
Changing of gamma-H2AX in peripheral blood mononuclear cells during concurrent chemoradiation in locally advanced rectal cancer patients: a potential response predictor
Highlight box
Key findings
• Gamma-H2AX (γ-H2AX) levels in peripheral blood mononuclear cells (PBMCs) show significant dynamic changes between responders and non-responders to concurrent chemoradiation (CCRT) in locally advanced rectal cancer patients.
What is known and what is new?
• γ-H2AX is a known marker for DNA double-strand breaks caused by radiation.
• This study adds evidence that γ-H2AX levels could be used as a biomarker for predicting treatment response in rectal cancer patients undergoing preoperative CCRT.
What is the implication, and what should change now?
• The findings suggest potential clinical use of γ-H2AX levels to tailor treatment strategies for rectal cancer patients.
• Further validation is required to confirm γ-H2AX as a reliable biomarker, which could lead to personalized and more effective cancer treatment protocols, improving patient outcomes and optimizing therapy efficiency.
Introduction
Rectal cancer is the eighth most commonly diagnosed cancer and the tenth leading cause of cancer-related deaths worldwide (1). The standard therapeutic approaches for locally advanced diseases is total neoadjuvant therapy (TNT) which includes preoperative concurrent chemoradiation (CCRT) and chemotherapy followed by total mesorectal excision (TME). The addition of chemotherapy following CCRT, had demonstrated significant improvements in the pathologic complete response (pCR) of patients during surgery (2,3). However, some patients may not tolerate consolidative chemotherapy as planned. Multidisciplinary treatments are now considered essential for the management of rectal cancer (4).
High resolution magnetic resonance imaging (MRI) of the rectum is a standard imaging option for preoperative rectal cancer assessment and can provide superior and accurate information on the primary tumor stage and mesorectal fascia involvement (5,6). Furthermore, post-CCRT MRI has been increasingly used for radiological response evaluation to assess tumor volume reduction and any signals of fibrosis. With regard to the MERCURY study, the clinical tumor response has been classified by the magnetic resonance tumor regression grade (mrTRG) system. Patients who achieved a good response exhibited significant outcomes in terms of being disease-free and improved chances for overall survival when compared with those who received a poor response (7). In terms of both clinical staging and the response after the administration of preoperative measures, CCRT could be a key step in developing more individualized treatment for each patient (8). Apart from MRI assessment, several biomarkers have increasingly been utilized for the prediction of treatment response for radiotherapy. Although many kinds of blood sample biomarkers have been tested in clinical trials, any prediction or correlation of the response to neoadjuvant CCRT have been controversial (9,10).
To define the radiosensitivity of a tumor, several biomarkers have been used in previous studies. Gamma-H2AX (γ-H2AX), one of the early indicators of DNA double strand breaks (DSBs), is a phosphorylated form of the H2AX protein in DNA histone cores that occurs after radiation has been used as the primary determinant for tumor response to irradiation in both normal and cancerous cells (11-15). Studies investigating γ-H2AX as a surrogate marker of DNA double-strand damage have been inconclusive in predicting tumor response in several cancer types (13,16). Only one study has collected peripheral blood mononuclear cells (PBMCs) from rectal cancer patients treated with radiotherapy, but it included only one PBMCs collection after five fractions of radiotherapy (11). Given the limitations of current biomarkers in predicting response to preoperative CCRT, there is a critical need for identifying novel biomarkers that can accurately assess treatment response. In this context, our study aims to investigate the dynamic change of γ-H2AX in PBMCs as a potential biomarker for monitoring treatment response in patients diagnosed with locally advanced rectal cancer undergoing preoperative CCRT. The expression percentage of γ-H2AX in PBMCs during preoperative CCRT was measured using the flow cytometry technique. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-488/rc).
Methods
Study population and participant recruitment
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Faculty of Medicine, Chiang Mai University Ethics Committee for Human Research (protocol code RAD-2563-07543, September 25th, 2020). Locally advanced rectal cancer patients who underwent preoperative CCRT treatment at the Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University between November 2020 and November 2021 were enrolled in this study. All patients had given verbal and written informed consent for their participation in this research study. Eligibility criteria included patients with newly diagnosed rectal adenocarcinoma confirmed by histopathology, who were classified as clinical stage T3 or T4, or had positive indications for regional lymph node involvement, and were candidates for preoperative CCRT. Patients were also included who exhibited normal liver function, displayed creatinine clearance for at least 30 mL/min, and had received an Eastern Cooperative Oncology Group (ECOG) performance status within the range of 0–2. Patients who had a history of previous pelvic irradiation, had recurrent rectal cancer, received any systemic cancer treatment, or who had exhibited uncontrolled medical conditions were totally excluded. All patients underwent complete laboratory and staging investigations including an MRI in the pelvic region and a computed tomography (CT) scan of the chest-abdomen-pelvis area.
Study protocol
All enrolled patients received preoperative CCRT that involved intensity modulated radiotherapy (IMRT) technique using helical tomotherapy and chemotherapy. Target volume [gross tumor volume (GTV); clinical target volume (CTV); planning treatment volume (PTV)] and organs at risk (OARs) were contoured according to International Consensus Guidelines on clinical target volume delineation for rectal cancer (17). The treatment regimen included simultaneous integrated boosts of radiation doses at 50 Gy to GTV (gross tumor and pathologic lymph node) and 45 Gy to regional lymph node CTV in 25 fractions. The chemotherapeutic agent that was given concurrently in this clinical study was capecitabine (825 mg/m2 twice daily) and was administered on the day that the patient received radiotherapy (18). Patients who had creatinine clearance of lower than 50 mL/min received 75% of the standard dose of capecitabine. During CCRT, all patients were routinely appointed for a physical examination once a week in order to monitor and record any acute radiation side effects using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (2017). The CONSORT flow-chart of the present study shown in Figure 1. After completing the study protocol, patients were referred to a colorectal surgeon for evaluation and consideration of oncologic surgery. Follow-up appointments were scheduled every three months.
γ-H2AX analysis
Patient’s blood samples of about 10 mL for serum γ-H2AX were collected 5 timepoints as showed in Figure 2. Immediately after blood collection, DNA damage evaluation based on γ-H2AX level in PBMCs through flow cytometry technique. Briefly, PBMCs were separated from the blood by centrifugation at 800 ×g for 30 minutes at 10 ℃. After the separation, a layer of PBMCs was isolated and wash three times with phosphate-buffered saline and centrifuged for five minutes at 800 ×g. The isolated PBMCs were counted (approximately 2×105 cells) and diluted with assay buffer. Afterwards, the cells were fixed, permeabilized and incubated with anti-phosphohistone H2AX and anti-H2AX, and then analyzed the percentage of phosphorylated Histone H2AX by the MuseTM cell analyzer following the manufacturer’s instructions. The report for the analyzer provided the percentage of γ-H2AX activation. Considering the variability in baseline γ-H2AX activation for each patient, we utilized the γ-H2AX activation ratio to compare levels at 2nd, 3rd, 4th, and 5th PBMCs collection to the baseline (1st) level.

Response assessment using MRI
All patients underwent high-resolution pelvic MRI before CCRT and 4–6 weeks after receiving CCRT using 1.5-T and 3.0-T magnetic resonance machines. The initial MRI protocol for tumor staging included large field of view (FOV) in sagittal and axial images and small FOV for high-resolution oblique axial image perpendicular to the long axis of tumor, sagittal and coronal on T2-weighted sequence, T1-weighted sequences, and diffusion weight image (DWI) sequences. Oblique coronal T2-weighted images were obtained for low rectal tumor. The same MRI protocol was obtained to assess tumor response after CCRT with additional pre- and dynamic post-gadolinium enhancement T1-weighted images.
The tumor (T) and nodal (N) staging, presence of extramural vascular invasion (EMVI) and the magnetic resonance circumferential resection margin (mrCRM) before and after CCRT were evaluated. For post CCRT MRI study, the extent and degree of post-treatment fibrosis was identified and the magnetic resonance tumor regression grade (mrTRG), using MERCURY system were assessed and recorded by the same experienced gastrointestinal radiologist, who is a specialist with 20 years of experience in radiology. This radiologist has been utilizing the MERCURY system for mrTRG assessments since 2019, bringing 2 years of specific experience with this criterion. Post treatment fibrosis was identified as low signal on T2-weighted image. The residual tumor appeared as intermediate signal intensity on T2-weighted image similar to those of tumor on pre-CCRT image. The MRI response was used to divide patients into 2 groups as showed in Figure 2; the first group was comprised of good responders who were defined as mrTRG 1–3 and clear mrCRM, while the second group was comprised of poor responders who were defined as mrTRG 4-5 or who had involved mrCRM results (7).
Statistical analysis
Statistical analyses were conducted using Stata version 16 for Windows. Fisher’s exact test and Mann-Whitney U test were used to identify differences between imaging response and patient characteristics. In the univariable analysis of γ-H2AX activation, t-test and repeated measures analysis of variance (ANOVA) were employed to test differences between group of response. Considering the variability in measurement intervals and multiple confounding factors, a multilevel linear regression model was utilized, incorporating random intercept and fixed slope for each patient to account for individual variability, and marginal prediction plots were generated to visualize the predicted trend of γ-H2AX activation ratio changes from the 1st to 5th PBMCs collection. A P value less than 0.05 was considered statistically significant.
Additionally, Cox proportional hazards models were used to examine the association between γ-H2AX activation ratios and distant metastasis-free survival as well as overall survival. Correlations between γ-H2AX activation ratios and white blood cell (WBC) counts and lymphocyte counts were assessed using Pearson correlation.
Results
Patient characteristics
Thirty locally advanced rectal cancer patients were enrolled in this study. All patients received preoperative CCRT without treatment interruption. Among the patients analyzed, 21 patients (70%) were male and 9 (30%) were female with a median age of 61 years (ranging from 23 to 79 years). Most patients had well-differentiated adenocarcinoma (56.67%). According to the MRI findings after CCRT using the MERCURY system, 63.33% (n=19) were classified as responders, while 36.67% (n=11) were classified as non-responders. Patient characteristics for each response group are summarized in Table 1.
Table 1
| Factors | Total (n=30) | Non-responder (n=11) | Responder (n=19) | P value |
|---|---|---|---|---|
| Age (years), median [IQR] | 61 [54–67] | 59 [48–71] | 62 [56–67] | 0.83b |
| Sex (%) | 0.42a | |||
| Male | 21 (70.00) | 9 (81.82) | 12 (63.16) | |
| Female | 9 (30.00) | 2 (18.18) | 7 (36.84) | |
| Histopathology (%) | 0.62a | |||
| Well-differentiated | 17 (56.67) | 5 (45.45) | 12 (63.16) | |
| Moderate-differentiated | 11 (36.67) | 5 (45.45) | 6 (31.58) | |
| Poor-differentiated | 2 (6.67) | 1 (9.09) | 1 (5.26) | |
| Smoking (%) | 0.02a* | |||
| No | 24 (80.00) | 6 (54.55) | 18 (94.74) | |
| Yes | 6 (20.00) | 5 (45.45) | 1 (5.26) | |
| Stage (%) | 0.67a | |||
| IIA | 1 (3.33) | 0 (0) | 1 (5.26) | |
| IIIB | 9 (30.00) | 2 (18.18) | 7 (36.84) | |
| IIIC | 16 (53.33) | 7 (63.64) | 9 (47.37) | |
| IV | 4 (13.33) | 2 (18.18) | 2 (10.53) | |
| Pre-CCRT CRM status (%) | <0.001a* | |||
| Clear | 12 (40.00) | 0 (0) | 12 (63.16) | |
| Involved | 18 (60.00) | 11 (100.00) | 7 (36.84) | |
| Pre-CCRT EMVI (%) | 0.64a | |||
| Negative | 24 (80.00) | 8 (72.73) | 16 (84.21) | |
| Positive | 6 (20.00) | 3 (27.27) | 3 (15.79) | |
| Pre-CCRT CEA (ng/mL), median [IQR] | 6.67 [3.13–18.32] | 11.00 [3.82–18.31] | 6.25 [2.65–19.31] | 0.38b |
| Post-CCRT CEA (ng/mL), median [IQR] | 3.34 [2.17–5.44] | 4.25 [2.79–9.28] | 2.71 [2.14–4.31] | 0.07b |
*, significant at P value of <0.05. a, Fisher’s exact test. b, Mann-Whitney U test. CCRT, concurrent chemoradiation; CEA, carcinoembryonic antigen; CRM, circumferential resection margin; EMVI, extramural vascular invasion; IQR, interquartile range.
γ-H2AX profile
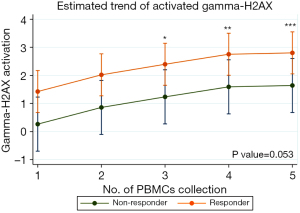
The γ-H2AX activation for all patients was illustrated in Figure 3. A substantial increased γ-H2AX activation in PBMCs was noted throughout the treatment course, as determined by repeated measures ANOVA (P=0.007, Figure 3A). Additionally, significant differences were observed between response groups (P=0.01, Figure 3B). The crude mean activation ratio of γ-H2AX showed in Table 2.

Table 2
| No. of PBMCs collection | Ratio of activated γ-H2AX, mean (SD) | Adjusted difference between groups (95% CI)# | P value# | ||
|---|---|---|---|---|---|
| Non-responder | Responder | P value$ | |||
| 2nd | 1.70 (1.54) | 1.54 (1.41) | 0.79 | 1.17 (−0.02 to 2.34) | 0.053 |
| 3rd | 1.49 (1.07) | 2.26 (1.60) | 0.46 | ||
| 4th | 1.85 (2.06) | 2.61 (2.74) | 0.02* | ||
| 5th | 2.11 (2.08) | 2.54 (3.05) | 0.19 | ||
$, difference of two means using t-test. #, multilevel linear regression model adjusted for age, sex, smoking, stage, EMVI and CRM status, and histopathology. *, significant at P value of <0.05. mrTRG, magnetic resonance tumor regression grade; PBMCs, peripheral blood mononuclear cells; SD, standard deviation; CI, confidence interval; EMVI, extramural vascular invasion; CRM, circumferential resection margin.
The ratio of γ-H2AX activation assessed using a multilevel linear regression model that accounted for confounding factors, revealed higher expression trend in the responder group compared to non-responder 1.17 [95% confidence interval (CI): −0.02 to 2.34, P=0.053] (Table 2). The differences between baseline (1st) and the 2nd (0.60, 95% CI: −0.21 to 1.40, P=0.15), the 3rd (0.97, 95% CI: 0.17 to 1.78, P=0.02), the 4th (1.33, 95% CI: 0.53 to 2.13, P=0.001), and the 5th (1.38, 95% CI: 0.57 to 2.18, P=0.001) PBMCs collections, as shown in Figure 4.
Pathological outcomes
The median follow-up time for this study was 3.06 years (interquartile range: 2.34–3.43 years). A total of 19 patients (67.86%) underwent oncologic surgery. The remaining 11 patients did not undergo oncologic surgery due to disease progression or distant metastasis in 5 patients, refusal of surgery in 4 patients, and loss follow-up in 2 patients. The interval between MRI and surgery was 57 days (interquartile range: 38–119 days). During the data collection and patient treatment period, a trend toward adopting TNT emerged. Five patients (26.32%) received chemotherapy with capecitabine and oxaliplatin as part of TNT while waiting for surgery. Four patients (21.06%) achieved a pCR. Additional instances of pathologic downstaging, along with Mandard’s five-tier tumor regression grading, were presented in Table 3. The association between pCR, non-pCR patients, and γ-H2AX activation was shown in Table 4 and Figure 5, similar to the distinction observed between responders and non-responders based on mrTRG. A trend of higher γ-H2AX activation was observed in patients who achieved a pCR compared to those who did not. The differentiation was significant higher in the 4th PBMCs collection (5.39 vs. 1.61, P=0.02). However, after adjusting for age, sex, smoking status, tumor stage, EMVI, CRM, and histopathology, no statistically significant difference was found (P=0.11). Significant difference from 1st (95% CI: 0.75 to 3.05, P=0.001).
Table 3
| Surgical outcome | No. of patients (n=19, %) |
|---|---|
| Pathologic outcome | |
| ypT0N0 (pCR) | 4 (21.06) |
| ypT1N0 | 3 (15.79) |
| ypT2N0 | 3 (15.79) |
| ypT3N0 | 5 (26.32) |
| ypT3N1 | 1 (5.26) |
| ypT3N2 | 3 (15.79) |
| ypCRM status | |
| Clear | 18 (94.74) |
| Involved | 1 (5.26) |
| Mandard’s five-tier tumor regression grading (n=13) | |
| TRG 1 | 4 (30.77) |
| TRG 2 | 1 (7.70) |
| TRG 3 | 7 (53.85) |
| TRG 4 | 1 (7.70) |
| TRG 5 | 0 |
pCR, pathologic complete response; CRM, circumferential resection margin; TRG, tumor regression grade.
Table 4
| No. of PBMCs collection | Ratio of activated γ-H2AX, mean (SD) | Adjusted difference between groups (95% CI)# | P value# | ||
|---|---|---|---|---|---|
| Non-pCR (n=15) | pCR (n=4) | P value$ | |||
| 2nd | 1.72 (1.56) | 2.44 (2.33) | 0.47 | 1.16 (−0.25 to 2.56) | 0.11 |
| 3rd | 2.00 (1.58) | 2.65 (1.30) | 0.46 | ||
| 4th | 1.61 (1.36) | 5.39 (5.23) | 0.02** | ||
| 5th | 2.40 (2.75) | 4.79 (4.51) | 0.19 | ||
$, difference of two means using t-test. #, multilevel linear regression model adjusted for age, sex, smoking, stage, EMVI and CRM status, and histopathology. **, significant at P value of <0.05. PBMCs, peripheral blood mononuclear cells; SD, standard deviation; pCR, pathologic complete response; CI, confidence interval; EMVI, extramural vascular invasion; CRM, circumferential resection margin.

Survival outcome
The survival outcomes and associations between γ-H2AX activation ratios and survival metrics, including distant metastasis-free survival and overall survival, were analyzed. The results of these analyses, which did not reveal significant correlations, were detailed in Tables S1,S2.
Toxicity
The maximal toxicities observed during treatment, based on the CTCAE version 5.0. Skin toxicities were observed in 10 patients, with 8 experiencing grade 1 toxicity and 2 experiencing grade 2 toxicity. Gastrointestinal toxicities were more prevalent, affecting 25 patients, with 6 patients experiencing grade 1 toxicity, 18 experiencing grade 2 toxicity, and only 1 patient experiencing grade 3 toxicity. Genitourinary toxicities were noted in 11 patients, all of whom experienced grade 1 toxicity. Hematologic toxicities were observed in 28 patients, with 20 experiencing grade 1 toxicity and 8 experiencing grade 2 toxicity.
Supplementary analyses were conducted to further explore the relationships between γ-H2AX activation ratios and various hematological parameters, including WBC count and lymphocyte count. Pearson correlation analysis showed no significant associations between γ-H2AX levels and either WBC count or lymphocyte count across five different time points (refer to Tables S3,S4 for detailed correlation results).
Discussion
TNT is considered the standard of care for locally advanced rectal cancer patients. The beneficial effect of adding more fluorouracil-based chemotherapy before surgery is to increase the pathological response in patients, especially in patients with poorer responses after receiving CCRT (19,20). Our study used mrTRG to evaluate the response to treatment after CCRT completion. Accordingly, our outcomes were consistent with those of an ongoing TRIGGER study that also used this parameter to further guide treatment for individual patients (21). The radiation technique employed in our treatment protocol was IMRT technique. The volume of the treatment and the dose treatment schedule followed the international consensus guidelines on target volume delineation in rectal cancer (17). The patients who were classified in the responder group was 63.33% in this study. This value is comparable to that of the MERCURY study which reported a value of 48.5% (7).
Apparently, γ-H2AX, a phosphorylated form of histone H2AX, is caused by a DNA double strand break. According to recent data, γ-H2AX has been used to monitor DNA damage that occurs either from chemotherapy or radiation (15,22). Another previous clinical study demonstrated that the mean number of γ-H2AX foci in PBMCs after radiation in breast and rectal cancer patients were significantly enhanced (11,16). However, none of the previous clinical studies demonstrated the dynamic changes during CCRT course.
In our study, we collected PBMCs at 5 different time points, from baseline through 6-week after CCRT, and confirmed significant dynamic changes in the ratio of γ-H2AX activation in PBMCs among locally advanced rectal cancer patients during CCRT. After we used the multilevel linear regression model, we found the significantly increased from baseline (1st) during CCRT caused and continue to increase to the end. The 2nd PBMCs collection did not show a statistically significant difference compared to baseline. This finding may be attributed to the DNA repair process occurring within 24 hours after receiving ionizing radiation. It is consistent with in vitro studies demonstrating that the half-life of γ-H2AX is approximately 2 to 7 hours, reflecting the DNA repair process (22,23). After repeated daily radiation fractions during treatment, the cumulative γ-H2AX values in PBMCs increased, showing significantly higher values in the 3rd to 5th collections.
Two clinical trials investigated the correlation between treatment response and γ-H2AX. Djuzenova et al. reported inconsistencies between the number of foci and the response of rectal tumors to treatment (11). In contrast, a study on non-small cell lung cancer found that 67% of patients with a complete response after radiation had consistent γ-H2AX foci numbers during and 12 weeks after treatment (13). The variation in results may be due to differences in the treatment scheme, histopathology, and the time points of assessment. In the present study, patients were categorized as responders or non-responders in this study utilizing the MERCURY system. Our analysis using t-test and repeated measure ANOVA revealed that the responders had significantly higher γ-H2AX values compared to the non-responders. Upon controlling for potential confounding factors, our model also demonstrated that the responder patient exhibited a trend towards increased expression during and after CCRT (P=0.053).
In our additional analysis, 19 patients underwent complete oncologic surgery after preoperative CCRT, with 4 of them (21.06%) showing pCR in the specimen, consistent with findings from a previous trial (19). However, due to the limited data on pathologic TRG, the sample size was too small to perform accurate discrimination. When comparing pCR and non-pCR patients, we observed a differentiation in the γ-H2AX ratio, with an increase in γ-H2AX levels found in patients with pCR, consistent with the responder group using mrTRG data. A significantly higher γ-H2AX ratio was observed in pCR patients during the 4th PBMC collection. Nevertheless, the limited sample size, no significant difference was detected in multilevel linear regression. Furthermore, we did not control the timing of surgery for each patient, and the emerging trend toward TNT during the study may have affected the accuracy of the pathologic response results in categorizing patients.
The study also found that active smokers and involved CRM on diagnostic MRI were statistically significantly related with poor responses to CCRT. In the large meta-analysis of 14 prospective cohort studies, issues of reduced cell-mediated immunity and relative tissue hypoxia were caused by patients who smoked during treatment and were associated with poorer overall survival rates when compared with non-smokers (24,25). In a previous study, CRM was identified as a key prognostic factor in patients with locally advanced disease (26). Notably, there have been limited studies involving CRM and the relevant response after CCRT. Due to the smoking and CRM status correlated with response of treatment, we adjusted these two factors in multilevel linear regression model.
Additionally, we performed a survival analysis. The 2-year overall survival rate in this study was 83.33%, which is comparable to historical data for patients receiving preoperative CCRT (27,28). Most recurrences occurred within the first two years after completing treatment (29). We also analyzed the association between γ-H2AX levels and overall survival, as well as distant metastasis-free survival, but found no significant correlations. This lack of association could be due to the short half-life of γ-H2AX, which may limit its predictive value for long-term outcomes. Key prognostic factors influencing survival included disease stage, tumor grading, performance status, hemoglobin levels, and genetic mutations. To enhance the accuracy of prognostic models, these factors should be incorporated alongside predictive biomarkers (30-32).
Acute toxicity levels that were recorded in our study were within a grade range of 0-2. Only one patient developed acute grade 3 radiation to induce enteritis on the 5th week of the CCRT course. Before the follow-up timepoint, this patient presented with immediate large bowel obstruction after CCRT was completed. Diarrhea, which occurred in the later weeks of CCRT, might be considered a retentive encopresis (overflow incontinence). The results of an analysis of the acute treatment related side effects in our study were comparable with those of other retrospective studies using IMRT for preoperative CCRT (33,34). We investigated the correlation between γ-H2AX values and both WBC count and lymphocyte count. Unfortunately, no significant correlations were identified.
The strength of this study resides in its clinical prospective design. We collected the patient’s PMBCs in several timepoints, from baseline to 6-week after CCRT completed, aimed to assess the dynamic change of γ-H2AX overtime. Moreover, we utilized automated flow cytometry techniques to help minimize potential human errors in foci counting. However, certain limitations should be noted. The small sample size prevented the establishment of a definitive cut-off point for the increasing of γ-H2AX. Additionally, the lack of histopathological specimens from TME restricted the depth of our analysis.
Conclusions
Our study demonstrated the possibility of γ-H2AX as a biomarker for response predictor in patients with locally advanced rectal cancer patients underwent CCRT. There was a trend toward increased expression between responders and non-responders after excluding confounding variables. However, more sample and cut-point level of γ-H2AX research are needed to be explored.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-488/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-488/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-488/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-488/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Evrik M, Lam F, et al. Global Cancer Observatory: Cancer Today. 2024. Available online: https://gco.iarc.who.int/today. Accessed 25 August 2024.
- Fokas E, Schlenska-Lange A, Polat B, et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol 2022;8:e215445. [Crossref] [PubMed]
- Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102. [Crossref] [PubMed]
- Creavin B, Ryan E, Martin ST, et al. Organ preservation with local excision or active surveillance following chemoradiotherapy for rectal cancer. Br J Cancer 2017;116:169-74. [Crossref] [PubMed]
- Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, et al. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019;39:367-87. [Crossref] [PubMed]
- Suzuki C, Torkzad MR, Tanaka S, et al. The importance of rectal cancer MRI protocols on interpretation accuracy. World J Surg Oncol 2008;6:89. [Crossref] [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-60. [Crossref] [PubMed]
- Martens MH, Maas M, Heijnen LA, et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst 2016;108:djw171. [Crossref] [PubMed]
- Morais M, Fonseca T, Machado-Neves R, et al. Can pretreatment blood biomarkers predict pathological response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer? Future Oncol 2021;17:4947-57. [Crossref] [PubMed]
- Perez RO, São Julião GP, Habr-Gama A, et al. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum 2009;52:1137-43. [Crossref] [PubMed]
- Djuzenova CS, Zimmermann M, Katzer A, et al. A prospective study on histone γ-H2AX and 53BP1 foci expression in rectal carcinoma patients: correlation with radiation therapy-induced outcome. BMC Cancer 2015;15:856. [Crossref] [PubMed]
- Park SH, Noh SJ, Kim KM, et al. Expression of DNA Damage Response Molecules PARP1, γH2AX, BRCA1, and BRCA2 Predicts Poor Survival of Breast Carcinoma Patients. Transl Oncol 2015;8:239-49. [Crossref] [PubMed]
- Yin X, Mason J, Lobachevsky PN, et al. Radiation Therapy Modulates DNA Repair Efficiency in Peripheral Blood Mononuclear Cells of Patients With Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2019;103:521-31. [Crossref] [PubMed]
- Avkshtol V, Arora S, Lesh R, et al. Peripheral Blood Lymphocyte Gamma-H2AX as a Predictor for Treatment Response in Rectal Cancer Patients. International Journal of Radiation Oncology, Biology, Physic 2017;99:E576-7. [Crossref]
- Redon CE, Dickey JS, Bonner WM, et al. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res 2009;43:1171-8. [Crossref] [PubMed]
- Djuzenova CS, Elsner I, Katzer A, et al. Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat Oncol 2013;8:98. [Crossref] [PubMed]
- Valentini V, Gambacorta MA, Barbaro B, et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol 2016;120:195-201. [Crossref] [PubMed]
- Craven I, Crellin A, Cooper R, et al. Preoperative radiotherapy combined with 5 days per week capecitabine chemotherapy in locally advanced rectal cancer. Br J Cancer 2007;97:1333-7. [Crossref] [PubMed]
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957-66. [Crossref] [PubMed]
- Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29-42. [Crossref] [PubMed]
- Battersby NJ, Dattani M, Rao S, et al. A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials 2017;18:394. [Crossref] [PubMed]
- Ivashkevich A, Redon CE, Nakamura AJ, et al. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 2012;327:123-33. [Crossref] [PubMed]
- Vasireddy RS, Sprung CN, Cempaka NL, et al. H2AX phosphorylation screen of cells from radiosensitive cancer patients reveals a novel DNA double-strand break repair cellular phenotype. Br J Cancer 2010;102:1511-8. [Crossref] [PubMed]
- Ordóñez-Mena JM, Walter V, Schöttker B, et al. Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: a meta-analysis of individual patient data from cohorts within the CHANCES consortium. Ann Oncol 2018;29:472-83. [Crossref] [PubMed]
- Japuntich SJ, Kumar P, Pendergast JF, et al. Smoking Status and Survival Among a National Cohort of Lung and Colorectal Cancer Patients. Nicotine Tob Res 2019;21:497-504. [Crossref] [PubMed]
- Liu Q, Luo D, Cai S, et al. Circumferential resection margin as a prognostic factor after rectal cancer surgery: A large population-based retrospective study. Cancer Med 2018;7:3673-81. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Okamura R, Itatani Y, Fujita Y, et al. Postoperative recurrence in locally advanced rectal cancer: how does neoadjuvant treatment affect recurrence pattern? World J Surg Oncol 2023;21:247. [Crossref] [PubMed]
- Martín-Carnicero A, Ramalle-Gomara E, Rubio-Mediavilla S, et al. Prognostic and Predictive Biomarkers in Patients with Locally Advanced Rectal Cancer (LARC) Treated with Preoperative Chemoradiotherapy. J Clin Med 2022;11:6091. [Crossref] [PubMed]
- Dayde D, Tanaka I, Jain R, et al. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int J Mol Sci 2017;18:573. [Crossref] [PubMed]
- Quah HM, Chou JF, Gonen M, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008;113:57-64. [Crossref] [PubMed]
- Jabbour SK, Patel S, Herman JM, et al. Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol 2012;2012:891067. [Crossref] [PubMed]
- Engels B, De Paoli A, Delmastro E, et al. Preoperative Radiotherapy with a Simultaneous Integrated Boost Compared to Chemoradiotherapy for cT3-4 Rectal Cancer: Long-Term Results of a Multicenter Randomized Study. Cancers (Basel) 2023;15:3869. [Crossref] [PubMed]



