
Clinical characteristics, survival and prognostic nomogram for patients with esophageal mucinous adenocarcinoma: a SEER population-based analysis
Highlight box
Key findings
• In this study, we explored the clinical characteristics and prognosis of esophageal mucinous adenocarcinoma (MAC) based on the Surveillance, Epidemiology, and End Results (SEER) database. Our results suggested no significant difference among these three types of esophageal adenocarcinoma (AC) patients. Therefore, esophageal MAC may have similar malignant behavior and prognosis to esophageal signet-ring cell carcinoma (SRC) and AC, and esophageal MAC may not need to be distinguished from AC and SRC.
What is known and what is new?
• Our study is the first population-based study to develop nomogram models for predicting the overall survival (OS) and cancer-specific survival (CSS) of patients with esophageal MAC.
• Our study established a model that can sufficiently predict the prognosis of MAC patients.
What is the implication, and what should change now?
• For patients with esophageal MAC, it is critical for clinicians to perform appropriate surgery on patients at an early stage to improve OS and CSS.
Introduction
Esophageal cancer is a common gastrointestinal cancer with 604,100 new cases and 544,076 deaths worldwide according to 2022 global cancer statistics (1). The two main histopathological types of esophageal cancer are squamous cell carcinoma and adenocarcinoma (AC) (2). Signet-ring cell carcinoma (SRC) is characterized by large intracellular mucin aggregates and compressed nuclei displaced toward one end of the cell, while mucinous AC (MAC) has abundant extracellular mucin (3).
Esophageal MAC is a rare subtype of AC characterized by an abundance of extracellular mucins in more than 50% of the tumor cell area. Esophageal SRC has more than 50% of the tumor area consisting of intracellular mucin (4,5). The various clinical characteristics and mortality rates of individuals with carcinoma are significantly influenced by the histological subgroup (6-9). Previous studies have shown that colorectal and small bowel cancer along with MAC, AC, and SRC are heterogeneous diseases with different biological features and clinical behaviors (9-11). However, owing to its rarity, the attention given to esophageal MAC is currently insufficient. Few studies have explored the incidence, survival and potential response to different esophageal MAC treatments. A previous study analyzed both esophageal MAC and SRC patients, revealing better survival in these patients compared with AC patients after preoperative chemoradiotherapy (3). Moreover, two independent phase III clinical trials showed that poor survival in esophageal MAC patients can be improved by neoadjuvant chemotherapy (4). Some small-scale studies have demonstrated that esophageal SRC has poor pathomorphological characteristics, often diagnosed at a later disease stage, and has a poor prognosis (12-16).
However, the 2019 World Health Organization (WHO) classification of esophageal carcinoma does not recommend the classification of MAC into different AC subtypes (17). To gain a deeper understanding of the clinicopathological characteristics and prognosis of MAC, we conducted a retrospective study to compare the clinical characteristics and survival of MAC patients with AC and SRC patients, and developed nomogram models for predicting the 1-, 3- and 5-year overall survival (OS) and cancer-specific survival (CSS) of esophageal MAC patients using data from the Surveillance, Epidemiology, and End Results (SEER) database. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-244/rc).
Methods
Data sources and patients
Patients who were histologically diagnosed with MAC, SRC, or AC in 2004–2015 were selected from the SEER database. The third edition of the International Classification of Diseases for Oncology (ICD-O-3) was used to identify patients. In esophageal carcinoma, the following histological codes were used: 8480/3 and 8481/3 for MAC, 8140/3 for AC, and 8490/3 for SRC. Patients with unknown surgical information, nonpathologically confirmed diagnosis, and unknown survival time were excluded from the current study. We included baseline demographics (sex, age at diagnosis, marital status, race, survival time and vital status), tumor characteristics [pathological grade, primary site of cancer, tumor node metastasis (TNM) stage, American Joint Committee on Cancer (AJCC)] and treatment strategies (surgery, radiation, chemotherapy). The survival endpoints were OS and CSS. The OS was described as the time period between the diagnosis and the final follow-up or death. The time period between an esophageal cancer diagnosis and mortality or the last follow-up was referred to as CSS. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Development of the nomograms
To identify independent predictive markers, univariate and multivariate Cox regression analyses were used. Furthermore, significant variables from the multivariate Cox regression analysis were used to develop the nomograms for CSS and OS prediction. Calibration curves were displayed to illustrate the precision of the nomograms’ predictive capacity. The discrimination capability of the nomograms was evaluated using time-dependent receiver operating characteristic (ROC) curves. Using decision curve analysis (DCA), the clinical usefulness of the nomograms relative to the AJCC TNM stage was evaluated.
Statistical analysis
The Chi-square test was used to evaluate initial traits between groups for categorical variables, which were represented as percentage-based numbers. The propensity score matching (PSM) method was used to balance the baseline characteristics between MAC and AC and MAC and SRC at a 1:1 ratio. The OS and CSS of esophageal cancer patients were calculated using Kaplan-Meier curves, and the difference in OS and CSS between MAC and other kinds of esophageal cancer was evaluated using the log-rank test. All statistical analyses were conducted by R software. All P values were two-sided, and a P value <0.05 was considered significant.
Results
Clinicopathological characteristics of patients
A total of 497 patients (2.2%) with MAC, 21,109 patients (92.8%) with AC and 1,144 patients (5%) with SRC were included in the study. Compared with AC, patients with MAC were more likely to have a higher pathological grade (grade III–IV: 45.07% vs. 42.65%, P<0.001) and a later T stage (T3–4: 50.30% vs. 39.51%, P<0.001) and AJCC stage (AJCC III–IV: 60.36% vs. 52.80%, P=0.003) (Table 1). Regarding treatment strategies, patients with MAC were more likely to receive surgery (37.83% vs. 33.29%, P=0.04), radiation (59.96% vs. 54.19%, P=0.01) and chemotherapy (68.01% vs. 63.40%, P=0.04) than AC patients. Compared with SRC patients, those with MAC were more likely to have a lower pathological grade (grade I–II: 32.60% vs. 3.50%, P<0.001) and to receive surgery (37.83% vs. 31.99%, P=0.03). After PSM at a 1:1 ratio, 460 patients with MAC were matched with 460 patients with AC (Table S1), and 280 patients with MAC were matched with 280 patients with SRC (Table S2).
Table 1
| Characteristics | MAC, N=497, n (%) | AC, N=21,109, n (%) | P† | SRC, N=1,144, n (%) | P‡ |
|---|---|---|---|---|---|
| Sex | 0.51 | >0.99 | |||
| Male | 435 (87.53) | 18,235 (86.38) | 1,000 (87.41) | ||
| Female | 62 (12.47) | 2,874 (13.62) | 144 (12.59) | ||
| Age, years | 0.27 | 0.18 | |||
| <65 | 225 (45.27) | 9,007 (42.67) | 475 (41.52) | ||
| ≥65 | 272 (54.73) | 12,102 (57.33) | 669 (58.48) | ||
| Marital | 0.10 | 0.15 | |||
| Married | 322 (64.79) | 12,771 (60.50) | 697 (60.93) | ||
| Unmarried | 156 (31.39) | 7,231 (34.26) | 381 (33.30) | ||
| Unknown | 19 (3.82) | 1,107 (5.24) | 66 (5.77) | ||
| Race | 0.12 | 0.042 | |||
| White | 478 (96.18) | 20,052 (94.99) | 1,078 (94.23) | ||
| Black | 13 (2.62) | 493 (2.34) | 27 (2.36) | ||
| Other | 6 (1.21) | 564 (2.67) | 39 (3.41) | ||
| Grade | <0.001 | <0.001 | |||
| I–II | 162 (32.60) | 8,543 (40.47) | 40 (3.50) | ||
| III–IV | 224 (45.07) | 9,004 (42.65) | 932 (81.47) | ||
| Unknown | 111 (22.33) | 3,562 (16.87) | 172 (15.03) | ||
| Site | 0.39 | 0.09 | |||
| Upper third | 7 (1.41) | 249 (1.18) | 9 (0.79) | ||
| Middle third | 33 (6.64) | 1,676 (7.94) | 77 (6.73) | ||
| Lower third | 405 (81.49) | 16,765 (79.42) | 936 (81.82) | ||
| Overlapping | 11 (2.21) | 754 (3.57) | 51 (4.46) | ||
| Esophagus NOS | 41 (8.25) | 1,665 (7.89) | 71 (6.21) | ||
| T | <0.001 | >0.99 | |||
| T1–2 | 161 (32.39) | 8,416 (39.87) | 370 (32.34) | ||
| T3–4 | 250 (50.30) | 8,341 (39.51) | 575 (50.26) | ||
| Unknown | 86 (17.30) | 4,352 (20.62) | 199 (17.40) | ||
| N | 0.12 | 0.14 | |||
| N0 | 193 (38.83) | 9,156 (43.37) | 441 (38.55) | ||
| N1 | 238 (47.89) | 9,470 (44.86) | 587 (51.31) | ||
| Unknown | 66 (13.28) | 2,483 (11.76) | 116 (10.14) | ||
| M | 0.20 | 0.92 | |||
| M0 | 316 (63.58) | 12,641 (59.88) | 716 (62.59) | ||
| M1 | 152 (30.58) | 7,272 (34.45) | 358 (31.29) | ||
| Unknown | 29 (5.84) | 1,196 (5.67) | 70 (6.12) | ||
| AJCC | 0.003 | 0.23 | |||
| I–II | 147 (29.58) | 7,587 (35.94) | 348 (30.42) | ||
| III–IV | 300 (60.36) | 11,146 (52.80) | 650 (56.82) | ||
| Unknown | 50 (10.06) | 2,376 (11.26) | 146 (12.76) | ||
| Surgery | 0.04 | 0.03 | |||
| No | 309 (62.17) | 14,082 (66.71) | 778 (68.01) | ||
| Yes | 188 (37.83) | 7,027 (33.29) | 366 (31.99) | ||
| Radiation | 0.01 | 0.33 | |||
| No | 199 (40.04) | 9,671 (45.81) | 427 (37.33) | ||
| Yes | 298 (59.96) | 11,438 (54.19) | 717 (62.67) | ||
| Chemotherapy | 0.04 | 0.96 | |||
| No | 159 (31.99) | 7,725 (36.60) | 363 (31.73) | ||
| Yes | 338 (68.01) | 13,384 (63.40) | 781 (68.27) |
†, MAC group vs. AC group; ‡, SRC group vs. AC group. MAC, mucinous adenocarcinoma; AC, adenocarcinoma; SRC, signet-ring cell carcinoma; NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
Survival analyses before and after PSM
Before PSM analysis, the P values for OS and CSS were 0.10 and 0.11 for MAC and AC patients, respectively (Figure 1A,1B). MAC patients had significantly longer OS (P=0.003) and CSS (P<0.001) than SRC patients (Figure 1C,1D). The 1-, 3-, and 5-year OS rates were 49.6%, 23.3%, and 16.0% in patients with MAC; 42.0%, 17.1%, and 12.6% in patients with SRC, respectively. The 1-, 3-, 5-year CSS rates were 54.4%, 27.2%, and 20.6% in patients with MAC and 45.2%, 20.9%, and 16.5% in patients with SRC, respectively. The 1-, 3-, and 5-year OS rates were 49.6%, 23.3%, and 16.0% in patients with MAC and 51.5%, 26.2%, and 19.3% in patients with AC, respectively. The 1-, 3-, and 5-year CSS rates were 54.4%, 27.2%, and 20.6% in patients with AC and 55.7%, 31.1%, and 25.1% in patients with SRC, respectively.

The survival of MAC patients was similar to that of AC and SRC patients after PSM analysis (Figure 2). The 1-, 3-, and 5-year OS rates were 47.0%, 21.5%, and 14.1% in patients with MAC and 46.1%, 21.3%, and 16.0% in patients with SRC, respectively. The 1-, 3-, and 5-year CSS rates were 52.6%, 26.0%, and 19.1% in patients with MAC and 48.7%, 23.9%, and 20.8% in patients with SRC, respectively. The 1-, 3-, and 5-year OS rates were 49.9%, 23.2%, and 16.0% in patients with MAC and 52.8%, 24.6%, and 18.4% in patients with AC, respectively. The 1-, 3-, and 5-year CSS rates were 52.8%, 24.6%, and 18.4% in patients with MAC and 54.7%, 28.6%, and 22.5% in patients with AC, respectively.

Univariate and multivariate Cox analyses in patients with MAC
Univariate and multivariate Cox analyses were performed to identify significant prognostic factors (Tables 2,3). For patients with MAC, according to univariate Cox analysis, age, T stage, N stage, M stage, AJCC stage, and surgery were significantly related to OS, and these factors were included in the multivariate Cox analysis. Sex, T stage, N stage, M stage, AJCC stage, and surgery were significantly associated with CSS, and these factors were also included in the multivariate Cox analysis. N stage, M stage, and surgery were found to be independent prognostic factors for both OS and CSS by multivariate Cox analysis. For OS, T stage was also an independent prognostic factor (HR, 1.417, 95% CI: 1.005–1.999, P =0.047).
Table 2
| Characteristics | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Sex | |||||||
| Male | 1.000 | 1.000 | |||||
| Female | 1.262 | 0.894–1.781 | 0.18 | ||||
| Age, years | |||||||
| <65 | 1.000 | 1.000 | 1.000 | ||||
| ≥65 | 1.328 | 1.043–1.69 | 0.02 | 1.099 | 0.857–1.411 | 0.45 | |
| Marital | |||||||
| Married | 1.000 | 1.000 | |||||
| Unmarried | 1.193 | 0.916–1.554 | 0.19 | ||||
| Race | |||||||
| White | 1.000 | 1.000 | |||||
| Black | 1.293 | 0.639–2.615 | 0.47 | ||||
| Other | 0.585 | 0.145–2.361 | 0.45 | ||||
| Grade | |||||||
| I–II | 1.000 | ||||||
| III–IV | 1.155 | 0.906–1.472 | 0.24 | ||||
| Site | |||||||
| Upper third | 1.000 | 1.000 | |||||
| Middle third | 0.570 | 0.216–1.505 | 0.26 | ||||
| Lower third | 0.601 | 0.266–1.359 | 0.22 | ||||
| Overlapping | 0.848 | 0.294–2.449 | 0.76 | ||||
| Esophagus NOS | 0.481 | 0.186–1.246 | 0.13 | ||||
| T | |||||||
| T1–2 | 1.000 | 1.000 | 1.000 | 1.000 | |||
| T3–4 | 1.433 | 1.115–1.841 | 0.005 | 1.417 | 1.005–1.999 | 0.047 | |
| N | |||||||
| N0 | 1.000 | 1.000 | 1.000 | 1.000 | |||
| N1 | 1.467 | 1.148–1.875 | 0.002 | 1.625 | 1.181–2.235 | 0.003 | |
| M | |||||||
| M0 | 1.000 | 1.000 | 1.000 | 1.000 | |||
| M1 | 2.094 | 1.586–2.764 | <0.001 | 1.685 | 1.198–2.37 | 0.003 | |
| AJCC | |||||||
| I–II | 1.000 | 1.000 | 1.000 | 1.000 | |||
| III–IV | 1.760 | 1.365–2.269 | <0.001 | 0.755 | 0.48–1.186 | 0.22 | |
| Surgery | |||||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | |||
| Yes | 0.297 | 0.23–0.385 | <0.001 | 0.304 | 0.23–0.401 | <0.001 | |
| Radiation | |||||||
| No | 1.000 | 1.000 | |||||
| Yes | 1.205 | 0.923–1.573 | 0.17 | ||||
| Chemotherapy | |||||||
| No | 1.000 | 1.000 | |||||
| Yes | 0.934 | 0.703–1.241 | 0.64 | ||||
MAC, mucinous adenocarcinoma; HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
Table 3
| Characteristics | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Sex | |||||||
| Male | 1.000 | 1.000 | |||||
| Female | 1.464 | 1.020–2.103 | 0.04 | 1.277 | 0.887–1.84 | 0.19 | |
| Age, years | |||||||
| <65 | 1.000 | ||||||
| ≥65 | 1.189 | 0.913–1.549 | 0.20 | ||||
| Marital | |||||||
| Married | 1.000 | ||||||
| Unmarried | 1.304 | 0.979–1.735 | 0.07 | ||||
| Race | |||||||
| White | 1.000 | ||||||
| Black | 1.186 | 0.527–2.669 | 0.68 | ||||
| Other | 0.404 | 0.057–2.880 | 0.36 | ||||
| Grade | |||||||
| I–II | 1.000 | ||||||
| III–IV | 1.172 | 0.897–1.531 | 0.24 | ||||
| Site | |||||||
| Upper third | 1.000 | ||||||
| Middle third | 0.821 | 0.264–2.554 | 0.73 | ||||
| Lower third | 0.776 | 0.287–2.099 | 0.62 | ||||
| Overlapping | 1.125 | 0.329–3.850 | 0.85 | ||||
| Esophagus NOS | 0.728 | 0.239–2.223 | 0.58 | ||||
| T | |||||||
| T1–2 | 1.000 | 1.000 | |||||
| T3–4 | 1.573 | 1.188–2.082 | 0.002 | 1.455 | 0.992–2.134 | 0.055 | |
| N | |||||||
| N0 | 1.000 | 1.000 | |||||
| N1 | 1.724 | 1.309–2.271 | <0.001 | 1.924 | 1.36–2.722 | <0.001 | |
| M | |||||||
| M0 | 1.000 | 1.000 | |||||
| M1 | 2.370 | 1.762–3.186 | <0.001 | 1.842 | 1.287–2.637 | 0.001 | |
| AJCC | |||||||
| I–II | 1.000 | 1.000 | |||||
| III–IV | 2.055 | 1.541–2.740 | <0.001 | 0.745 | 0.454–1.223 | 0.25 | |
| Surgery | |||||||
| No | 1.000 | 1.000 | |||||
| Yes | 0.283 | 0.214–0.375 | <0.001 | 0.286 | 0.213–0.383 | <0.001 | |
| Radiation | |||||||
| No | 1.000 | ||||||
| Yes | 1.345 | 0.995–1.820 | 0.054 | ||||
| Chemotherapy | |||||||
| No | 1.000 | ||||||
| Yes | 0.996 | 0.722–1.373 | 0.98 | ||||
MAC, mucinous adenocarcinoma; HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
Construction and evaluation of OS and CSS nomograms for patients with MAC
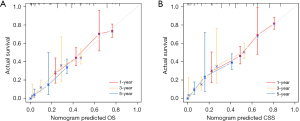
To predict the survival of patients with MAC, OS and CSS nomogram models were created using the significant factors found by multivariate Cox analysis (Figure 3). The most significant influence on the OS and CSS nomograms was surgery. The area under the curve (AUC) values of the nomogram for 1-, 3-, and 5-year OS were 0.723, 0.793, and 0.815, respectively, and those for 1-, 3-, and 5-year CSS were 0.757, 0.804, and 0.818, respectively (Figure 4). The calibration curve of the OS and CSS nomograms indicated that the anticipated results were consistent with the actual observation, indicating that the models had a precise prediction effect (Figure 5). DCA suggested that the OS and CSS nomogram models had better clinical utility than TNM stage (Figure 6).



Discussion
Mucin-secreting AC (including MAC and SRC) is more common in breast, colorectal and ovarian cancer (18-23). A previous study showed that it is important to differentiate the subtypes of breast SRC and MAC because their prognoses are different (8). However, few studies have focused on esophageal MAC as it is a rare type. Using information obtained from the SEER database, we examined the clinicopathological characteristics and prognosis of esophageal MAC, AC, and SRC patients in the current study.
We found that esophageal MAC, AC and SRC patients had many similarities, such as sex, age, marital status, tumor site, and N and M stage. A previous study comparing the clinical features of breast MAC and SRC patients suggested that the age and sex ratio between the two groups were not significantly different (8). Esophageal MAC patients also had some specific clinicopathological features. Our results indicated that compared with the AC group, the MAC group was characterized by high-grade tumors and a later T stage. The proportion of grade I–II tumors in the MAC group was higher than that in the SRC group. Consistent with our study, previous a study reported that high-grade tumors were more common in breast SRC patients than in the MAC patients (8). Our findings indicated that the incidence of lymph node metastasis and distal metastasis in esophageal MAC patients was comparable to that in SRC and AC patients. Regarding treatment strategies, the surgery, radiotherapy and chemotherapy rates of MAC patients were higher than those of AC patients. The surgery rate of MAC patients was also higher than that of SRC patients.
After PSM analysis, our study did not report any differences in OS and CSS between MAC and AC or MAC and SRC patients. Survival analyses of breast MAC and SRC patients have not been consistent with our study. Wu et al. reported that the 5-year OS of breast SRC patients (54.5%) was significantly poorer than that of MAC patients (88%) (24). Wang et al. also concluded that the OS and CSS of breast SRC patients were significantly lower than the OS and CSS of breast MAC patients (25). Our results indicated that esophageal MAC may have comparable malignant behavior to esophageal SRC and AC. Therefore, esophageal MAC may not need to be distinguished from AC and SRC, which is consistent with the WHO classification of digestive tumors (5th ed., 2019). The classification does not recommend classification of esophageal MAC into different histological subtypes, which means esophageal MAC is no longer an independent esophageal cancer type (17).
The prognostic factors for esophageal MAC patients were determined using Cox survival analysis. In patients with esophageal MAC, N stage, M stage, and surgery were all independent prognostic factors for both OS and CSS. N1 and M1 were the risk factors for OS and CSS. For OS, T1 was also a risk factor. Surgery was associated with better prognosis for both OS and CSS. Consistent with our results, one study on colorectal MAC demonstrated that surgery was the most important protective factor for survival outcomes (11), it is recommended that nearly all colorectal MAC patients receive surgery to improve their survival rate. Wang et al. reported that surgery significantly improved OS and CSS in both breast MAC and SRC groups (8). Our findings suggested that surgery was also an important protective factor for both OS and CSS in patients with esophageal MAC, suggesting early surgery to improve survival rate.
Our statistical analysis revealed that patients with MAC were more likely to receive surgery, radiotherapy, and chemotherapy compared to those with AC. This trend may be attributed to MACs typically being diagnosed at more advanced stages than standard ACs. Cancers detected at later stages commonly necessitate more aggressive treatment approaches, including surgery, radiotherapy, and chemotherapy. Such distinct treatment patterns underscore the need for further research to elucidate the underlying causes and to refine treatment strategies. Future studies might involve clinical trials tailored specifically to MAC, exploring novel therapeutic options and combinations. Therefore, these observations may have potential clinical implications.
To the best of our knowledge, our study is the first population-based study to develop nomogram models for predicting the OS and CSS of patients with esophageal MAC. The nomogram showed that surgery had the greatest impact on OS and CSS prediction. For patients with esophageal MAC, it is critical for clinicians to perform appropriate surgery on patients at an early stage to improve OS and CSS.
There are some limitations in this study. First, patients were from the United States, and there was a lack of patients from other countries. Second, detailed data about the treatment strategies of patients were not available. Third, the retrospective nature of the study led to inevitable selection bias. Finally, even though the nomograms’ high accuracy was confirmed by ROC analysis, calibration plot, and DCA, the repeatability and dependability of these nomograms could not be proven since there was no independent esophageal MAC cohort.
Conclusions
In summary, we explored the clinical characteristics and prognosis of esophageal MAC based on the SEER database. Our results suggested no significant difference among these three types of esophageal AC patients. Therefore, esophageal MAC may have similar malignant behavior and prognosis to esophageal SRC and AC, and esophageal MAC may not need to be distinguished from AC and SRC. Furthermore, the nomogram provides 1-, 3- and 5-year OS and CSS predictions for patients with esophageal MAC and contributes to clinical management decision-making.
Acknowledgments
The authors thank AJE for their language assistance during the writing of this article.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-244/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-244/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-244/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zaidi N, Kelly RJ. The management of localized esophageal squamous cell carcinoma: Western approach. Chin Clin Oncol 2017;6:46. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Correa AM, et al. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res 2005;11:2229-36. [Crossref] [PubMed]
- Wang L, Li C, Wang J, et al. Transformable ECM Deprivation System Effectively Suppresses Renal Cell Carcinoma by Reversing Anoikis Resistance and Increasing Chemotherapy Sensitivity. Adv Mater 2022;34:e2203518. [Crossref] [PubMed]
- Bleaney CW, Barrow M, Hayes S, et al. The relevance and implications of signet-ring cell adenocarcinoma of the oesophagus. J Clin Pathol 2018;71:201-6. [Crossref] [PubMed]
- Kang H, O'Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161-8. [Crossref] [PubMed]
- Razenberg LG, van Gestel YR, Lemmens VE, et al. The Prognostic Relevance of Histological Subtype in Patients With Peritoneal Metastases From Colorectal Cancer: A Nationwide Population-Based Study. Clin Colorectal Cancer 2015;14:e13-9. [Crossref] [PubMed]
- Wang S, Zhang Y, Yin F, et al. Prognostic Analysis of Primary Breast Signet Ring Cell Carcinoma and Mucinous Breast Adenocarcinoma: A SEER Population-Based Study. Front Oncol 2021;11:783631. [Crossref] [PubMed]
- Zhou YW, Xia RL, Chen YY, et al. Clinical features, treatment, and prognosis of different histological types of primary small bowel adenocarcinoma: A propensity score matching analysis based on the SEER database. Eur J Surg Oncol 2021;47:2108-18. [Crossref] [PubMed]
- Hugen N, Brown G, Glynne-Jones R, et al. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol 2016;13:361-9. [Crossref] [PubMed]
- Li ZP, Liu XY, Kao XM, et al. Clinicopathological characteristics and prognosis of colorectal mucinous adenocarcinoma and nonmucinous adenocarcinoma: a surveillance, epidemiology, and end results (SEER) population-based study. Ann Transl Med 2020;8:205. [Crossref] [PubMed]
- Nafteux PR, Lerut TE, Villeneuve PJ, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg 2014;260:1023-9. [Crossref] [PubMed]
- Chen L, Liu X, Gao L, et al. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: A 10-year retrospective study in China. PLoS One 2017;12:e0176637. [Crossref] [PubMed]
- Tang A, Rappaport J, Raja S, et al. Signet Ring Cell Histology Confers Worse Overall Survival in Treated Esophageal Adenocarcinoma. Ann Thorac Surg 2021;111:214-22. [Crossref] [PubMed]
- Solomon D, Abbas M, Feferman Y, et al. Signet Ring Cell Features are Associated with Poor Response to Neoadjuvant Treatment and Dismal Survival in Patients with High-Grade Esophageal Adenocarcinoma. Ann Surg Oncol 2021;28:4929-40. [Crossref] [PubMed]
- Corsini EM, Foo WC, Mitchell KG, et al. Esophageal adenocarcinoma with any component of signet ring cells portends poor prognosis and response to neoadjuvant therapy. J Thorac Cardiovasc Surg 2021;162:1404-1412.e2. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Marrazzo E, Frusone F, Milana F, et al. Mucinous breast cancer: A narrative review of the literature and a retrospective tertiary single-centre analysis. Breast 2020;49:87-92. [Crossref] [PubMed]
- El Amine El Hadj O, Ayadi M, Goucha A, et al. Mucinous breast carcinoma a rare entity to be known: clinico-pathological study of 48 cases. Tunis Med 2016;94:525-30. [PubMed]
- Luo C, Cen S, Ding G, et al. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond) 2019;39:13. [Crossref] [PubMed]
- Huang A, Yang Y, Shi JY, et al. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J Gastrointest Surg 2021;13:1567-83. [Crossref] [PubMed]
- Craig O, Salazar C, Gorringe KL. Options for the Treatment of Mucinous Ovarian Carcinoma. Curr Treat Options Oncol 2021;22:114. [Crossref] [PubMed]
- Marko J, Marko KI, Pachigolla SL, et al. Mucinous Neoplasms of the Ovary: Radiologic-Pathologic Correlation. Radiographics 2019;39:982-97. [Crossref] [PubMed]
- Wu X, Zhang Z, Li X, et al. Poorer Prognosis of Primary Signet-Ring Cell Carcinoma of the Breast Compared with Mucinous Carcinoma. PLoS One 2016;11:e0162088. [Crossref] [PubMed]
- Wang T, Shen B, Wang L, et al. Primary signet ring cell carcinoma of the breast: A rare entity with unique biological behavior-A clinical study based on pure signet ring cell carcinoma cohort. Pathol Res Pract 2020;216:152948. [Crossref] [PubMed]


