independent pathologists (A Alkhasawneh, TZ Toro, and LV Duckworth). After review of all H&E slides, a representative block with invasive tumor was selected for IHC studies. Cases were classified as pancreatobiliary (glands with cuboidal lining), intestinal (glands with columnar lining and/or cribriform architecture) or other variants (cases that did not fit into either category) based on their morphologic characteristics on H&E (see Figure 1). The other variants category consisted of cases with mixed cuboidal/columnar epithelium, solid sheet-like growth pattern, mucinous tumors, and tumors with a prominent inflammatory component.
Immunohistochemistry
IHC studies for CK7, CK17, CK20, and SMAD4 were performed using standard avidin-biotin-peroxidase complex procedure with a matching positive and negative control for each batch. A summary of the antibodies, clone, company, dilution, and antigen retrieval is listed in Table 1.

Full table
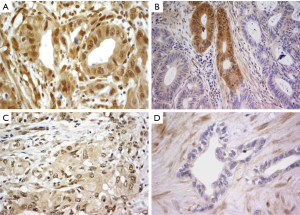
IHC studies for CK7, CK17, and CK20 were interpreted as positive in cases with cytoplasmic/membranous reactivity in ≥5% of tumor cells and negative when <5% of tumor cells were immunoreactive. SMAD4 immunoreactivity was in general both nuclear and cytoplasmic, and four categories were reported [adapted from Tascilar et al. (16)], as follows: absent (no staining), trace (weak reactivity relative to surrounding pancreas), focal (two cell population with a subset of clearly negative cells) and diffuse (strong staining comparable to surrounding pancreas) as illustrated in Figure 2. Cases with absent, trace or focal SMAD4 expression were considered negative and those with diffuse SMAD4 expression were considered positive. Internal positive controls included background fibroblasts, lymphocytes, and non-neoplastic pancreatic tissue.
Statistical analysis
The clinical characteristics and IHC data were summarized by sample size and the corresponding sample proportion. Chi-square exact test was used to test the association between clinical characteristics with IHC variables. Overall survival curves were compared by log rank test, and the corresponding cumulative survival rates were estimated using Kaplan-Meier method. All statistical analysis was performed using SAS 9.2 software.
Results
Clinicopathologic characteristics
Sixty-three cases were identified and all were included in this analysis. Our patient cohort presented at a mean age of 68, with a slight male predominance (55%). Detailed clinicopathologic characteristics including age, sex, clinical presentation, laboratory data, and tumor parameters are summarized in Table 2. Thirty-two of sixty-three patients (51%) had died at the time of this study with a mean survival of 23 months (range, 0.6–111 months) and an overall survival of 49%.
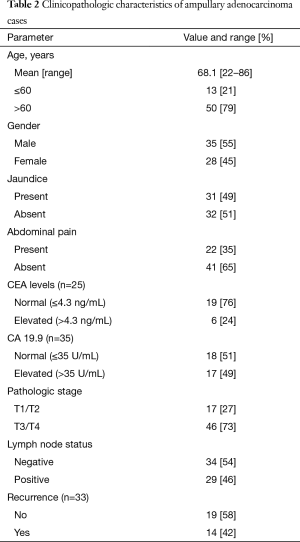
Full table
Tumor histopathologic subtypes and IHC profile
Nearly half of our cases (49%) were pancreatobiliary subtype, 29% were intestinal subtype, and 22% were other variants. Nearly all cases of pancreatobiliary type demonstrated a CK7+/CK20− profile in tumors, while most intestinal type were CK20+ and half of the intestinal type were CK7+. Tumors of other variants category showed a variety of IHC profiles including six CK7+/CK20−, five dual positive (CK7+/CK20+), and three dual negative (CK7−/CK20−) tumors. CK17, a marker seen in tumors of the pancreatobiliary tract, was present in 51% (32/63) of our cases. Though most of these cases showed pancreatobiliary morphology (69%), three had intestinal morphology, and seven had other variants morphology.
SMAD4
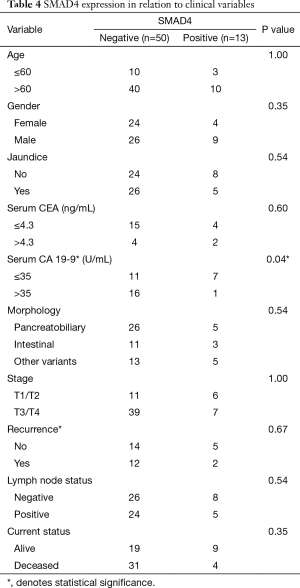
Clinical characteristics according to SMAD4 expression are summarized in Table 3. Similar clinicopathologic features were seen among patients with absent or trace SMAD4 expression in comparison to patients with focal or diffuse SMAD4 expression (data not shown). Complete absence of SMAD4 by IHC was seen in 22 (35%) cases, showing a higher predominance of SMAD4 loss in pancreatobiliary type than in intestinal type tumors (48% vs. 22%). Fifty (79%) of ampullary adenocarcinomas had negative (absent, trace or focal) SMAD4 expression by IHC, and 52% were pancreatobiliary type, 22% intestinal type, and 26% other variants type.
Full table
Correlation of clinicopathologic variables with outcomes
Tumors with pancreatobiliary morphology showed a worse overall survival than tumors with intestinal and other variants type (P=0.03) (see Table 3, Figure 3).
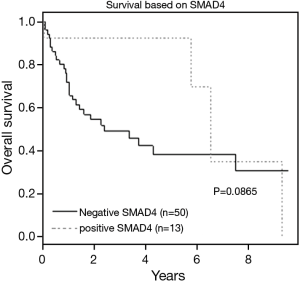
Negative (absent, trace or focal) SMAD4 expression by IHC portrayed a trend for higher tumor stage, recurrence, and death, though none of these parameters was statistically significant (Table 4, Figure 4). After multivariate analysis, CK17 positivity and age >60 were found to be independent predictors of shorter survival (P=0.0092 and P=0.0038, respectively).
Full table
Discussion
SMAD4 is a nuclear transcription factor that is inactivated in a subset of malignant neoplasms of the pancreatobiliary tract and is an independent predictor of poor prognosis in pancreatic adenocarcinomas (16,18). Loss of SMAD4 is seen in 55% of pancreatic and distal common bile duct, 36% of ampullary, and 15% of proximal bile duct carcinomas (13,20). In colorectal cancer, loss of SMAD4 expression correlated with poor survival, but did not correlate with tumor stage, type, or growth pattern (21). In gliomas, higher WHO grade and lower survival rates were seen in patients without SMAD4 staining (22).
The role of SMAD4 in signaling and tumor progression is more complex. For instance, nuclear and cytoplasmic expression of SMAD4 in prostatic adenocarcinoma was shown to correlate with aggressive tumor behavior (higher grade, higher stage, and postsurgical biochemical recurrence) (23). In breast carcinoma, a trend of longer survival was noted in SMAD4-negative patients (24). Also, strong SMAD4 expression was found to be an independent factor for predicting malignant transformation in oral hairy leukoplakia (25).
Immunohistochemistry for SMAD4 is a highly specific and sensitive marker for SMAD4 gene inactivation. Tascilar et al. showed that uniform SMAD4 expression in pancreatic adenocarcinoma was associated with wild-type SMAD4 gene, while absent or non-uniform (trace or focal staining) SMAD4 expression was associated with SMAD4 gene inactivation, and correlated with worse survival (16). However, two of the four (50%) patients with focal SMAD4 expression in this study were wild-type for the SMAD4 gene. Also, Wilentz et al. (26) found diffuse SMAD4 expression in the majority of wild-type SMAD4 cases and loss of SMAD4 expression in SMAD4 inactivated patients. However, one of the two patients with focal SMAD4 expression had wild-type SMAD4 gene. These findings raise concern about the relationship between focal SMAD4 expression and gene status, and suggest a more complex mechanism of SMAD4 gene regulation. In our cohort, similarity in tumor behavior in patients with diffuse and focal SMAD4 expression was noted (data not shown), but no statistical significance was found in relation to survival.
In our study, 35% of ampullary adenocarcinomas showed complete absence of SMAD4, similar in incidence to McCarthy et al. (13). However, we found that a substantial number of cases (n=28; 44%) showed either trace or focal expression of SMAD4 by IHC. We found a trend for worse survival and biologic behavior between patients with diffuse SMAD4 expression and patients with absent, trace, or focal SMAD4 expression. For example, the death rate was higher for patients with negative (absent, trace or focal) SMAD4 expression compared to patients with positive (diffuse) SMAD4 expression (62% vs. 31%). Although not statistically significant, the survival trend is limited by our small sample size and must be validated in a larger patient cohort. Higher pathologic stage (T3/T4) and more recurrence were also noted in patients with negative SMAD4 expression in comparison to positive SMAD4 cases. Moreover, among patients who recurred, most had SMAD4 negative tumors (86%).
CK17, a low molecular weight keratin often used as a marker of pancreatobiliary origin, was expressed in 51% of the cases in our study. The majority of CK17-positive tumors demonstrated pancreatobiliary morphology by H&E (69%, P=0.002) and showed a higher incidence of LN metastasis (59%, P=0.04). A prior study showed greater CK17 expression in pancreatobiliary-type ampullary adenocarcinoma, but the relationship between CK17 expression with survival was not evaluated (4). Since CK17 expression appears to be an independent predictor of shorter survival, this marker may be useful in risk stratification of ampullary adenocarcinomas.
In summary, our study demonstrated poorer outcomes for ampullary adenocarcinoma containing pancreaticobiliary morphology and negative SMAD4 expression. Negative SMAD4 expression was associated with higher tumor stage, recurrence, and death. Additional, larger scale studies are needed to validate the prognostic significance of SMAD4 in ampullary adenocarcinoma.
Acknowledgements
This research was supported by a Clinical Research Committee Grant from the University of Florida, Department of Pathology, Immunology, and Laboratory Medicine.
Footnote
Conflicts of Interest: Abstract was presented at the 102nd Annual United States and Canadian Academy of Pathology meeting, Baltimore, MD, March 2013.
Ethical Statement: This study was approved by the University of Florida Institutional Review Board and ethics committee (FWA00005790) with a waiver of informed consent given the de-identified nature of the data collected (Protocol ID# 452-2011).
References
- Carter JT, Grenert JP, Rubenstein L, et al. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg 2008;207:210-8. [Crossref] [PubMed]
- Westgaard A, Tafjord S, Farstad IN, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008;8:170. [Crossref] [PubMed]
- Chang DK, Jamieson NB, Johns AL, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol 2013;31:1348-56. [Crossref] [PubMed]
- Chu PG, Schwarz RE, Lau SK, et al. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol 2005;29:359-67. [Crossref] [PubMed]
- Zhou H, Schaefer N, Wolff M, et al. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol 2004;28:875-82. [Crossref] [PubMed]
- Ang DC, Shia J, Tang LH, et al. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am J Surg Pathol 2014;38:1371-9. [Crossref] [PubMed]
- Hansel DE, Maitra A, Lin JW, et al. Expression of the caudal-type homeodomain transcription factors CDX 1/2 and outcome in carcinomas of the ampulla of Vater. J Clin Oncol 2005;23:1811-8. [Crossref] [PubMed]
- Leo JM, Kalloger SE, Peixoto RD, et al. Immunophenotyping of ampullary carcinomata allows for stratification of treatment specific subgroups. J Clin Pathol 2016;69:431-9. [Crossref] [PubMed]
- Schueneman A, Goggins M, Ensor J, et al. Validation of histomolecular classification utilizing histological subtype, MUC1, and CDX2 for prognostication of resected ampullary adenocarcinoma. Br J Cancer 2015;113:64-8. [Crossref] [PubMed]
- Imai Y, Oda H, Tsurutani N, et al. Frequent somatic mutations of the APC and p53 genes in sporadic ampullary carcinomas. Jpn J Cancer Res 1997;88:846-54. [Crossref] [PubMed]
- Howe JR, Klimstra DS, Cordon-Cardo C, et al. K-ras mutation in adenomas and carcinomas of the ampulla of vater. Clin Cancer Res 1997;3:129-33. [PubMed]
- Kohler I, Jacob D, Budzies J, et al. Phenotypic and genotypic characterization of carcinomas of the papilla of Vater has prognostic and putative therapeutic implications. Am J Clin Pathol 2011;135:202-11. [Crossref] [PubMed]
- McCarthy DM, Hruban RH, Argani P, et al. Role of the DPC4 tumor suppressor gene in adenocarcinoma of the ampulla of Vater: analysis of 140 cases. Mod Pathol 2003;16:272-8. [Crossref] [PubMed]
- Agaram NP, Shia J, Tang LH, et al. DNA mismatch repair deficiency in ampullary carcinoma: a morphologic and immunohistochemical study of 54 cases. Am J Clin Pathol 2010;133:772-80. [Crossref] [PubMed]
- Ruemmele P, Dietmaier W, Terracciano L, et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am J Surg Pathol 2009;33:691-704. [Crossref] [PubMed]
- Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res 2001;7:4115-21. [PubMed]
- Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674-9. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- de Paiva Haddad LB, Patzina RA, Penteado S, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg 2010;14:719-28. [Crossref] [PubMed]
- Argani P, Shaukat A, Kaushal M, et al. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer 2001;91:1332-41. [Crossref] [PubMed]
- Isaksson-Mettävainio M, Palmqvist R, Forssell J, et al. SMAD4/DPC4 expression and prognosis in human colorectal cancer. Anticancer Res 2006;26:507-10. [PubMed]
- He SM, Zhao ZW, Wang Y, et al. Reduced expression of SMAD4 in gliomas correlates with progression and survival of patients. J Exp Clin Cancer Res 2011;30:70. [Crossref] [PubMed]
- Sheehan GM, Kallakury BV, Sheehan CE, et al. Smad4 protein expression correlates with grade, stage, and DNA ploidy in prostatic adenocarcinomas. Hum Pathol 2005;36:1204-9. [Crossref] [PubMed]
- Stuelten CH, Buck MB, Dippon J, et al. Smad4-expression is decreased in breast cancer tissues: a retrospective study. BMC Cancer 2006;6:25. [Crossref] [PubMed]
- Xia RH, Song XM, Wang XJ, et al. The combination of SMAD4 expression and histological grade of dysplasia is a better predictor for the malignant transformation of oral leukoplakia. PLoS One 2013;8:e66794. [Crossref] [PubMed]
- Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J Pathol 2000;156:37-43. [Crossref] [PubMed]