
Regorafenib monotherapy as the later-line treatment for elderly patients with metastatic colorectal cancer: a multicenter real-world study
Highlight box
Key findings
• For elderly patients (70 years > aged ≥60 years) with metastatic colorectal cancer (mCRC), maintaining the final dose of regorafenib monotherapy at 120 mg/day may have prognostic advantages.
What is known and what is new?
• The standard starting dose for regorafenib monotherapy is 160 mg/day in mCRC.
• The final daily dose is significantly associated with overall survival in elderly patients (aged ≥60 years) with mCRC who received regorafenib monotherapy.
What is the implication, and what should change now?
• Our findings may contribute to the refined management of regorafenib monotherapy and deserve further exploration in large-scale clinical trials.
Introduction
According to a report released by the National Cancer Center of China in 2022, the annual incidence of colorectal cancer (CRC) in China reached 592,000 cases, with 309,000 deaths, accounting for 28% of the global incidence (1,2). Since the emergence of multitarget tyrosine kinase inhibitors against the vascular endothelial growth factor receptor (VEGFR) axis represented by regorafenib, there has been some progress in the treatment of metastatic colorectal cancer (mCRC). Regorafenib inhibits tumor proliferation, metastasis, angiogenesis, and immune escape by targeting the VEGFR axis and its alternative signaling pathways (3). Based on the phase III CORRECT and CONCUR trials, regorafenib is currently the standard regimen for the third-line treatment of mCRC (4,5). While there is a trend towards a younger onset, the elderly population remains the main group at risk (6,7). Due to differences in the tumor molecular characteristics of patients in different age groups, more specific anti-tumor treatments are required (8). Given that the elderly have a more complex and diverse range of comorbidities, they are more susceptible to the adverse effects of drugs. Drug therapy in this group of patients should be based on robust data and good clinical evidence. However, real-world data on the application of regorafenib in elderly patients with mCRC is relatively limited.
The standard starting dose for regorafenib monotherapy is 160 mg/day in mCRC (4,5). However, in clinical practice, it is necessary to adjust the dose based on patient conditions and combination treatment plans. Bekaii-Saab et al. evaluated a dose escalation strategy (starting with a lower dose of 80 mg/day and gradually increasing to the standard dose), and found that it did not affect the drug activity and had a lower incidence of adverse events (AEs), and thus represents an optimized regimen (9). Argilés et al. compared the effects of starting doses of 120 and 160 mg/day or an intermittent dosing strategy (1 week on/1 week off) on prognosis. No significant differences in progression-free survival (PFS) and overall survival (OS) were observed between the different groups. Additionally, the most clinically relevant AEs (such as hand-foot skin reactions and fatigue) showed a more significant improvement in the intermittent dosing group (10). Petrioli et al. found that using a 2/1 intermittent dosing strategy (2 weeks on/1 week off) in an elderly population aged ≥75 years can maintain prognosis while ensuring drug tolerance (11). Another study found that in elderly patients aged ≥70 years who had previously failed treatment, regorafenib monotherapy achieved similar median PFS and OS compared to previous phase III clinical trials and real-world studies (12). These findings provide more possibilities for the clinical application of regorafenib, including in elderly patients.
We conducted this multicenter real-world study to evaluate the dosing characteristics, treatment prognosis, and safety of regorafenib monotherapy in elderly Chinese patients (aged ≥60 years) with mCRC. We also aimed to explore the dose selection suitable for different age groups within the elderly population. We present this article in accordance with the STROBE checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-464/rc).
Methods
Study design and outcome assessment
This real-world study was based on an original study that enrolled mCRC patients treated with regorafenib from August 2017 to June 2020 in 10 hospitals, including the Cancer Hospital of Chinese Academy of Medical Sciences (CAMS). The original study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The original study was approved by the ethics committee of the Cancer Hospital of CAMS (reference number: 2021010711241202). All participating hospitals were informed and agreed with this study. The informed consent of all patients was waived by the ethics committee. The original study was registered at https://clinicaltrials.gov/ (registration number: NCT04835324). Among the included population of the original study, we further selected those of them who were treated with regorafenib monotherapy and aged ≥60 years for further analysis.
The main inclusion criteria were: (I) cytologically or pathologically confirmed colorectal adenocarcinoma; (II) advanced unresectable metastatic disease; (III) failure of at least two standard treatments, including a fluoropyrimidine plus oxaliplatin or irinotecan (previous treatment with bevacizumab, cetuximab, or panitumumab was allowed for patients who had KRAS wild-type tumors); and (IV) treatment with regorafenib monotherapy for at least one cycle (28 days). The main exclusion criteria were: (I) participating in another interventional clinical trial during the regorafenib treatment; (II) presence of other malignant tumors within 5 years before receiving regorafenib treatment, except for locally curable cancer (cured malignant melanoma, basal cell carcinoma, and carcinoma in situ of the bladder and cervix); (III) previous treatment of regorafenib in the first or second line; and/or (IV) an age <60 years.
The data of the patients’ baseline characteristics were extracted by searching the medical records, including age, sex, body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) performance status, primary site, liver metastasis, lung metastasis, pathological type, Rat sarcoma (RAS) gene mutation status, V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) gene mutation status, mismatch repair (MMR) gene status, prior anti-vascular endothelial growth factor (VEGF) therapy, lines of therapy, and the initial and final daily dose. The primary endpoint was OS, and the secondary endpoints included PFS and safety. OS was defined as the time from the initiation of regorafenib monotherapy to death from any cause. PFS was defined as the time from the initiation of regorafenib monotherapy to disease progression or death from any cause, whichever occurred first. The follow-ups were conducted by telephone monthly. The last follow-up occurred on March 31, 2022. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03 (NCI-CTCAE 4.03).
Statistical analysis
The statistical analysis was performed by R software (version 4.2.0). Missing data were not included in the data analysis process. The continuous variables were compared using the Student’s t-test/Wilcoxon rank-sum test, or the Kruskal-Wallis H test. The categorical data were compared using the chi-square test/Fisher’s exact test or the Cochran-Mantel-Haenszel test. The Kaplan-Meier method was used to plot the survival curves and calculate the median PFS and OS. The log-rank test was used to compare the median PFS and OS. Univariate and multivariate Cox proportional hazards regression model survival analyses were performed. We have relaxed the P value threshold to 0.20 in univariate analysis to identify more potential prognostic factors. We developed two multivariate models to elucidate the relationship between drug dosage and clinical outcomes. Variables with a P value <0.20 in the univariate analysis were adjusted in the Model 1. Variables with a P value <0.20 in the univariate analysis and those with a P value <0.05 from the differential analysis were adjusted in the Model 2. The 95% confidence interval (CI) was calculated using the Clopper-Pearson method based on the normal distribution approximation.
Three sets of sensitivity analyses were undertaken. First, propensity score matching (PSM) was performed to match patients in the high-dose group with patients in the low-dose group. The propensity score data set was constructed using the multivariate logistic regression model, which included variables with a P value <0.20 from the univariate analysis and those with a P value <0.05 from the differential analysis. We used caliper matching with the caliper 0.02 of the pooled standard deviation of the logit of the propensity score. Patients in the high-dose group were matched 1:1 to patients in the low-dose group. We assessed the balance of the post-PSM data set by generating histograms and jitter plots and calculating the standardized mean differences (SMDs) between groups. The propensity data set generated the inverse probability of treatment weighting (IPTW) data set. To balance those observable characteristics, each patient was weighted by the inverse probability of being in the high-dose group compared to the low-dose group. We analyzed the PSM dataset and the IPTW dataset with Cox regression. The results are expressed as the adjusted hazard ratios (HRs) with 95% CIs. Second, we conducted a repeated analysis of elderly patients receiving regorafenib monotherapy as a third-line treatment. Third, considering the differences in sex distribution across various final daily dose groups, we conducted a subgroup analysis by sex to eliminate confounding effects. Forest plots were used to present the multivariate Cox regression results after subgroup analysis.
All the tests were two-tailed, and P values <0.05 were considered statistically significant.
Results
Patient characteristics
As of March 31, 2022, 768 patients with mCRC were selected from 10 centers. Among them, 98 patients were excluded from the analysis because they received regorafenib as a first- or second-line treatment, 49 patients were excluded due to missing clinical data, 303 patients were excluded because they were aged <60 years, and 115 patients were excluded as they had received combination chemotherapy and/or immunotherapy. Ultimately, 203 patients were enrolled in the study (Figure 1, Table S1).
The baseline characteristics of the patients are shown in Table 1. The mean age of the included patients was 68.03 years old at diagnosis (range, 60.00 to 83.71 years), and the mean BMI was 23.34 kg/m2 (range, 16.53 to 31.48 kg/m2). Of all patients, 152 (74.88%) were male, 190 (93.60%) patients had an ECOG performance status of 0–1, 134 (82.21%) patients had the primary tumor located in the left side, 103 (50.74%) patients had liver metastasis, and 118 (58.13%) patients had lung metastasis. RAS wild type (51.30%, n=59), BRAF wild type (96.43%, n=81), and pMMR (94.90%, n=93) were the most common types of genetic status. The majority of patients had received anti-VEGF targeted therapy (n=146, 73.00%) and were treated with regorafenib monotherapy as a third-line therapy (n=123, 60.59%). In terms of dosage, 80 mg/day was the most common daily initial dose (n=76, 44.71%) and final daily dose (n=51, 43.97%). The differential analysis among various dose groups indicated no significant differences in the baseline characteristics of patients receiving different initial daily doses (Table S2). However, patients receiving different final daily doses exhibited a significant difference in sex distribution (P=0.049) (Table S3).
Table 1
| Subgroup | Values |
|---|---|
| Age (years) | 68.03 (60.00–83.71) |
| Sex | |
| Male | 152 (74.88) |
| Female | 51 (25.12) |
| BMI (kg/m2) | 23.34 (16.53–31.48) |
| ≥22 | 85 (65.89) |
| <22 | 44 (34.11) |
| Unknown | 74 |
| ECOG score | |
| 0/1 | 190 (93.60) |
| 2/3 | 13 (6.40) |
| RAS gene mutation status | |
| Wild type | 59 (51.30) |
| KRAS | 54 (46.96) |
| NRAS | 2 (1.74) |
| Unknown | 88 |
| BRAF gene mutation status | |
| Wild type | 81 (96.43) |
| Mutant | 3 (3.57) |
| Unknown | 119 |
| Primary site | |
| Left | 134 (82.21) |
| Right | 29 (17.79) |
| Unknown | 40 |
| Liver metastasis | |
| Positive | 103 (50.74) |
| Negative | 100 (49.26) |
| Lung metastasis | |
| Positive | 118 (58.13) |
| Negative | 85 (41.87) |
| MMR gene status | |
| dMMR | 5 (5.10) |
| pMMR | 93 (94.90) |
| Unknown | 105 |
| Prior anti-VEGF therapy | |
| Used | 146 (73.00) |
| Not used | 54 (27.00) |
| Unknown | 3 |
| Lines of therapy | |
| Third-line | 123 (60.59) |
| Fourth-line | 52 (25.62) |
| Fifth-line | 19 (9.36) |
| ≥ Sixth-line | 9 (4.43) |
| Initial daily dose (mg) | |
| 40 | 5 (2.94) |
| 80 | 76 (44.71) |
| 120 | 43 (25.29) |
| 160 | 46 (27.06) |
| Unknown | 33 |
| Final daily dose (mg) | |
| 40 | 0 (0.00) |
| 80 | 51 (43.97) |
| 120 | 37 (31.90) |
| 160 | 28 (24.14) |
| Unknown | 87 |
Data are presented as mean (range) or n (%). BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; RAS, Rat sarcoma; KRAS, Kirsten rats arcomaviral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; BRAF, V-Raf Murine Sarcoma Viral Oncogene Homolog B; MMR, mismatch repair; dMMR, deficiency of MMR; pMMR, proficiency of MMR; VEGF, vascular endothelial growth factor.
Survival analysis
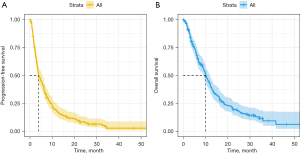
As of March 31, 2022, the median follow-up time was 30.9 months (95% CI: 28.9–34.5). The median PFS for elderly patients with mCRC treated with regorafenib monotherapy was 3.88 months (95% CI: 3.48–5.65), and the median OS time was 10.1 months (95% CI: 8.94–12.1) (Figure 2). No significant differences in PFS and OS were observed between the patients using different initial or final daily dosages (Figure S1A-S1D). Subsequently, we divided the initial dose into a standard-dose group (160 mg/day) and a reduced-dose group (40–120 mg/day). The survival analysis showed no significant differences in PFS and OS between the two groups (Figure S1E,S1F). Additionally, no significant association was observed between prior anti-VEGF therapy and prognosis (Figure S1G,S1H).
In the Cox multivariate analysis, the number of positive outcome events has reached at least ten times the number of variables in the multivariable model to ensure statistical power (13,14). We observed no significant association between the initial daily dose and OS (Table 2). Reducing the initial dose (40–120 mg/d) did not result in a significant change in OS compared to the standard dose [HR (95% CI): 1.26 (0.73–2.15), P=0.41]. While no significant association was observed between the final dose and OS, there was a trend toward shorter OS with a final dose of 80 mg/day compared to 160 mg/day in the Model 1 [HR (95% CI): 1.96 (0.92–4.18), P=0.08] and Model 2 [HR (95% CI): 2.04 (0.94–4.43), P=0.07] (Table 2). The results of two models are very similar. Given the relatively small sample size, we have opted to utilize Model 1 for the subsequent analysis. No association was found between the secondary endpoint PFS and drug dosage in both the univariate and multivariate Cox analyses (Table S4).
Table 2
| Subgroup | N (%) | Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Final daily dose-Model 1 | Final daily dose-Model 2 | Initial daily dose | ||||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||
| Sex | ||||||||||||
| Male | 152 (74.88) | 1 | 1 | |||||||||
| Female | 51 (25.12) | 0.90 (0.62–1.30) | 0.56 | 0.83 (0.43–1.62) | 0.59 | |||||||
| BMI (kg/m2) | ||||||||||||
| ≥22 | 85 (65.89) | 1 | ||||||||||
| <22 | 44 (34.11) | 1.26 (0.83–1.92) | 0.27 | |||||||||
| ECOG score | ||||||||||||
| 0–1 | 190 (93.60) | 1 | ||||||||||
| 2–3 | 13 (6.40) | 0.87 (0.44–1.71) | 0.69 | |||||||||
| Tumor location | ||||||||||||
| Left | 134 (82.21) | 1 | ||||||||||
| Right | 29 (17.79) | 0.78 (0.48–1.27) | 0.33 | |||||||||
| Liver metastasis | ||||||||||||
| Positive | 103 (50.74) | 1 | ||||||||||
| Negative | 100 (49.26) | 0.92 (0.67–1.26) | 0.61 | |||||||||
| Lung metastasis | ||||||||||||
| Positive | 118 (58.13) | 1 | 1 | 1 | 1 | |||||||
| Negative | 85 (41.87) | 0.80 (0.58–1.10) | 0.16 | 1.08 (0.60–1.94) | 0.79 | 1.10 (0.61–1.98) | 0.75 | 0.85 (0.53–1.35) | 0.49 | |||
| RAS gene mutation status | ||||||||||||
| Wild type | 59 (51.30) | 1 | 1 | 1 | 1 | |||||||
| Mutant | 56 (48.70) | 1.38 (0.91–2.10) | 0.13 | 2.29 (1.24–4.23) | 0.008 | 2.40 (1.26–4.56) | 0.007 | 1.77 (1.08–2.91) | 0.02 | |||
| BRAF gene mutation status | ||||||||||||
| Wild type | 81 (96.43) | 1 | ||||||||||
| Mutant | 3 (3.57) | 1.20 (0.37–3.88) | 0.76 | |||||||||
| MMR gene status | ||||||||||||
| dMMR | 5 (5.10) | 1 | ||||||||||
| pMMR | 93 (94.90) | 1.18 (0.47–2.94) | 0.72 | |||||||||
| Prior-anti VEGF therapy | ||||||||||||
| Used | 146 (73.00) | 1 | 1 | 1 | 1 | |||||||
| Not used | 54 (27.00) | 1.37 (0.96–1.94) | 0.08 | 1.72 (0.90–3.26) | 0.10 | 1.70 (0.89–3.24) | 0.11 | 1.93 (1.10–3.38) | 0.02 | |||
| Final daily dose (mg) | ||||||||||||
| 160 | 28 (24.14) | 1 | 1 | 1 | ||||||||
| 120 | 37 (31.90) | 0.95 (0.53–1.72) | 0.87 | 0.83 (0.38–1.78) | 0.63 | 0.80 (0.37–1.74) | 0.58 | |||||
| 80 | 51 (43.96) | 1.27 (0.73–2.21) | 0.39 | 1.96 (0.92–4.18) | 0.08 | 2.04 (0.94–4.43) | 0.07 | |||||
| Initial daily dose (mg) | ||||||||||||
| 160 | 46 (27.06) | 1 | 1 | |||||||||
| 120 | 43 (25.29) | 0.81 (0.50–1.31) | 0.38 | 0.85 (0.43–1.67) | 0.64 | |||||||
| 80 | 76 (44.71) | 1.00 (0.66–1.51) | 0.98 | 1.60 (0.90–2.86) | 0.11 | |||||||
| 40 | 5 (2.94) | 1.38 (0.54–3.54) | 0.50 | 1.64 (0.37–7.28) | 0.52 | |||||||
We initially identified the variables associated with outcomes (P<0.20) through a univariate analysis. Variables with a P value <0.20 in the univariate analysis were adjusted in the Model 1. Variables with a P value <0.20 in the univariate analysis and those with a P value <0.05 from the differential analysis were adjusted in the Model 2. OS, overall survival; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; RAS, Rat sarcoma; KRAS, Kirsten rats arcomaviral oncogene homolog; NRAS, neuroblastoma RAS viraloncogene homolog; BRAF, V-Raf Murine Sarcoma Viral Oncogene Homolog B; MMR, mismatch repair; dMMR, deficiency of MMR; pMMR, proficiency of MMR; VEGF, vascular endothelial growth factor; HR, hazard ratio; CI, confidence interval.
We further defined patients with a final daily dose of 120 or 160 mg/day as the high-dose group, and patients with a final daily dose of 80 mg/day as the low-dose group. The OS of the grouped patients was described using survival curves (Figure S1I), and no significant difference in OS was observed between the high- and low-dose groups. In the multivariate model (Model 1), it was found that OS extended as the final daily dose of regorafenib increased, and patients who could tolerate a high dose of 120–160 mg/day after dose adjustment benefited significantly in terms of OS [HR (95% CI): 0.45 (0.25–0.84), P=0.01].
Sensitive analysis
First, we analyzed the PSM dataset and the IPTW dataset with Cox regression for sensitivity analysis. The PSM was used to reduce selection bias and evaluate the accuracy of the results. The pre- and post-match cohorts are shown in Table S5. The histogram and jitter plot both indicated that the propensity score distributions of the high-dose group and the low-dose group after matching were more similar. The SMDs for each matched variable did not exceed 0.1, further affirming the balance of matched groups and the acceptable quality of matching (Figures S2,S3 and Table S5). In the PSM analysis, OS was insignificantly prolonged in the high-dose group compared to the low-dose group [HR (95% CI): 0.58 (0.30–1.12), P=0.09] (Figure S4). After IPTW, the baseline characteristics of the two groups were balanced, and significant differences between the groups still existed [HR (95% CI): 0.48 (0.26–0.91), P=0.03].
Second, an increased line of therapy may indicate that patients are less likely to benefit from and tolerate the treatment. We primarily focused on patients who had failed at least two standard treatments, most (n=123, 60.59%) of whom were treated with regorafenib monotherapy as third-line therapy. We proceeded with the same analysis within the specified group. The results of the multivariate analysis did not show any significant changes. Compared to the low-dose group, patients in the high-dose group experienced longer OS [HR (95% CI): 0.26 (0.11–0.64), P=0.003].
Third, considering the significant differences in sex across various final daily dose groups, and the close association between sex and cancer incidence as well as mortality, we excluded the confounding effect of sex through subgroup analysis. The conclusions drawn for male patients aligned with the original findings [HR (95% CI): 0.35 (0.16–0.75), P=0.007]. However, no association was observed between the final daily dose and OS in female patients [HR (95% CI): 0.80 (0.23–2.79), P=0.73] (Figure S5).
Age subgroup analysis
The elderly patients treated with regorafenib monotherapy had a broad age range. Based on the age distribution (Figure S6), it was observed that the majority of elderly patients were aged around 65–70 years. We divided the elderly population into “younger elderly” (n=128, 63.05%) and “older elderly” (n=75, 36.95%) using the age of 70 years as the dividing point. The “younger elderly” patients often received higher doses than the “older elderly” patients (initial dose of 160 mg/day: 30.39% vs. 22.06%; final dose of 120–160 mg/day: 59.42% vs. 51.05%).
To further explore the optimal final dose for patients in different age groups, we conducted an age subgroup analysis. We found that among those aged ≥70 years, no significant association was observed between the final dose and OS (Figure S7A). In those aged <70 years, the application of higher final doses (120–160 mg/day) was significantly associated with a prolongation of OS compared to a final dose of 80 mg/day [HR (95% CI): 0.38 (0.16–0.91), P=0.03] (Figure S7B), and the prolongation of OS was predominantly observed in the 120 mg/day dose group [HR (95% CI): 0.24 (0.09–0.67), P=0.006] and not in the 160 mg/day dose group [HR (95% CI): 0.66 (0.25–1.72), P=0.39].
Safety
According to data from six centers (i.e., Beijing Hospital, The Affiliated Hospital of Hebei University, The Fourth Hospital of Hebei Medical University, Henan Cancer Hospital, Shandong Tumor Hospital, and Tianjin People’s Hospital; n=77), 36 (46.75%) patients experienced AEs, of which 11.69% were classified as grade III AEs. Fatigue (18.18%), loss of appetite (18.18%), and hand-foot skin reactions (10.39%) were the most common AEs (Table S6). We observed a statistically insignificant increase in the incidence of AEs in the higher dose group compared to the lower dose group. In the high-dose group, the majority of patients experienced AEs. In contrast, in the low-dose group, the majority of patients had no adverse reactions. No association was found between the baseline characteristics, such as age, sex, BMI, and ECOG performance status, and the occurrence of AEs (Table S7).
Discussion
This real-world study examined the dosage and prognosis of regorafenib monotherapy in elderly patients with mCRC. The World Health Organization (WHO) defines individuals aged ≥60 years as elderly. Our study showed that regorafenib monotherapy resulted in a median PFS time of 3.88 months and a median OS time of 10.1 months in the elderly population, which is broadly consistent with the findings of previous phase III randomized controlled trials and real-world studies (4,5,15-19), and demonstrates the value of regorafenib monotherapy in elderly patients with mCRC.
In our study, 46 patients (27.06%) initiated treatment with the standard initial daily dose of 160 mg/day. After dose adjustment, 28 (24.14%) patients continued treatment at this dosage. The majority opted for a reduced daily dose of 80 or 120 mg/day, which is consistent with the current usage of regorafenib in the Chinese population (17,20). In a phase III clinical trial, Asian patients had a higher incidence of AEs than non-Asian patients (5). In clinical practice, choosing a lower initial dose or an intermittent dosing strategy is an effective way to alleviate AEs and improve drug tolerance (9-11,21), which provide more possibilities for the clinical application of regorafenib. Our multivariate analysis did not show any association between the initial daily dose and prognosis in the elderly population. Given the association between AEs and the medication dose, the elderly population should be monitored more closely during the initial phase and it is necessary to adjust the dosage based on patient conditions and reactions.
In addition, our study also examined the final daily dose, which is gradually adjusted based on the efficacy and AEs during medication. The final daily dose may be influenced by a number of factors, including the subjective perception of patients. A real-world study of regorafenib in Chinese patients with mCRC showed that the median OS was prolonged in the high final daily dose group represented by 120 mg/day compared to the other dose groups (22). This conclusion was subsequently validated in elderly patients aged ≥60 years (23), indicating that a final daily dose of 120 mg/day could achieve a favorable risk/benefit ratio and may be an appropriate treatment dose for Chinese patients with mCRC. The above two studies mainly focused on the population treated with regorafenib monotherapy, but included a small number of patients receiving combination therapy. Our study was conducted in an entirely monotherapy cohort. The Cox multivariate analysis showed that no difference in OS was observed between the final daily doses of 120 and 160 mg/day, and reducing the dose to 80 mg/day may be associated with poorer OS compared to the dose of 160 mg/day. Accordingly, patients receiving a final daily dose of 120 or 160 mg/day were defined as the high-dose group. Compared to the patients receiving a low dose of 80 mg/day, those receiving the high final daily dose benefited significantly in terms of OS.
Multiple sensitivity analyses were conducted to confirm the reliability of the conclusions. Initially, we utilized PSW and IPTW to match and adjust for potential confounding variables. The relationship between final daily dose and OS remained unchanged. Subsequently, considering that an increased line of therapy may indicate that patients are less likely to benefit from and tolerate the treatment, a reanalysis was carried out among patients receiving third-line therapy, yielding similar conclusions. Finally, sex was found to be closely associated with cancer incidence and mortality rates (2,24), with significant difference among various final daily dose groups in our study. Subgroup analysis was performed, with conclusions in male patients aligning with the original results. However, no association between the final daily dose and OS was observed in female patients. Given the limited number of female patients in our study, it is imperative to develop clinical studies with a balanced sex ratio to further validate the reliability of this conclusion. These findings collectively suggested that opting for a higher tolerable final daily dose may lead to prognostic benefits, even in elderly patients.
In the age subgroup analysis, the application of higher final doses (120–160 mg/day) was significantly associated with the prolongation of OS compared to a final dose of 80 mg/day in those aged <70 years, and the prolongation of OS was predominantly observed in the 120 mg/day dose group and not in the 160 mg/day dose group, which once again highlights that 120 mg/day is an optimal dose for elderly mCRC patients in China. Therefore, we recommend that in elderly patients aged <70 years, maintaining the final daily dose at 120 mg/day if the patient can tolerate may result in prognostic benefits.
Regarding safety, the main AEs in elderly patients were fatigue, loss of appetite, and hand-foot skin reactions. The overall incidence of AEs was lower than that reported in previous studies, which may be related to the lower dose of medication (5,17,20,25,26). The dosage of 160 mg/day was associated with a higher incidence of AEs, although the results were not statistically significant. Combined with the prognostic analysis, we further recommend a dosage of 120 mg/day as the treatment dose for elderly Chinese patients with mCRC using regorafenib monotherapy.
This study had several limitations. First, it was a multicenter real-world study, and there might have been differences in the quality of the data collection among centers, especially in the recording of medication discontinuation caused by AEs or patient subjective preferences. As a result, our time to treatment failure (TTF) data were incomplete, preventing us from using this parameter commonly employed in real-world studies to represent drug efficacy, which could potentially lead to unforeseen biases. Otherwise, while our study included several large medical institutions in China, it only covered a small portion of urban areas. Therefore, the generalizability of the results to other regions remains to be considered. Future efforts should focus on conducting larger-scale clinical studies or verifying the applicability of these findings in different regions or populations. The robust statistical analysis was also limited by the relatively small sample size and missing data.
Conclusions
Regorafenib monotherapy has been shown to improve prognosis in the elderly population with mCRC, but optimal dosage strategy has yet to be established. This research represents a multicenter real-world investigation of the different dosing regimens in this group of patients. We observed that the final daily dose was significantly associated with OS. Maintaining the final dosage at 120 mg/day whenever possible in elderly patients aged <70 years may result in prognostic benefits. For patients aged ≥70 years, dosage adjustments should be made according to individual conditions and responses.
Acknowledgments
For their encouragement, support, and research assistance, we would like to thank Zimin Liu from Department of Medical Oncology, Affiliated Hospital of Qingdao University, Xiaobing Chen from Department of Medical Oncology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Bo Liu from Department of Medical Oncology, Shandong Cancer Hospital, Hao Yan from Department of Oncology, Tianjin Union Medical Center, Xiujuan Qu from Department of Oncology, The First Hospital of China Medical University, Shengmian Li from Department of Gastroenterology and Hepatology, Fourth Hospital of Hebei Medical University, Aimin Zang from Department of Oncology, Affiliated Hospital of Hebei University and Liangjun Zhu from Department of Medical Oncology, Jiangsu Cancer Hospital who have contributed substantially to the completion of this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-464/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-464/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-464/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-464/coif). N.Z.T. receives honorarium from Medtronic Singapore for lecture presentation and conducting workshop, and Device Technologies Asia for robotic case proctoring. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The original study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The original study was approved and the informed consent was waived for all patients by the ethics committee of the Cancer Hospital of Chinese Academy of Medical Science (reference number: 2021010711241202). All participating hospitals were informed and agreed with this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Arai H, Battaglin F, Wang J, et al. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev 2019;81:101912. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022;7:627-47. [Crossref] [PubMed]
- Siegel RL, Wagle NS, Cercek A, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- Shah Y, Verma A, Marderstein AR, et al. Pan-cancer analysis reveals molecular patterns associated with age. Cell Rep 2021;37:110100. [Crossref] [PubMed]
- Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 2019;20:1070-82. [Crossref] [PubMed]
- Argilés G, Mulet N, Valladares-Ayerbes M, et al. A randomised phase 2 study comparing different dose approaches of induction treatment of regorafenib in previously treated metastatic colorectal cancer patients (REARRANGE trial). Eur J Cancer 2022;177:154-63. [Crossref] [PubMed]
- Petrioli R, Chirra M, Messuti L, et al. Efficacy and Safety of Regorafenib With 2/1 Schedule for Patients ≥ 75 Years With Metastatic Colorectal Cancer (mCRC) After Failure of 2 Lines of Chemotherapy. Clin Colorectal Cancer 2018;17:307-12. [Crossref] [PubMed]
- Aparicio T, Darut-Jouve A, Khemissa Akouz F, et al. Single-arm phase II trial to evaluate efficacy and tolerance of regorafenib monotherapy in patients over 70 with previously treated metastatic colorectal adenocarcinoma FFCD 1404 - REGOLD. J Geriatr Oncol 2020;11:1255-62. [Crossref] [PubMed]
- Concato J, Peduzzi P, Holford TR, et al. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol 1995;48:1495-501. [Crossref] [PubMed]
- Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. [Crossref] [PubMed]
- Ducreux M, Petersen LN, Öhler L, et al. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer in routine clinical practice in the prospective, observational CORRELATE study. Eur J Cancer 2019;123:146-54. [Crossref] [PubMed]
- Metges JP, Genet D, Tougeron D, et al. Real-world safety and effectiveness of regorafenib in metastatic colorectal cancer: the French CORRELATE cohort. Future Oncol 2021;17:3343-53. [Crossref] [PubMed]
- Yeh KH, Yang TS, Hsu TC, et al. Real-world evidence of the safety and effectiveness of regorafenib in Taiwanese patients with metastatic colorectal cancer: CORRELATE Taiwan. J Formos Med Assoc 2021;120:2023-31. [Crossref] [PubMed]
- Deng YY, Zhang XY, Zhu PF, et al. Comparison of the efficacy and safety of fruquintinib and regorafenib in the treatment of metastatic colorectal cancer: A real-world study. Front Oncol 2023;13:1097911. [Crossref] [PubMed]
- He WZ, Wang L, Yin CX, et al. Regorafenib with or without a programmed cell death protein 1 antibody as third-line treatment for microsatellite stable metastatic colorectal cancer. Cancer Med 2023;12:6488-98. [Crossref] [PubMed]
- Xu D, Liu Y, Tang W, et al. Regorafenib in Refractory Metastatic Colorectal Cancer: A Multi-Center Retrospective Study. Front Oncol 2022;12:838870. [Crossref] [PubMed]
- Tabchi S, Ghosn M. Regorafenib: start low and go slow. Target Oncol 2015;10:445-7. [Crossref] [PubMed]
- Jiang ZC, Sun YK, Zhang W, et al. Analysis of metastatic colorectal cancer patients treated with regorafenib in real-world practice. Zhonghua Yi Xue Za Zhi 2020;100:2018-22. [PubMed]
- Liu WB, Zhao YB. Effectiveness and safety of Regorafenib in elderly patients with metastatic colorectal cancer. Chinese Journal of Geriatrics 2021;40:5.
- Rubin JB. The spectrum of sex differences in cancer. Trends Cancer 2022;8:303-15. [Crossref] [PubMed]
- Lam KO, Lee KC, Chiu J, et al. The real-world use of regorafenib for metastatic colorectal cancer: multicentre analysis of treatment pattern and outcomes in Hong Kong. Postgrad Med J 2017;93:395-400. [Crossref] [PubMed]
- Yamaguchi K, Komatsu Y, Satoh T, et al. Large-Scale, Prospective Observational Study of Regorafenib in Japanese Patients with Metastatic Colorectal Cancer in a Real-World Clinical Setting. Oncologist 2019;24:e450-7. [Crossref] [PubMed]



