
Development and validation of a prognostic nomogram for elderly-onset pancreatic neuroendocrine carcinoma: a prospective cohort study from the SEER database
Highlight box
Key findings
• We identified independent prognostic factors for pancreatic neuroendocrine carcinoma (PanNEC) patients aged 50 years and older through the Surveillance, Epidemiology, and End Results (SEER) database, and subsequently developed a clinical prediction model to assist clinicians in better predicting cancer-free survival for these patients.
What is known and what is new?
• PanNEC occurring in individuals aged ≥50 years is referred to as elderly-onset PanNEC. It is characterized by poor prognosis and its incidence is increasing annually.
• We conducted the first exploration of prognostic factors for elderly-onset PanNEC patients and established a predictive model based on their independent prognostic factors.
What is the implication, and what should change now?
• We developed a relatively simple yet highly accurate clinical prediction model, which can assist clinicians in understanding patients' prognosis and making appropriate decisions.
Introduction
The incidence of pancreatic neuroendocrine neoplasm (PanNEN) has increased over the past 40 years and the incidence of pancreatic neuroendocrine carcinoma (PanNEC) has also increased (1,2). According to the 2019 World Health Organization (WHO) classification, PanNENs are divided into well-differentiated pancreatic neuroendocrine tumor (PanNET), PanNEC with histological types of large-cell neuroendocrine carcinoma and small-cell neuroendocrine carcinoma, and mixed neuroendocrine–non-neuroendocrine neoplasm of the pancreas (3). PanNEN is a very rare type of tumor, with recent guidelines reporting an incidence of approximately 0.8/100,000 in the United States (4). PanNEC accounts for only 10–20% of PanNEN cases (5). There is a significant difference in prognosis between PanNET and PanNEC, primarily due to the higher invasiveness and frequent distant metastasis observed in PanNEC (6-9).
With the age-associated increase in lifespan, the incidence of PanNENs is much higher in older people (aged ≥50 years) than in younger individuals (2,10). Moreover, clinical features and prognosis differ between early-onset PanNEC (<50 years old) and elderly-onset PanNEC (≥50 years old) (11-14). Due to the rarity of the tumor and the poor prognosis of elderly-onset PanNEC, there is currently a lack of large sample studies exploring the clinical characteristics and prognosis of elderly-onset PanNEC classified according to the new WHO criteria.
This study investigated the clinical features and prognosis of elderly-onset PanNEC and constructed a nomogram model that could accurately predict cancer-specific survival (CSS) based on an analysis of the Surveillance, Epidemiology, and End Results (SEER) database which collected data from 17 regions of the United States, covering approximately 28% of the total U.S. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-344/rc).
Methods
Patient selection
Eligible patients were screened from the SEER database using SEER*Stat version 8.4.3. According to the latest WHO classification criteria, we collected data from the SEER database for patients diagnosed between 2010 and 2021 with primary pancreatic tumors and histological codes from the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) 8013 (large cell neuroendocrine carcinoma) and 8041 (small cell carcinoma, not otherwise specified). The exclusion criteria were (I) patients with missing demographic and crucial clinical information, (II) patients aged <50 years at diagnosis, and (III) patients with incomplete survival data. The patient selection flowchart is illustrated in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Assessment of covariates
The following demographic and clinicopathological data were extracted from the SEER database: age, sex, race (white, black, others), marital status (married, others), primary site (body and tail, head, others), tumor size (≤20, 21–40, >40 mm, unknown), primary site surgery (yes, no), radiation (yes, no/unknown), chemotherapy (yes, no/unknown), the tumor, nodes, and metastasis (TNM) stage (I, II/III, or IV), lymph nodes metastasis (yes, no, unknown), liver metastasis (yes, no), bone metastasis (yes, no, unknown), brain metastasis (yes, no, unknown), lung metastasis (yes, no, unknown), survival time, the status of survival, and cause-specific death. Tumor node metastasis (TNM) staging was based on the following codes proposed by the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th edition): derived AJCC T, AJCC 6th edition (2004–2015); derived AJCC T, combined T (SEER 2016–2017); EOD 2018 T (2018+); derived AJCC N, AJCC 6th edition (2004–2015); derived SEER combined N (SEER 2016–2017), derived EOD 2018 N (2018+); derived AJCC M, 6th edition (2004–2015); derived SEER combined M (SEER 2016–2017), derived EOD 2018 M (2018+), regional nodes examined (1988+), positive regional nodes (1988+), CS extension (2004–2015), CS lymph nodes (2004–2015), CS Mets at DX (2004–2015). The study endpoint is defined as either death due to the primary tumor or the last follow-up date before November 2023. The primary outcome was the death due to the cancer.
Statistical analysis
The study population was randomly assigned to a training set (N=244) and a validation set (N=163) (ratio of 6: 4). CSS in patients with elderly-onset PanNEC was estimated using the Kaplan-Meier method and compared between groups using the log-rank test. We performed multivariate Cox regression analyses both including and excluding treatments in the training set. The results of univariate and multivariate Cox proportional hazards regression were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Variables with significance (P<0.05) from the multivariate Cox regression were used to construct nomograms, either incorporating treatments or excluding them. The nomogram was generated using the rms package based on independent variables of the training set and was validated in the validation set. The accuracy and discrimination of the nomogram were evaluated by the area under the receiver operating characteristic curve (AUC) and calibration curves. The clinical benefit was assessed using decision curve analysis (DCA). Statistical analyses were conducted using R statistical software version 4.2.2. A two-sided P value of less than 0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 407 patients with elderly-onset PanNEC were selected from the SEER database according to the inclusion and exclusion criteria (Figure 1). In this cohort, 55.8% were males, 76.9% were white, and 69.0% were married. Of the tumor specimens, 46.7% were larger than 40 mm, and 84.3% of the patients were classified as stage IV, 318 individuals (78.1%) died due to the tumor. The median CSS time was 6 months. The percentage of patients receiving chemotherapy, surgery, and radiation was 61.7%, 11.6%, and 11.6%, respectively. The clinical features of the patients are summarized in Table 1.
Table 1
| Variables | Total set (n=407) | Training set (n=244) | Validation set (n=163) | P value |
|---|---|---|---|---|
| Age (years) | 0.37 | |||
| <75 | 282 (69.29) | 165 (67.62) | 117 (71.78) | |
| ≥75 | 125 (30.71) | 79 (32.38) | 46 (28.22) | |
| Sex | 0.70 | |||
| Female | 180 (44.23) | 106 (43.44) | 74 (45.40) | |
| Male | 227 (55.77) | 138 (56.56) | 89 (54.60) | |
| Race | 0.94 | |||
| White | 313 (76.90) | 189 (77.46) | 124 (76.07) | |
| Black | 49 (12.04) | 29 (11.89) | 20 (12.27) | |
| Others | 45 (11.06) | 26 (10.66) | 19 (11.66) | |
| Marital status | 0.74 | |||
| Married | 281 (69.04) | 170 (69.67) | 111 (68.10) | |
| Others | 126 (30.96) | 74 (30.33) | 52 (31.90) | |
| Primary site | 0.96 | |||
| Body and tail | 112 (27.52) | 66 (27.05) | 46 (28.22) | |
| Head | 183 (44.96) | 110 (45.08) | 73 (44.79) | |
| Others | 112 (27.52) | 68 (27.87) | 44 (26.99) | |
| Tumor size (mm) | 0.92 | |||
| ≤20 | 29 (7.13) | 16 (6.56) | 13 (7.98) | |
| 21–40 | 124 (30.47) | 76 (31.15) | 48 (29.45) | |
| >40 | 190 (46.68) | 115 (47.13) | 75 (46.01) | |
| Unknown | 64 (15.72) | 37 (15.16) | 27 (16.56) | |
| Surgery | 0.71 | |||
| Yes | 47 (11.55) | 27 (11.07) | 20 (12.27) | |
| No | 360 (88.45) | 217 (88.93) | 143 (87.73) | |
| Radiation | 0.10 | |||
| Yes | 47 (11.55) | 23 (9.43) | 24 (14.72) | |
| No/unknown | 360 (88.45) | 221 (90.57) | 139 (85.28) | |
| Chemotherapy | 0.92 | |||
| Yes | 251 (61.67) | 150 (61.48) | 101 (61.96) | |
| No/unknown | 156 (38.33) | 94 (38.52) | 62 (38.04) | |
| TNM stage | 0.99 | |||
| I | 18 (4.42) | 11 (4.51) | 7 (4.29) | |
| II/III | 46 (11.30) | 28 (11.48) | 18 (11.04) | |
| IV | 343 (84.28) | 205 (84.02) | 138 (84.66) | |
| Liver metastasis | 0.65 | |||
| No | 125 (30.71) | 77 (31.56) | 48 (29.45) | |
| Yes | 282 (69.29) | 167 (68.44) | 115 (70.55) | |
| Lymph nodes metastasis | 0.19 | |||
| No | 270 (66.34) | 156 (63.93) | 114 (69.94) | |
| Yes | 122 (29.98) | 76 (31.15) | 46 (28.22) | |
| Unknown | 15 (3.69) | 12 (4.92) | 3 (1.84) | |
| Bone metastasis | 0.82 | |||
| No | 351 (86.24) | 209 (85.66) | 142 (87.12) | |
| Yes | 47 (11.55) | 30 (12.30) | 17 (10.43) | |
| Unknown | 9 (2.21) | 5 (2.05) | 4 (2.45) | |
| Brain metastasis | 0.01 | |||
| No | 373 (91.65) | 230 (94.26) | 143 (87.73) | |
| Yes | 23 (5.65) | 7 (2.87) | 16 (9.82) | |
| Unknown | 11 (2.70) | 7 (2.87) | 4 (2.45) | |
| Lung metastasis | 0.78 | |||
| No | 332 (81.57) | 198 (81.15) | 134 (82.21) | |
| Yes | 67 (16.46) | 42 (17.21) | 25 (15.34) | |
| Unknown | 8 (1.97) | 4 (1.64) | 4 (2.45) |
Data are presented as n (%). PanNEC, pancreatic neuroendocrine carcinoma; TNM, tumor, nodes, and metastasis.
Independent risk factors for CSS in patients with elderly-onset PanNEC
We conducted univariate and multivariate Cox regression analyses in the training group, which consisted of 244 individuals, 184 (75.4%) of whom experienced the outcome event. Univariate Cox regression analysis showed that age, TNM stage, liver metastasis, surgery, chemotherapy and lung metastasis were significantly (P<0.05) correlated with CSS. Multivariate Cox analysis showed that age (HR: 1.56, 95% CI: 1.10–2.22, P=0.01), surgery (HR: 2.32, 95% CI: 1.27–4.23, P=0.006), chemotherapy (HR: 2.39, 95% CI: 1.68–3.38, P<0.001), TNM stage (HR: 3.96, 95% CI: 1.19–13.19, P=0.03), and liver metastasis (HR: 1.75, 95% CI: 1.16–2.65, P=0.008) were independent prognostic factors for CSS in patients with elderly-onset PanNEC (Table 2).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| <75 | 1.00 (reference) | 1.00 (reference) | |||
| ≥75 | 2.09 (1.54–2.85) | <0.001 | 1.56 (1.10–2.22) | 0.01 | |
| Sex | |||||
| Female | 1.00 (reference) | ||||
| Male | 0.97 (0.72–1.30) | 0.83 | |||
| Race | |||||
| White | 1.00 (reference) | ||||
| Black | 1.06 (0.69–1.64) | 0.79 | |||
| Others | 1.05 (0.64–1.71) | 0.86 | |||
| Marital status | |||||
| Married | 1.00 (reference) | ||||
| Others | 0.84 (0.61–1.16) | 0.29 | |||
| Primary site | |||||
| Body and tail | 1.00 (reference) | ||||
| Head | 1.26 (0.88–1.80) | 0.21 | |||
| Others | 1.32 (0.89–1.97) | 0.17 | |||
| Tumor size (mm) | |||||
| ≤20 | 1.00 (reference) | ||||
| 21–40 | 0.74 (0.39–1.38) | 0.34 | |||
| >40 | 0.83 (0.45–1.51) | 0.54 | |||
| Unknown | 0.89 (0.45–1.75) | 0.73 | |||
| Surgery | |||||
| Yes | 1.00 (reference) | 1.00 (reference) | |||
| No | 2.56 (1.52–4.30) | <0.001 | 2.32 (1.27–4.23) | 0.006 | |
| Radiation | |||||
| Yes | 1.00 (reference) | ||||
| No/unknown | 1.44 (0.86–2.40) | 0.17 | |||
| Chemotherapy | |||||
| Yes | 1.00 (reference) | 1.00 (reference) | |||
| No/unknown | 2.02 (1.49–2.72) | <0.001 | 2.39 (1.68–3.38) | <0.001 | |
| TNM stage | |||||
| I | 1.00 (reference) | 1.00 (reference) | |||
| II/III | 3.51 (1.04–11.79) | 0.042 | 3.28 (0.96–11.27) | 0.06 | |
| IV | 5.97 (1.89–18.87) | 0.002 | 3.96 (1.19–13.19) | 0.03 | |
| Liver metastasis | |||||
| No | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 2.04 (1.46–2.84) | <0.001 | 1.75 (1.16–2.65) | 0.008 | |
| Lymph nodes metastasis | |||||
| No | 1.00 (reference) | ||||
| Yes | 0.94 (0.68–1.29) | 0.70 | |||
| Unknown | 0.93 (0.48–1.77) | 0.82 | |||
| Bone metastasis | |||||
| No | 1.00 (reference) | ||||
| Yes | 1.29 (0.82–2.01) | 0.27 | |||
| Unknown | 2.34 (0.95–5.72) | 0.06 | |||
| Brain metastasis | |||||
| No | 1.00 (reference) | ||||
| Yes | 1.34 (0.55–3.28) | 0.52 | |||
| Unknown | 2.18 (0.96–4.94) | 0.06 | |||
| Lung metastasis | |||||
| No | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 0.99 (0.66–1.50) | 0.98 | 0.83 (0.55–1.27) | 0.40 | |
| Unknown | 3.70 (1.35–10.12) | 0.01 | 2.21 (0.80–6.07) | 0.13 | |
CSS, cancer-specific survival; PanNEC, pancreatic neuroendocrine carcinoma; HR, hazard ratio; CI, confidence interval; TNM, tumor, nodes, and metastasis.
Furthermore, we also excluded several treatment variables in both univariate and multivariate Cox regression analyses. We found that age (HR: 2.25, 95% CI: 1.63–3.10, P<0.001), TNM stage (HR: 4.12, 95% CI: 1.27–13.40, P=0.02), and liver metastasis (HR: 1.76, 95% CI: 1.17–2.66, P=0.007) were independent risk factors affecting patient prognosis (Table S1).
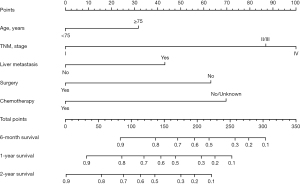
Construction and validation of a nomogram for predicting CSS in patients with elderly-onset PanNEC
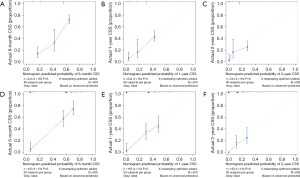
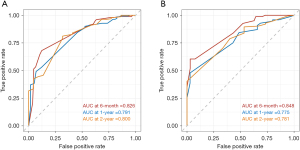
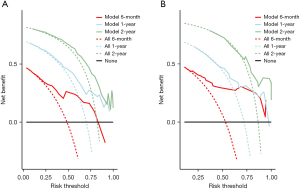
The nomogram was based on independent variables of the training set (Figure 2). The most significant risk factor for CSS was TNM stage (Figure S1). We also developed a nomogram that excluded treatment, as shown in Figure S2. The calibration curve based on independent prognostic factors incorporating treatment demonstrated consistent predicted and observed results for 6-month, 1-year, and 2-year CSS (Figure 3). The calibration curve excluding treatments yielded similar results (Figure S3). The AUCs for 6-month, 1-year, and 2-year CSS in the model incorporating treatment were 0.826, 0.791, and 0.800 in the training set and 0.848, 0.775, and 0.781 in the validation set (Figure 4), while the model excluding treatment achieved AUCs of 0.725, 0.758, and 0.807 in the training set and 0.692, 0.683, and 0.695 in the validation set (Figure S4), demonstrating high accuracy in predicting CSS. Furthermore, DCA indicated that the nomogram had clinical benefits (Figure 5, Figure S5).
Discussion
This study developed a nomogram containing independent prognostic factors (age, TNM stage, liver metastasis, surgery and chemotherapy) for CSS in patients with elderly-onset PanNEC. The AUCs were higher than 0.8, indicating the high accuracy and discrimination of this model.
We excluded PanNEC patients under 50 years old from our study due to literature indicating that PanNEC can be categorized by age into early-onset PanNEC (under 50 years) and classic or late-onset PanNEC (50 years and older), with differences in prognosis and clinical characteristics (11). The early-onset PanNEC constitutes less than 10% of cases in our study. To ensure the accuracy of our results, we focused solely on elderly-onset PanNEC patients aged 50 and older.
In our study group, we observed that age was a significant predictor of survival rates, with decreasing relative survival rates observed as age increased. These findings are consistent with previous studies that have reported similar outcomes (4,15). The poorer ability to tolerate the extensive treatment and genetic differences might be the causes.
PanNENs are treated with surgery and systemic therapy including cytotoxic chemotherapy, local radiation, peptide receptor radiotherapy (PRRT), somatostatin analogs and targeted therapy (5,16-18). Compared with PanNET, PanNEC has more limited treatment options due to its rarity and diagnostic novelty and the treatment of PanNEC is more personalized according to tumor grade, KI-67, functionality and tumor behavior (19). For localized PanNEC, radical surgery is usually recommended (18). For PanNEC patients with metastasis, a small number of cases with relatively slow progression may still be eligible for surgical treatment (18). Previous studies have revealed that surgery was associated with improved survival for PanNEC (20,21). However, the studies that adopted the 2010 WHO classification of PanNEC did not distinguish G3 PanNET from PanNEC. Currently, Yoshida et al. demonstrated that the survival of PanNEC with surgery was longer with no statistical significance (22). Our data showed that surgery significantly improved the prognosis of elderly-onset PanNEC patients, consistent with a previous study (23).
Platinum-based chemotherapy is the first-line treatment for PanNEC in several guidelines, and FOLIFIRI (fluorouracil, folinic acid, and irinotecan), FOLFOX (folinic acid, fluorouracil, and oxaliplatin) and CAPTEM (capecitabine and temozolomide) are considered as the second-line selection (5,24-26). Chemotherapy based on platinum compounds, including cisplatin and etoposide, is indicated in cases of unresectable PanNEC (18,27,28). Sorbye et al. found that additional systemic chemotherapy after surgery improved survival for unresectable PanNEC (29). In our study, chemotherapy was also found to improve the CSS of PanNEC patients. However, there are no prospective clinical trials about the effect of the different types of chemotherapy.
The information regarding the effect of the radiotherapy in PanNEC is limited. Iwata et al. found that the radiotherapy could improve the survival probability in localized PanNEC (30). Radiotherapy is often palliative care for unresectable PanNEC. PRRT is a novel treatment modality that primarily targets PanNEN with overexpressed somatostatin receptors (17), but the effect of PRRT for PanNEC is unclear. In our study, radiotherapy can not improve the CSS for PanNEC; however, the effects of radiotherapy need to be further studied.
The clinical features of PanNEC are similar to exocrine pancreatic tumors (31), so the 8th AJCC stage originally applied to exocrine pancreatic tumors is also applicable for PanNEC. In addition, the TNM stage was a good prognostic factor for CSS in PanNEC in our study, and stage IV had the worst prognosis, probably because tumors at this stage have metastasized.
In our study, nearly 70% of PanNEC patients had liver metastasis, and liver metastasis was identified as an independent risk factor for poor prognosis, which is consistent with previous research findings (18,32,33). Liver metastasis had also been found to be associated with the survival of PanNEC in our study, which might contribute to an increased tumor progression and a lower chance of receiving surgical treatment (18).
To our knowledge, this study is the first to build a nomogram based on a multicenter database to predict the prognosis of elderly-onset PanNEC. Our findings improve clinical management by allowing the identification of independent prognostic factors in patients with elderly-onset PanNEC.
Our study has limitations. First, family history, history of drinking and smoking, and immunotherapy were not obtained from the SEER database. Second, the study’s subjects are primarily white, so caution is needed when applying the findings to other ethnic groups, especially Asian populations. Third, our model was not validated externally. Large scale prospective studies are needed to validate the results.
Conclusions
We built and validated a nomogram that could accurately predict the prognosis of patients with elderly-onset PanNEC, enabling clinicians to predict CSS in these patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-344/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-344/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-344/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu Z, Wang L, Dai S, et al. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open 2021;4:e2124750. [Crossref] [PubMed]
- Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589-97. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844-60. [Crossref] [PubMed]
- Kawasaki K, Fujii M, Sato T. Gastroenteropancreatic neuroendocrine neoplasms: genes, therapies and models. Dis Model Mech 2018;11:dmm029595. [Crossref] [PubMed]
- Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: New insights and treatment implications. Cancer Treat Rev 2016;50:61-7. [Crossref] [PubMed]
- Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012;36:173-84. [Crossref] [PubMed]
- Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 2011;29:2372-7. [Crossref] [PubMed]
- Lee MR, Harris C, Baeg KJ, et al. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin Gastroenterol Hepatol 2019;17:2212-2217.e1. [Crossref] [PubMed]
- Goksu SY, Ozer M, Kazmi SMA, et al. Distinct Clinical Characteristics in Young-Onset Pancreatic Neuroendocrine Tumor. Cancers (Basel) 2020;12:2501. [Crossref] [PubMed]
- Liu M, Sun X, Zhang Z, et al. The clinical characteristics and survival associations of pancreatic neuroendocrine tumors: does age matter? Gland Surg 2021;10:574-83. [Crossref] [PubMed]
- Beeghly-Fadiel A, Luu HN, Du L, et al. Early onset pancreatic malignancies: Clinical characteristics and survival associations. Int J Cancer 2016;139:2169-77. [Crossref] [PubMed]
- Shi M, Zhou B. Clinical Characteristics and Prognostic Factors of Early-Onset Pancreatic Neuroendocrine Tumors. Cancer Control 2021;28:1073274820986827. [Crossref] [PubMed]
- Boyar Cetinkaya R, Aagnes B, Myklebust TÅ, et al. Survival in neuroendocrine neoplasms; A report from a large Norwegian population-based study. Int J Cancer 2018;142:1139-47. [Crossref] [PubMed]
- Liu DJ, Hua R, Sun YW. Current treatment status in pancreatic neuroendocrine neoplasms. Chin Clin Oncol 2019;8:20. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Sorbye H, Grande E, Pavel M, et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J Neuroendocrinol 2023;35:e13249. [Crossref] [PubMed]
- Rodriguez-Freixinos V, Thawer A, Capdevila J, et al. Advanced Pancreatic Neuroendocrine Neoplasms: Which Systemic Treatment Should I Start With? Curr Oncol Rep 2021;23:80. [Crossref] [PubMed]
- Haugvik SP, Janson ET, Österlund P, et al. Surgical Treatment as a Principle for Patients with High-Grade Pancreatic Neuroendocrine Carcinoma: A Nordic Multicenter Comparative Study. Ann Surg Oncol 2016;23:1721-8. [Crossref] [PubMed]
- Crippa S, Partelli S, Bassi C, et al. Long-term outcomes and prognostic factors in neuroendocrine carcinomas of the pancreas: Morphology matters. Surgery 2016;159:862-71. [Crossref] [PubMed]
- Yoshida T, Hijioka S, Hosoda W, et al. Surgery for Pancreatic Neuroendocrine Tumor G3 and Carcinoma G3 Should be Considered Separately. Ann Surg Oncol 2019;26:1385-93. [Crossref] [PubMed]
- Luo X, Yang M, Tian B, et al. Surgical Outcomes and Prognostic Factors of G3 Pancreatic Neuroendocrine Carcinomas: A Consecutive Analysis Based on Previous Study Results. J Clin Med 2022;11:3176. [Crossref] [PubMed]
- Singh S, Dey C, Kennecke H, et al. Consensus Recommendations for the Diagnosis and Management of Pancreatic Neuroendocrine Tumors: Guidelines from a Canadian National Expert Group. Ann Surg Oncol 2015;22:2685-99. [Crossref] [PubMed]
- Halfdanarson TR, Strosberg JR, Tang L, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:863-81. [Crossref] [PubMed]
- Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103:153-71. [Crossref] [PubMed]
- Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016;103:186-94. [Crossref] [PubMed]
- Ito T, Masui T, Komoto I, et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol 2021;56:1033-44. [Crossref] [PubMed]
- Sorbye H, Strosberg J, Baudin E, et al. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014;120:2814-23. [Crossref] [PubMed]
- Iwata T, Ueno H, Itami J, et al. Efficacy of radiotherapy for primary tumor in patients with unresectable pancreatic neuroendocrine tumors. Jpn J Clin Oncol 2017;47:826-31. [Crossref] [PubMed]
- Guilmette JM, Nosé V. Neoplasms of the Neuroendocrine Pancreas: An Update in the Classification, Definition, and Molecular Genetic Advances. Adv Anat Pathol 2019;26:13-30. [Crossref] [PubMed]
- Chen XY, Guo NJ, Guo PL, et al. Clinical features and prognosis of advanced intra- and extra-pulmonary neuroendocrine carcinomas. J Cancer Res Ther 2023;19:951-6. [Crossref] [PubMed]
- Park HK, Kwon GY. Comparison of Metastatic Patterns Among Neuroendocrine Tumors, Neuroendocrine Carcinomas, and Nonneuroendocrine Carcinomas of Various Primary Organs. J Korean Med Sci 2023;38:e85. [Crossref] [PubMed]


