Summary of emerging targets in anal cancer: the case for an immunotherapy based-approach
Introduction
Squamous cell carcinoma of the anal canal (SCCA) accounts for approximately 2% of all gastrointestinal malignancies (1). Despite the successful introduction of primary human papillomavirus (HPV) vaccination to prevent development of this disease, the annual incidence continues to rise in the United States, with no foreseeable decline in the coming years. Therefore, patients afflicted with this orphan disease are in dire need of novel effective therapies, especially as no standard of care exists for the metastatic setting. This review seeks to detail emerging immunotherapeutic approaches which hold promise for patients with SCCA.
Altered immunity in the development of anal cancer
Risk factors associated with the development of anal cancer include immunosuppressive therapies following organ transplantation, concomitant autoimmune diseases, and infection with human immunodeficiency virus (HIV) (2,3). Altered immunity increases the likelihood of anal cancer, presumably by predisposing the anal epithelium to infection and weakened clearance of HPV, which can integrate into the host cell DNA to promote oncogenesis. Prior work at our institution detected the presence of HPV in approximately 95% of patients with metastatic anal cancer (4), and this virus has also been linked to other squamous cell cancers in the head/neck, cervix, penis, and vagina/vulva (5,6). Of the many high- and low-risk subtypes of HPV known to exist, HPV-16 appears the most common in patients with SCCA. Oncogenesis associated with HPV infection is related to the various viral oncoproteins generated by the viral DNA, and these “non-self” proteins present within the human host tumor cell have important implications in the application towards rational design of targeted immunotherapies emerging for SCCA. For example, E6 and E7 are genes activated “early” in the HPV life cycle that initially may counteract interferon to promote immune evasion in infected anal epithelial cells. Intracellularly, E6 efficiently binds p53 to promote avid ubiquitin-mediated degradation of this tumor suppressor with subsequent avoidance of apoptosis (7). Alternatively, E7 binding of phosphorylated Rb enables upregulated cell cycle activity through increased DNA synthesis in the S-phase (8). Once integrated into the host genome, the effects of E6 and E7 are far more deleterious, leading to “immortalized” tumor cells which develop into anal cancer.
L1, on the other hand, is in HPV oncoprotein which appears “late” in the viral replication life cycle and plays an important role in capsid formation of virion packaging necessary for reinfection into adjacent squamous epithelia. Prophylactic/preventative vaccinations against HPV like Gardasil and the nine-valent HPV vaccine utilize “virus-like particles” that are structurally similar to L1 ultimately to prevent viral exposure/entry in people with no prior exposure to the virus (9). In a preventative trial of men-who-have-sex-with-men, a higher-risk population for development of SCCA, vaccination with a quadrivalent vaccine (covering HPV-16) led to fewer cases of precancerous, high-grade anal intraepithelial neoplasia relative to people who received placebo, and no cases of invasive carcinoma (10). Therefore, manipulation of the immunogenic “non-self” properties has proven benefit in primary prevention of SCCA. Recent studies have suggested that fewer than 30% of eligible females in the United States have received the complete HPV vaccination series (even lower in males) (11), and until further improvement in vaccination efforts, the incidence of SCCA will likely continue to worsen both in the United States and globally in the coming decades. In the meantime, further understanding of the viral biology relevant to the tumorigenesis of HPV-associated malignancies like SCCA has generated promising novel immune therapies as potential treatments for this disease.
Immune checkpoint agents in the treatment of metastatic anal cancer
While the majority of patients with anal cancer present with locoregional disease and can be cured by concurrent chemoradiation, approximately 10–20% of this population will recur to develop distant metastases, and an additional 10% of patients with anal cancer will have stage IV disease at initial presentation (12). Because of the low prevalence of metastatic disease in this orphan malignancy, no prospective clinical trial has thus far been completed for patients with metastatic SCCA. Rather, historically much of the treatment approach for the management of metastatic disease has been extrapolated from other, better-studied, HPV-associated malignancies like head/neck and cervical cancers. Indeed, few case series exist regarding the efficacy of cytotoxic chemotherapeutic agents in this setting. Combination approaches using cisplatin/5-fluorouracil and carboplatin/paclitaxel have been associated with activity in small, retrospective cohorts (13,14). While the InterAACT ECOG #2133 trial (NCT02560298) will compare these two combinations in the front-line setting for metastatic SCCA, at present there remain no consensus standard-of-care recommendations for the management of treatment naïve and refractory metastatic anal cancer.
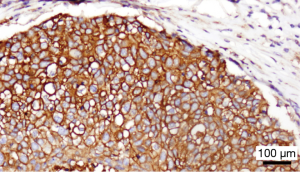
In order to offer patients with incurable anal cancer novel and effective therapies, attention his shifted recently to utilization of immunotherapeutic agents, given the association with HPV infection in anal tumorigenesis. Immune checkpoint inhibitors such as programmed death 1 (PD-1) receptor reside on the surface of host T cells and downregulate T-cell effector function when bound to their respective ligands (15). Within the tumor microenvironment, PD-1 on the surface of T cells interacts with PD-L1 on adjacent tumor cells to turn off an immune-mediated response, so that tumor cells can thereby escape T-cell mediated cytotoxicity. At our institution, we have observed the presence of PD-L1 diffusely on the surface of tumor cells primary resected specimen of a patient with recurrent SCCA following prior chemoradiation (Figure 1). To date, one retrospective series has described the prevalence of PD-L1 expression in patients with SCCA (16). In this group of 41 patients with stage I–IV disease, 93% of tumors were HPV-positive by p16 expression. PD-L1 positivity, defined as the detection of any membranous or cytoplasmic staining positive for PD-L1 by immunohistochemistry, was observed in 23 of 41 (56%) patients with SCCA. No significant survival differences were noted between the PD-L1-positive and PD-L1-negative groups. However, the population was heterogeneous, and no patient received an immune-based therapy. Nonetheless, this report does provide important initial data that SCCA tumors do express this protein as a potential mediator for evasion against immune-based antitumor activity.
This finding lends further support to the study of checkpoint inhibitors, such as nivolumab and pembrolizumab, monoclonal antibodies targeting PD-1 on the surface of T cells, in the management of this disease. In vitro, impairment of PD-1 and PD-L1 binding by such antibodies perpetuates T-cell activity against tumor cells. Anti-PD1 therapies have demonstrated significant clinical activity and improvement in survival for other advanced malignancies like melanoma, non-small cell lung cancer, and renal cell carcinoma. Considering this virally mediated element of HPV in SCCA tumorigenesis, the use of extending the role of anti-PD-1 therapy in metastatic SCCA is plausible. Recently, early results were reported from a phase Ib expansion cohort trial of pembrolizumab for patients with metastatic anal cancer (17). Tumors were required to be PD-L1-positive (defined as greater than 1% of cells at the cell membrane staining positive by immunohistochemistry for PD-L1). Of the 43 patients screened, PD-L1 expression was detected in 34 (74%), and 25 patients were enrolled for treatment. Notably, squamous histology (88%) was not required for participation, as patients with carcinoid (4%), endometrioid (4%), and muco-epidermoid (4%) anal tumors also received treatment. The majority of patients on this study (80%) had at least one prior line of systemic therapy for distant metastases. Overall, pembrolizumab was well-tolerated, with diarrhea (20%), fatigue (16%), and nausea (12%) reported as the most frequent adverse events. Grade 3 toxicities were uncommon and included hypothyroidism (4%), autoimmune-mediated colitis (4%), and diarrhea (4%), findings consistent with the prior side effect profiles of anti-PD-1 therapies for other malignancies. According to RECIST 1.1 criteria, partial radiographic responses were observed in 5/26 patients [response rate 20%; 95% confidence interval (CI), 7–41%], and stable disease was seen in 11 patients (response rate 44%; 95% CI, 24–65%). The mean progression-free survival was 3.0 months (95% CI, 1.7–7.3), with an estimated 20% of patients still on study after 12 months. It should be emphasized that the sample size was underpowered for efficacy of therapy. Nonetheless, there appears to be a subset of patients on this study did demonstrate a dramatic, sustained clinical response to anti-PD1 therapy.
These results provide a very optimistic, early rationale to support the use of anti-PD-1 therapy as a backbone for further studies of immune-mediated therapies in the treatment of metastatic anal cancer. NCI9673, the first prospective phase II clinical trial ever for metastatic anal cancer, is evaluating nivolumab for patients with previously treated metastatic SCCA. In addition, HIV-positive patients with CD4 counts greater than 300/microliter are eligible. Accrual for the study is now complete, with results expected to be reported in 2016 (18). Given the encouraging findings from the aforementioned phase Ib study with pembrolizumab, the efficacy of single-agent nivolumab from NCI9673 is anticipated. This trial also is expected to provide correlative studies which may give insights into tumor characteristics associated with response to anti-PD-1 agents.
Adoptive T-cell transfer
Adoptive T-cell transfer utilizes T-cell specific to an antigen of interest (e.g., HPV-E6 or E7), either selected directly from the patient or genetically engineered to express the given antigen, are expanded ex vivo and reinfused as tumor-infiltrating lymphocytes (TILs) autologously back into the patient (19). Recently, a series of 9 women with metastatic cervical cancer who had received prior chemoradiation and/or platinum-based chemotherapy were treated with HPV-TILs recognizing E6 and E7 (20). Two patients here with this otherwise difficult-to-treat disease experienced complete radiographic responses, and another demonstrated a partial response to HPV-TIL infusion. Depth of response correlated to duration of response, and those two patients with complete responses remained without recurrence at last reporting. Given that patients with refractory metastatic SCCA likewise have few treatment options with demonstrated efficacy, extension in studying HPV-TIL therapy to patients with metastatic anal cancer, using the same engineered targeted E6 and E7 viral proteins in SCCA oncogenesis, is warranted.
Chimeric antigen T-cells
T cells engineered with chimeric antigen receptors (CARs) consist of three principal regions which are genetically engineered and inserted into the patient’s T cells to be expressed and to reside at the surface of the affected T-cell. Here, an extracellular domain is comprised of an antibody region specific to a tumor neo-antigen particular to the cancer of interest and is linked with a hydrophobic transmembrane domain responsible for intracellular activation of signaling pathways necessary for antitumor T-cell function. Given the extracellular antibody component of the CAR, these receptors are able to bind more specifically than a processed (shorter) peptide being presented to a T-cell via that MHC cleft on the MHC receptor of the tumor cell (21). Other advantages of the CAR are the intracellular costimulatory and stimulatory components, the former designed to include molecules like OX40, 4-1BB, and ICOS, which upon extracellular binding of the specific ligand of interest, can increase the CAR T-cell activity of the stimulatory ζ chain, important to survival, expansion, and function of the given engineered T-cell population (22,23). Because of the multiple functional domains present within the single polypeptide, a second costimulatory interaction between a T-cell and its complementary tumor cell is not required in order for CAR T cells to enact their helper T-cell or cytotoxic T-cell activity. Preliminary results from a trial of patients with acute lymphoblastic leukemia who were treated with infusions of CAR T cells engineered to recognize CD19 (an extracellular B-cell protein present in these malignant cells) demonstrated complete responses in 27 of 29 patients (24). Although treatment related side effects are serious/potentially lethal and associated with inflammatory cytokine release, these findings generate optimism for expansion of this approach to solid tumors as well. Indeed, given at the majority of anal cancers are HPV positive and therefore capable of expressing viral proteins like E6 and E7 on the tumor cell surface, engineering of CAR T cells recognizing these targets is a worthy pursuit. Clinical trials testing HPV-16 E6 CAR T cells are being planned and expected to enroll patients in the near future.
Novel Listeria-based immune vaccines
ADXS 11-001 is a novel immune-based treatment for cancers in which a live, attenuated Listeria monocytogenes (Lm) bacteria is engineered to express a fusion Lm-LLO-E7 protein (25). Notably, these bacteria have been genetically modified not to express any virulence factors and thereby are unable to propagate and cause infection in the infused host. Rather, dendritic cells of the patient recognize and process this modified Listeria, which are broken down intracellularly in the phagosome, and released peptide fragments of the Lm-LLO-E7 fusion protein are presented as tumor-specific antigens to activate cytotoxic T-cell response against other cells expressing E7. In one trial of 110 heavily pretreated patients with recurrent/metastatic cervical cancer administered ADXS 11-001 with or without cisplatin, complete responses were seen in 6 patients, and partial responses were seen in an additional 6 patients (response rate 11%) (26). An additional 35 patients had stable disease, for a disease control rate of 43%. The addition of cisplatin to the immune vaccine did not appear to generate added antitumor activity already seen with ADXS 11-001. At 18 months, 28% of patients were still alive on this study. ADXS 11-001 was overall safe and well tolerated, with drug-related serious adverse effects occurring in 2% of patients. The phase II FAWCETT trial (NCT02399813) is testing ADXS11-001 in patients with metastatic SCCA and will be open for enrollment shortly. Given the dramatic success in a fraction of the those patients with metastatic cervical cancer, these findings further support the notion that immune targeting of HPV viral oncoproteins can generate profound responses not typically seen with standard available chemotherapeutic agents.
Conclusions
Despite a growing prevalence, SCCA is a rare and poorly understood malignancy for which no standard of care exists for those with metastatic disease. Traditional cytotoxic doublet combinations have more activity in treatment-naive patients when compared to heavily pretreated patients, and the rationale for their use is often extrapolated from other, better studied advanced squamous malignancies pending the completion of InterAACT ECOG #2133 trial. However, with a paucity of effective treatments dispensable to the treating medical oncologist, novel immunotherapies provide sound rationale for their potential promise given the strong correlation between the development of the HPV infection and SCCA. While formal efficacy results tailored to SCCA patients are eagerly awaited, preliminary findings with immune checkpoint agents, bacteria-based immunotherapies, TILs, and CAR-T cells appear to be effective and safe for subpopulations of patients affected with HPV associated malignancies. Further studies to optimize immune-based approaches for the treatment of patients with metastatic SCCA are warranted. We highly encourage patients with metastatic SCCA and their treating oncologists to consider participation in such immune-based clinical trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [Crossref] [PubMed]
- Sunesen KG, Nørgaard M, Thorlacius-Ussing O, et al. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978-2005. Int J Cancer 2010;127:675-84. [Crossref] [PubMed]
- Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997;337:1350-8. [Crossref] [PubMed]
- Morris VK, Rashid A, Rodriguez-Bigas M, et al. Clinicopathologic Features Associated With Human Papillomavirus/p16 in Patients With Metastatic Squamous Cell Carcinoma of the Anal Canal. Oncologist 2015;20:1247-52. [Crossref] [PubMed]
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92:709-20. [Crossref] [PubMed]
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010;102:315-24. [Crossref] [PubMed]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990;248:76-9. [Crossref] [PubMed]
- Ruiz S, Santos M, Segrelles C, et al. Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development 2004;131:2737-48. [Crossref] [PubMed]
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372:711-23. [Crossref] [PubMed]
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011;365:1576-85. [Crossref] [PubMed]
- Stokley S, Cohn A, Dorell C, et al. Adolescent vaccination-coverage levels in the United States: 2006-2009. Pediatrics 2011;128:1078-86. [Crossref] [PubMed]
- Eng C. Anal cancer: current and future methodology. Cancer Invest 2006;24:535-44. [Crossref] [PubMed]
- Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget 2014;5:11133-42. [Crossref] [PubMed]
- Faivre C, Rougier P, Ducreux M, et al. 5-fluorouracile and cisplatinum combination chemotherapy for metastatic squamous-cell anal cancer. Bull Cancer 1999;86:861-5. [PubMed]
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. [PubMed]
- Gujja S, Williamson SK, Batra A, et al. Programmed cell death-Ligand 1 (PD-L1) expression and outcome in patients with squamous cell cancer of anal canal (SCCAC). J Clin Oncol 2015;33:abstr 3523.
- Ott PA, Piha-Paul SA, Munster P, et al. Pembrolizumab (MK-3475) for PD-L1-positive squamous cell carcinoma (SCC) of the anal canal: Preliminary safety and efficacy results from KEYNOTE-028. European Cancer Congress. Vienna, Austria, 2015.
- Morris V, Mahvash A, Vence L, et al. Abstract CT131: NCI#9673 phase II study of nivolumab in refractory metastatic squamous cell carcinoma of the anal canal: Immunologic correlates of response. Cancer Res 2016;76:CT131. [Crossref]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [Crossref] [PubMed]
- Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015;33:1543-50. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Hombach AA, Heiders J, Foppe M, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology 2012;1:458-66. [Crossref] [PubMed]
- Song DG, Ye Q, Carpenito C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res 2011;71:4617-27. [Crossref] [PubMed]
- Riddell S. Engineering T Cells for Safe and Effective Cancer Immunotherapy. AAAS 2016 Annual Meeting. Washington DC, USA, 2016.
- Cory L, Chu C. ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccin Immunother 2014;10:3190-5. [Crossref] [PubMed]
- Basu P, Mehta AO, Jain MM, et al. ADXS11-001 immunotherapy targeting HPV-E7: Final results from a phase 2 study in Indian women with recurrent cervical cancer. J Clin Oncol 2014;32:abstr 5610.


