
Unexpected peritoneal metastases diagnosed at the time of primary colon cancer resection: controversies regarding options for management
Introduction
Synchronous peritoneal metastases can occur in 5–7% of patients with colorectal cancer; some may unexpectedly present during the planned colorectal resection (1,2). Peritoneal metastases diagnosed at the time of a colon or rectal cancer resection places the patient at a high risk of disease progression within the abdomen and pelvis (3). In the past, most surgeons faced with this situation would resect the primary, consult the medical oncologist, and treat peritoneal metastases with systemic chemotherapy. This would be followed by a surveillance computed tomography (CT) scan and carcinoembryonic antigen (CEA) follow-up. Many health boards including the United Kingdom would not support cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal metastases. In other venues, close surveillance with CT as well as serial CEA assays would guide the decision for possible second-look surgery (4). In this approach, the technique of primary resection of the colon was essentially unchanged. This standard of care has been successfully challenged by the adoption of specialized surgical techniques for peritoneal metastases called CRS (5). This strategy employs a series of peritonectomy procedures combined with visceral resections to remove all visible evidence of the primary cancer and the peritoneal metastases (6,7). The complete cytoreduction is followed during the same operation by HIPEC with a goal to eradicate small volume residual disease (8). Rather than palliative systemic chemotherapy being the standard of care for primary colorectal cancer with peritoneal metastases, a potentially curative approach employing CRS and HIPEC along with systemic chemotherapy can now be considered (9). Controversies regarding the options for optimal management of these patients continue to exist.
An important concept in the management of these patients is data concerning tumor cell entrapment that follows primary cancer resection in patients with known peritoneal metastases (10-13). Sugarbaker reported that primary colon or rectal cancer resection performed in patients with peritoneal metastases in an absence of HIPEC contributed to the process of cancer dissemination. Approximately 50% of patients having CRS for peritoneal metastases sometime after primary resection were found to have peritoneal dissemination to the abdominal incision or laparoscopy port sites. Two-thirds of these patients had cancer progression within the resection site of the primary cancer. The primary cancer resection in the presence of peritoneal metastases was, in reality, a part of the pattern of progression of the colon cancer (14).
With these new concepts in mind, asymptomatic patients or those presenting with obstruction or bowel perforation and unexpected synchronous peritoneal metastases diagnosed at the time of colon resection are discussed. Options for the multidisciplinary management of colon cancer patients with peritoneal metastases are presented (Figure 1).

Prognostic indicators needed to evaluate colon cancer with peritoneal metastases
In order to adequately evaluate peritoneal metastases from colon cancer, there are some prognostic indicators which must operate at the time of the initial evaluation of all patients. This would include patients with peritoneal metastases identified prior to colon resection but also to those patients with unresected peritoneal metastases diagnosed at the time of primary colon cancer resection. The Peritoneal Cancer Index (PCI) is a necessary evaluation in order to determine if the extent of disease is compatible with a potentially curative approach using CRS and HIPEC or whether the patient should go straight to palliative systemic chemotherapy treatments with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CAPOX). Figure 2 shows how the PCI uses the distribution of the disease in 13 abdominopelvic regions along with the extent of disease in each of these regions as an estimate of the volume of abdominal and pelvic cancer dissemination (15). Goéré and colleagues presented data to show that patients with a PCI greater than 17 rarely, if ever, profit long term from CRS plus HIPEC (16). When patients with unexpected peritoneal metastases from colon cancer are being evaluated intraoperatively, it is extremely important to determine the PCI.
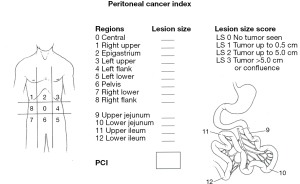
One of the most reliable prognostic indicators for peritoneal metastases is the completeness of cytoreduction score (15). Those patients in whom a CRS is possible with no visible disease remaining after surgery may show a prolonged disease-free and overall survival. Those patients who have sites of unresectable disease, such as encasement of the porta hepatis, will not show long-term survival. These patients with sites of unresectable disease are candidates for systemic chemotherapy but not for a potentially curative treatment approach using CRS and HIPEC.
A third group of patients for special consideration if unexpected peritoneal metastases are documented are those patients with hepatic metastases. Since our patients had a CT scan prior to their colon cancer resection, hepatic metastases in these patients would be small, less than 1 cm in diameter, and limited in their distribution within the liver. The data is well established that patients with peritoneal metastases and liver metastases can be long-term survivors. However, the likelihood of a long-term favorable outcome is reduced by at least 50% if peritoneal metastases and liver metastases need to be treated. A reasonable opinion for these patients with unexpected peritoneal metastases would follow the recommendations of Elias et al. (17,18). These authors suggested that a limited number [1–3] of liver metastases that can be resected without a major hepatic lobectomy may continue as candidates for CRS and HIPEC. If major hepatic surgery is indicated, the combined disease (liver metastases and peritoneal metastases) is better approached using systemic chemotherapy treatments.
In all patients, a careful description of the peritoneal disease must be made a part of the patient’s permanent record. The PCI must be documented and its role in the decision-making process described in the operative report.
Colon cancer with no overt symptoms diagnosed with peritoneal metastases at the time of colon resection with CRS and HIPEC available
In this situation, the management of the patient will depend on several factors. These contributions include but are not limited to whether the patient is undergoing a laparoscopic or robotic procedure or a laparotomy, the resectability of the primary tumor, the PCI score, whether the patient has been counselled and consented for a HIPEC procedure, experience of the operating team in cytoreductive procedures and ability to perform an unscheduled HIPEC procedure at short notice. As diagrammed in Figure 1, in some patients the situation can be managed as a one-step procedure (immediate definitive treatment) and in others a two-step procedure is required (delayed definitive treatment). In both groups of patients, the peritoneal metastases must be confirmed by frozen section.
In the fit patient being treated at an institution where CRS and HIPEC are immediately available, the peritoneal metastases can be definitively treated along with the primary colon cancer as a one-step procedure. Pestieau et al. and Braam et al. presented data to suggest that this surgical approach provides the patient with the best outcome (19,20). Synchronous peritoneal metastases unexpectedly diagnosed at the time of resection of colorectal cancer usually has some favorable characteristics like a low PCI (median 3–5) and a high rate of complete cytoreduction both of which are associated with improved survival after CRS and HIPEC (21,22). Pestieau and colleagues identified intraoperatively peritoneal metastases in five patients and were able to perform CRS, resection of the primary colon cancer with anastomosis, and HIPEC. All but one of Pestieau’s patients were 5-year survivors and 2 patients went on to be cured of their disease (19). This approach assumes that the institution can provide a previously unscheduled HIPEC procedure within a few hours and that operating room time is available for a more extensive surgical procedure. An extended consent for surgery that includes CRS and HIPEC if peritoneal metastases are intraoperatively detected is an important consideration as well as the additional cost of the procedure to the patient and the healthcare system. This approach may be referred to as immediate definite treatment of peritoneal metastases from colon cancer.
Definite technical and institutional factors will determine if a previously unplanned CRS plus HIPEC is to be considered. The pharmacy must be able to produce the chemotherapy agent within a few hours. Also, if a perfusionist is needed, they must be available on short notice. At least 2 hours of additional operating room time needs to be available. Only if the HIPEC procedure can be performed safely and effectively when it is previously unscheduled should it be considered. It is possible that the selection criteria to undergo a previously unscheduled CRS and HIPEC are more demanding than an elective CRS plus HIPEC.
To our knowledge, a direct comparison of the adverse events associated with an unscheduled CRS plus HIPEC procedure as compared to an elective CRS plus HIPEC procedure is not available. Only experienced groups that perform HIPEC on a routine basis and can complete the treatment without compromise of safety or efficacy should consider the unscheduled CRS and HIPEC.
If peritoneal metastases are definitively diagnosed and are to be treated by immediate definitive treatment, the cancer resection should be modified. Firstly, the primary tumor should be widely excised with a complete regional lymph node dissection. If tumor involves the peritoneal surface of the colon, the adjacent inflammation on parietal peritoneum should be removed using peritonectomy. Secondly, a total omentectomy should be performed. Even when the greater omentum appears to be completely normal, occult omental metastasis may be seen in 12–15% of patients so that peritoneal metastases will be diagnosed in this organ at a later time (23). The round ligament and falciform ligament should be resected and the gastrohepatic ligament divided to open the lesser sac. In the post-menopausal woman, the ovaries and Fallopian tubes should be removed as there is a 17% risk of occult metastasis in the ovaries in the presence of peritoneal metastases of colorectal origin (24). In a pre-menopausal woman, the ovaries need to be carefully inspected and, in most instances, preserved unless an abnormality is visualized (25). The peritoneal metastases that were biopsied and determined by frozen section to be cancer need to be widely excised using a visceral resection or peritonectomy (26). Thirdly, following this primary colon cancer resection plus CRS, HIPEC, usually with mitomycin C, is administered. After the anastomosis the abdomen is closed and the patient is expected to have a slightly prolonged postoperative course. Adjuvant chemotherapy using FOLFOX or CAPOX will be recommended (27).
Although the availability of CRS with HIPEC is steadily increasing around the world as a treatment option for peritoneal metastases from colon cancer, it is not universally available to treat unexpected peritoneal metastases. The number of treatment centers that can add several hours of surgery and arrange for HIPEC to be administered with only a few hours notice to the pharmacy, to a perfusionist, and to the surgical team may be quite limited. The expertise necessary for a safe and effective CRS and HIPEC added to the operating schedule may be limited to HIPEC centers. It is well known that centers who have overcome the learning curve, HIPEC is rarely associated with increased morbidity in low PCI patient. Hence, CRS with HIPEC in such patients could be performed after full discussion with the patients and their family.
Alternatively, a modified CRS that would completely resect all visible peritoneal metastases but not employ HIPEC is a reasonable consideration. The PRODIGE 7 trial failed to show efficacy of HIPEC in colon cancer patients treated by complete CRS for peritoneal metastases (28). In this trial, a 30-minute HIPEC with oxaliplatin showed benefit in a subgroup of patients with a PCI between 10 and 15. These improved outcomes may expand when a mitomycin C HIPEC is used for a longer duration. Nevertheless, PRODIGE 7 data showed that complete CRS in the absence of HIPEC resulted in excellent survival of colon cancer peritoneal metastases patients. When HIPEC is not available, the PCI is low and the surgical team is experienced in CRS, a definitive CRS in the absence of HIPEC may be considered.
Colon cancer with no overt symptoms diagnosed with peritoneal metastases at the time of colon resection but CRS and HIPEC not available
If the surgeon who is operating on this patient with unexpected peritoneal metastases cannot arrange for CRS and the administration of HIPEC at the time of the colon resection, a major change in management in the form of a two-stage strategy is indicated (Figure 1). In this strategy, the peritoneal nodules detected by minimally invasive or open surgical exploration should be biopsied and confirmed by frozen section to be metastatic disease. Nodules on the parietal or visceral peritoneum that are thought compatible with peritoneal metastases by the surgeon should not be assumed to have this diagnosis (29,30). These nodules must be subjected to frozen section and confirmed as metastatic disease. In this situation the number and position of peritoneal metastases should be carefully recorded using a PCI chart. Pictures or videos are of considerable value for the knowledgeable referral of the patient. The primary colon cancer is left in place, the majority of the peritoneal metastases are left in place, and the abdomen is closed.
These patients with unexpected peritoneal metastases at institutions where CRS and HIPEC are not available on an unscheduled basis come to the operating room expecting to have a definitive surgical procedure but no resection occurs. A diagnosis of peritoneal metastases is established and a PCI carefully recorded but the colon cancer is left in place. If the operative approach was laparoscopic or robotic, these patients do not need to heal a major abdominal incision that was performed with only diagnostic information gathered. If the patient had a major laparotomy, these biopsies and PCI determination are of benefit but the major abdominal incision is not of benefit to the patient. It may seem more difficult to the surgeon who has performed a laparotomy as compared to a laparoscopic or robotic approach to cancel the colon resection that may take place at a later time. Certainly, careful explanation to the patient and family regarding the cancellation of the colon cancer resection is indicated.
At this point the management may not be completely straightforward. The surgeon has several options: to schedule a planned CRS and HIPEC at the earliest convenient date, to refer the patient to a center with expertise for CRS and HIPEC (early definitive treatment), or to offer neoadjuvant chemotherapy and subsequently schedule CRS and HIPEC. If CRS and HIPEC are used after a short course of neoadjuvant chemotherapy, it is referred to as delayed definitive treatment. The results of the FOXTROT trial show that 4 cycles of neoadjuvant chemotherapy will facilitate the subsequent cancer resection. These data may indicate that neoadjuvant chemotherapy may be recommended (31). The NeoCol trial showed safety with a suggestion of efficacy (32). These studies, however, did not include patients with peritoneal diseases. Therefore, benefit from disease progression cannot be assured from these two trials. The manuscript by Devilee et al. was a retrospective study suggesting improved survival from neoadjuvant chemotherapy in patients with resectable peritoneal metastases. It had drawbacks in that it included only patients who underwent surgery. Those patients who progressed on chemotherapy were not included. This study also excluded patients with signet tumors and those undergoing chemoradiation (33). With these limitations in mind, the Devilee manuscript and the FOXTROT randomized trial provide the rationale to use neoadjuvant chemotherapy.
It is important at this point to establish that the neoadjuvant chemotherapy recommended for these patients at high risk for treatment failure has not been definitively accepted as standard of care. In the trials to date, three or four cycles of FOLFOX or CAPOX were recommended as the neoadjuvant treatment. This short course of neoadjuvant chemotherapy has not been shown to cause harm. Unsalvageable progression in poor responders has not been reported. However, after the full extent of the disease has been staged with a definitive resection of the primary colon cancer and the metastatic disease, a decision for subsequent adjuvant chemotherapy can be made. In most of these patients a total of eight cycles of systemic chemotherapy will be recommended in order to optimize the systemic chemotherapy treatments of colon cancer. Neoadjuvant chemotherapy and adjuvant chemotherapy are combined at the proper time in this group of high risk patients.
In Figure 1, neoadjuvant chemotherapy is recommended when possible, however, other considerations such as cost may need to be considered. The pilot arm of CAIRO 6 trial supported neoadjuvant chemotherapy along with bevacizumab, because there was no progression of disease, response rates were high and neoadjuvant treatment made no difference in subsequent resectability or morbidity. The final results of phase 3 CAIRO 6 trial will hopefully further elucidate the proper role for neoadjuvant chemotherapy (34).
Our manuscript may be criticized in that neoadjuvant chemotherapy is recommended in all of these peritoneal metastases patients unless CRS and HIPEC are used as immediate definitive treatment. In these patients treated definitively with CRS and HIPEC, the chemotherapy would be given as adjuvant treatment. Data to support neoadjuvant chemotherapy in all patients with colon cancer certainly does not exist. In contrast, patients who clearly demonstrate signs of poor prognosis may be selectively treated with neoadjuvant chemotherapy. Of course, randomized studies and definitive data as outlined above are preliminary. Further justification for neoadjuvant chemotherapy beyond that presented in this manuscript is not currently available. However, the high risk for recurrence of peritoneal metastases patients makes neoadjuvant chemotherapy a reasonable treatment option which we have proposed in the treatment algorithm (Figure 1). Because of the high risk of these patients for rapidly recurring disease, our preference is to preserve neoadjuvant chemotherapy as a part of this treatment algorithm.
Following the neoadjuvant chemotherapy, the patient is taken back to the operating room for an open colon resection which may be preceded by laparoscopic staging. In this delayed definitive treatment, the primary tumor is removed and a modified CRS as discussed earlier is performed. HIPEC with mitomycin C is administered. The anastomosis is performed following/or prior to the HIPEC procedure and the patient is expected to have an uneventful recovery. The length of stay may be prolonged 2 or 3 days over that which is expected for colon resection without CRS and HIPEC.
It is important to emphasize that the 2-step approach must not involve a resection of the primary colon cancer at the first procedure. Some groups have performed the HIPEC days or weeks following the colon cancer resection with CRS. When compared to a single step procedure, this late definitive treatment was associated with a significant delay in performing CRS and HIPEC (20). There were more extended bowel resections in that 60% of earlier anastomoses were resected and 55–66% of such anastomoses had malignancy on histopathological examination. Other problems with late definitive treatment were a significantly higher rate of relaparotomy after a resection of a previous anastomosis and a higher rate of creation of permanent stomas. However, survival rates between the two approaches were equivalent (20). Hence, this strategy is not to be considered optimal treatment. By the tumor cell entrapment hypothesis, cancer cells will be entrapped in scar tissue that is caused by tissue dissection (9,10). Also, cancer cells will be trapped at the anastomotic site. A delayed HIPEC will not treat these entrapped cancer cells and is not an acceptable treatment option (35,36).
Unexpected peritoneal metastases in a patient with obstructing colon cancer
In patients with colonic obstruction from colon cancer the management plan is often dependent on the capability of the institutions to safely and effectively place a colonic stent at the anatomic site of the colonic obstruction (Figure 1). Stent placement in patients with left-sided obstructing colon cancer has been critically reviewed by a systematic review and meta-analysis. Amelung and coworkers in 2018 collected data to show no difference in overall survival, disease-free survival or local recurrence rate when a colonic stent was used as a bridge to surgery (37). Xu and coworkers in 2020 reported greater benefits of a colonic stent bridge to surgery as compared to a transanal colorectal tube. The stent had improved quality of decompression and safety (38). In 2021, Tan and coworkers reported a survival advantage when an emergency diverting stoma was compared to a colonic stent (39). A colonic stent or a diverting stoma resulted in better overall survival when compared to emergency resection. Tan and coworkers in their systematic review concluded that a diverting ostomy as a bridge to surgery was the superior treatment strategy. Ishibe and coworkers suggest a diverting ostomy for obstructing cancer in order to facilitate neoadjuvant chemotherapy. In 45 patients who had an R0 resection after downstaging with neoadjuvant chemotherapy, they report a 3-year relapse-free survival of 76.5%. This is far better than patients treated with resection alone following a diverting ostomy (40).
In some unusual patients it is possible that a bypass or resection with primary anastomosis in the acute setting may be considered. Emergency resection of an obstructing colon cancer is not often recommended (39). If this option is used to treat the obstruction, patients would become candidates for CRS and HIPEC at some time in the future. This delayed definitive treatment has not shown acceptable results (20,35,36). If a definitive resection is performed with obstruction and peritoneal metastases, the surgical team would need to make a judgement that these patients would not be a candidate for CRS plus HIPEC at some time in the future.
Suzuki and coworkers summarized the possible disadvantages of a self-expandable stent placement in a right-sided obstructing colon cancer. There was a lower technical success rate and longer procedure time (41). In 2020, van Hooft and coworkers published guidelines for the European Society of Gastrointestinal Endoscopy (ESGE) (42). The ESGE guidelines cited too many difficulties of stenting an obstructed colon proximal to the splenic flexure. They stated that placement of a self-expandable metal stent as a bridge to surgery is recommended only for obstructed left-sided colon cancer.
Although the oncologic outcome of a self-expanding metal stent has been questioned by some authors (39), the ESGE guidelines recommend stenting as a bridge to surgery for obstructing left colon cancer (42). After successful bowel decompression by the stent and the resumption of oral nutrition, the multidisciplinary team must make a decision regarding the initiation of neoadjuvant chemotherapy versus a recommendation to proceed with a wide resection of the left-sided cancer (31-33). In a fit patient an obstructing colon cancer may be regarded as sufficient risk of surgical treatment failure and an adequate indication for neoadjuvant chemotherapy. However, as discussed earlier, after resolution of the obstruction by the self-expandable metal stent, CRS with the patient prepared for HIPEC may be recommended without neoadjuvant chemotherapy. The fitness of the patient for chemotherapy treatment may be the deciding factor.
If the colon is obstructed and ischemic, resection is necessary. These cases require damage control laparotomy in order to remove the focus of life endangering sepsis. This strategy is with the hope of saving a life which unfortunately must prioritize the outcome of peritoneal metastases (43).
The proper placement of the diverting ostomy in relation to the obstructing cancer is an important consideration. The distal portion of the loop ostomy should be positioned so it can be resected as part of the colon resection. For a right colon obstruction, the loop ileostomy should be approximately 15 cm from the ileocecal valve. The distal portion of the loop ileostomy becomes a part of the colon cancer specimen and the proximal part of the loop is used in the ileocolic anastomosis. For a splenic flexure or descending colon cancer, a midline loop transverse colostomy is recommended (44). The distal portion of the loop transverse colostomy is resected as part of the colon cancer specimen. The proximal portion of the loop colostomy is the proximal portion of the colocolic anastomosis. For a sigmoid or rectosigmoid primary, a loop sigmoid colostomy is optimal. The distal portion of the colostomy is resected as part of the colon cancer specimen and the proximal part of the loop is the proximal portion of the colocolic anastomosis.
After the patient becomes a fit candidate for surgery, the colon cancer should be removed and definitive cytoreduction performed and HIPEC used. In almost all situations a primary anastomosis can be performed without additional diversion. The one situation in which a delayed ostomy closure must be performed may occur in patients who had a diverting loop ileostomy for a left-sided primary cancer. Optimal management occurs if the patient does not need to return for stoma closure as a third procedure at a later time.
Unexpected peritoneal metastases in a patient with perforated colon cancer
An emergency situation in colorectal surgery is the perforation of the bowel wall as a result of cancer progression. In a large proportion of these patients the primary tumor is advanced with full thickness invasion of the bowel wall and extensive involvement of the regional lymph nodes. Control of the colon perforation with a definitive irrigation of the entire peritoneal cavity becomes a lifesaving emergency procedure. Peritoneal metastases may be observed and biopsied at the time of exploration. If peritoneal metastases are observed, they must be biopsied. However, if the perforation has occurred through the primary tumor, contamination of the peritoneal space with colon cancer cells is highly likely (45). Consequently, in all colon cancer perforations, it is wise to treat the patient as a peritoneal surface malignancy patient.
The recommendations for control of the perforation must include a resection of the colon cancer (Figure 1). One very reasonable recommendation is a limited resection (segmental removal) of the colon for approximately 5 cm on either side of the perforation. This resection does not involve a definitive wide resection of the adjacent lymph nodes which is a requirement with advanced disease. In a very few patients it may be safe to perform an anastomosis after this resection. In other patients an end colostomy proximally with a mucus fistula distally may be the optimal plan for managing the residual colon.
Assuming that the patient recovers without abscess or major wound infection, systemic chemotherapy should be considered (31-34). Additional colon removal with wide resection of the relevant lymph nodes is indicated and a CRS should be performed; if at all possible HIPEC with mitomycin C initiated. In most patients a primary colocolic anastomosis would be indicated.
The initial resection of the perforated colon should be minimal in order to prevent the deep implantation of cancer cells in the tissues that border on the lymph node dissection. Although dividing potentially contaminated lymphatic channels close to the primary tumor is to be avoided whenever possible, it is necessary in this situation. A wide resection of the initial anatomic site of colon resection and relevant lymphatics would be undertaken at the subsequent second operation. This wide resection should remove the cancer cells that may be implanted at the anastomosis or at the ostomy sites that were constructed in order to deal definitively with the perforated bowel.
Conclusions
If peritoneal metastases are anticipated, the surgeon will be able to address the metastatic disease detected during surgical resection of colorectal cancer in a single step. Risk factors for peritoneal metastases in colorectal cancers include young age, T4 or node positive tumors, poorly differentiated or mucinous histology, presence of ovarian metastasis or suspicious findings on preoperative imaging (2,3). In patients with these high-risk factors, pre-operative counselling and consenting for CRS and HIPEC should be performed and arrangements made for performing the CRS and HIPEC as a planned part of the primary tumor resection if peritoneal disease is intraoperatively detected. However, a common practice in the management of a primary colon cancer with unexpected peritoneal metastases is standard colon resection without special treatment of the peritoneal metastases. This resection is followed by referral to the medical oncologist for systemic chemotherapy. This approach may not provide the optimal results that synchronous primary colon resection with CRS plus HIPEC may offer in this situation. Long-term favorable results may be expected when the primary cancer and the unexpected synchronous peritoneal metastases are concomitantly treated with CRS and HIPEC. If unexpected peritoneal metastases are found and CRS plus HIPEC is not available then the resection should be discontinued. The distribution and volume of peritoneal metastases should be carefully recorded and a definitive biopsy confirmed by frozen section. After neoadjuvant chemotherapy, definitive CRS and HIPEC with resection of the primary cancer should be performed. Patients with obstructing cancer or a perforated cancer require modifications of the treatment strategy not only to save the patient from multiple procedures but also to provide the optimal possibility for a long-term survival. An essential role for HIPEC has not been established with unexpected peritoneal metastases. However, the complete CRS is standard if a curative approach is the goal of treatment (46,47).
Acknowledgments
We appreciate secretarial and administrative support from the Foundation for Applied Research in Gastrointestinal Oncology (FARGO).
Funding: None.
Footnote
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-258/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-258/coif). S.D.W. is a consultant for Baxter, Becton, Dickinson and Co, Glaxo Smith Kline, Intuitive Surgical, Livsmed, Medtronic, OstomyCure, Stryker, Takeda, and Virtual Ports; is a member of the Data Safety Monitoring Board of JSR/WCG/ACI (chair), Polypoid (chair), and Boomerang; and receives royalties from Intuitive Surgical, Karl Storz Endoscopy America Inc., and Unique Surgical Solutions, LLC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Segelman J, Granath F, Holm T, et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012;99:699-705. [Crossref] [PubMed]
- Lemmens VE, Klaver YL, Verwaal VJ, et al. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 2011;128:2717-25. [Crossref] [PubMed]
- Honoré C, Goéré D, Souadka A, et al. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol 2013;20:183-92. [Crossref] [PubMed]
- Attiyeh FF, Stearns MW Jr. Second-look laparotomy based on CEA elevations in colorectal cancer. Cancer 1981;47:2119-25. [Crossref] [PubMed]
- Yan TD, Black D, Savady R, et al. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006;24:4011-9. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Sugarbaker PH, van der Speeten K. An overview of peritonectomy, visceral resection, and therapeutic laparoscopy for peritoneal surface malignancy. In: Sugarbaker PH. editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd Edition. Cine-Med Publishing: Woodbury, CT; 2017:17-46.
- Van der Speeten K, Stuart OA, Sugarbaker PH. Cancer chemotherapy for peritoneal metastases: Pharmacology and treatment. In: Sugarbaker PH. editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd Edition. Cine-Med Publishing: Woodbury, CT; 2017:47-82.
- Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999;43:S15-25. [Crossref] [PubMed]
- Zoetmulder FA. Cancer cell seeding during abdominal surgery: experimental studies. Cancer Treat Res 1996;82:155-61. [Crossref] [PubMed]
- Sethna KS, Sugarbaker PH. New prospects for the control of peritoneal surface dissemination of gastric cancer using perioperative intraperitoneal chemotherapy. Cancer Therapy 2004;2:79-84.
- Raa ST, Oosterling SJ, van der Kaaij NP, et al. Surgery promotes implantation of disseminated tumor cells, but does not increase growth of tumor cell clusters. J Surg Oncol 2005;92:124-9. [Crossref] [PubMed]
- Sugarbaker PH, Glehen O. Management of unexpected peritoneal metastases with primary colorectal cancer using second-look surgery with HIPEC. Can Surg 2015;1:101.
- Sugarbaker PH, Chang D. Anatomic sites of disease in colorectal cancer patients recorded at the time of cytoreductive surgery for peritoneal metastases. Eur J Surg Oncol 2022;48:946-55. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 1996;15:49-58.
- Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 2015;22:2958-64. [Crossref] [PubMed]
- Elias D, Benizri E, Pocard M, et al. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol 2006;32:632-6. [Crossref] [PubMed]
- Elias D, Faron M, Goéré D, et al. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol 2014;21:2052-8. [Crossref] [PubMed]
- Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum 2000;43:1341-6; discussion 1347-8. [Crossref] [PubMed]
- Braam HJ, Boerma D, Wiezer MJ, et al. Hyperthermic intraperitoneal chemotherapy during primary tumour resection limits extent of bowel resection compared to two-stage treatment. Eur J Surg Oncol 2013;39:988-93. [Crossref] [PubMed]
- van Oudheusden TR, Braam HJ, Nienhuijs SW, et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy: a feasible and effective option for colorectal cancer patients after emergency surgery in the presence of peritoneal carcinomatosis. Ann Surg Oncol 2014;21:2621-6. [Crossref] [PubMed]
- Braam HJ, van Oudheusden TR, de Hingh IH, et al. Patterns of recurrence following complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. J Surg Oncol 2014;109:841-7. [Crossref] [PubMed]
- Canda AE, Arslan C, Terzi C, et al. Treatment of intraoperatively detected peritoneal carcinomatosis of colorectal origin with cytoreductive surgery and intraperitoneal chemotherapy. World J Surg Oncol 2018;16:70. [Crossref] [PubMed]
- Mehta AM, Bignell MB, Alves S, et al. Risk of Ovarian Involvement in Advanced Colorectal or Appendiceal Tumors Involving the Peritoneum. Dis Colon Rectum 2017;60:691-6. [Crossref] [PubMed]
- Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg 2011;98:287-92. [Crossref] [PubMed]
- Bhatt A, Yonemura Y, Mehta S, et al. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? Eur J Surg Oncol 2020;46:582-9. [Crossref] [PubMed]
- Rovers KP, Bakkers C, van Erning FN, et al. Adjuvant Systemic Chemotherapy vs Active Surveillance Following Up-front Resection of Isolated Synchronous Colorectal Peritoneal Metastases. JAMA Oncol 2020;6:e202701. [Crossref] [PubMed]
- Quénet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:256-66. [Crossref] [PubMed]
- Berger Y, Jacoby H, Kaufmann MI, et al. Correlation Between Intraoperative and Pathological Findings for Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2019;26:1103-9. [Crossref] [PubMed]
- Bhatt A, Yonemura Y, Mehta S, et al. The Pathologic Peritoneal Cancer Index (PCI) Strongly Differs From the Surgical PCI in Peritoneal Metastases Arising From Various Primary Tumors. Ann Surg Oncol 2020;27:2985-96. [Crossref] [PubMed]
- Morton D, Seymour M, Magill L, et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol 2023;41:1541-52. [Crossref] [PubMed]
- Jensen LH, Kjaer ML, Larsen FO, et al. Phase III randomized clinical trial comparing the efficacy of neoadjuvant chemotherapy and standard treatment in patients with locally advanced colon cancer: The NeoCol trial. 2023 ASCO Annual Meeting. Abstract LBA3503. Presented June 4, 2023.
- Devilee RA, Simkens GA, van Oudheusden TR, et al. Increased Survival of Patients with Synchronous Colorectal Peritoneal Metastases Receiving Preoperative Chemotherapy Before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2016;23:2841-8. [Crossref] [PubMed]
- Rovers KP, Bakkers C, Nienhuijs SW, et al. Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial. JAMA Surg 2021;156:710-20. [Crossref] [PubMed]
- Chua TC, Liauw W, Zhao J, et al. Upfront compared to delayed cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei is associated with considerably lower perioperative morbidity and recurrence rate. Ann Surg 2011;253:769-73. [Crossref] [PubMed]
- Veld JV, Wisselink DD, Amelung FJ, et al. Synchronous and Metachronous Peritoneal Metastases in Patients with Left-Sided Obstructive Colon Cancer. Ann Surg Oncol 2020;27:2762-73. [Crossref] [PubMed]
- Amelung FJ, Burghgraef TA, Tanis PJ, et al. Critical appraisal of oncological safety of stent as bridge to surgery in left-sided obstructing colon cancer; a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;131:66-75. [Crossref] [PubMed]
- Xu J, Zhang S, Jiang T, et al. Transanal drainage tubes vs metallic stents for acute malignant left-sided bowel obstruction: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e18623. [Crossref] [PubMed]
- Tan L, Liu ZL, Ran MN, et al. Comparison of the prognosis of four different treatment strategies for acute left malignant colonic obstruction: a systematic review and network meta-analysis. World J Emerg Surg 2021;16:11. [Crossref] [PubMed]
- Ishibe A, Watanabe J, Suwa Y, et al. A Prospective, Single-arm, Multicenter Trial of Diverting Stoma Followed by Neoadjuvant Chemotherapy Using mFOLFOX6 for Obstructive Colon Cancer: YCOG 1305 (PROBE Study). Ann Surg 2022;276:140-5. [Crossref] [PubMed]
- Suzuki H, Tsujinaka S, Sato Y, et al. Oncologic impact of colonic stents for obstructive left-sided colon cancer. World J Clin Oncol 2023;14:1-12. [Crossref] [PubMed]
- van Hooft JE, Veld JV, Arnold D, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy 2020;52:389-407. [Crossref] [PubMed]
- Boeding JRE, Ramphal W, Rijken AM, et al. A Systematic Review Comparing Emergency Resection and Staged Treatment for Curable Obstructing Right-Sided Colon Cancer. Ann Surg Oncol 2021;28:3545-55. [Crossref] [PubMed]
- Sugarbaker PH. Diverting colostomy in a midline incision, a case report. Int J Surg Open 2019;16:14-7. [Crossref]
- Cheynel N, Cortet M, Lepage C, et al. Incidence, patterns of failure, and prognosis of perforated colorectal cancers in a well-defined population. Dis Colon Rectum 2009;52:406-11. [Crossref] [PubMed]
- Shida D, Tsukamoto S, Ochiai H, et al. Long-Term Outcomes After R0 Resection of Synchronous Peritoneal Metastasis from Colorectal Cancer Without Cytoreductive Surgery or Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2018;25:173-8. [Crossref] [PubMed]
- Shida D, Yoshida T, Tanabe T, et al. Prognostic Impact of R0 Resection and Targeted Therapy for Colorectal Cancer with Synchronous Peritoneal Metastasis. Ann Surg Oncol 2018;25:1646-53. [Crossref] [PubMed]


